Abstract
Multidetector computed tomography has come a long way in a short time, quickly becoming a standard tool in the cardiac imaging armamentarium. The promise of plaque imaging, combined with both anatomical visualization and stenosis detection, has made this a preferred first line test of many cardiologists and radiologists. This test is well suited to rule out coronary artery disease (obstruction) and still diagnosing subclinical plaque, with may be a good target for anti-atherosclerotic therapies. There has been recent criticism against CT imaging, and cardiac CT specifically, due to the high radiation doses that being employed. New advances have allowed for dramatic dose reductions. These include more routinely performed methods such as dose modulation, and newer methods such as prospective gating or minimizing the field of view. This paper will review the different applications to reduce cardiac CT radiation doses to nominal levels, potentially expanding the applications of cardiac CT by removing one of the biggest barriers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decade great strides have been made in the field of cardiac imaging, particularly in the ability to view the coronary artery lumen with ample diagnostic accuracy [1]. Current 64-MDCT (multi-row detector computed tomography) systems have faster gantry rotation speed resulting in better temporal resolution. Better Z-axis spatial resolution is made possible by thin collimations with extensive volumetric acquisitions [2, 3]. The technique of retrospective image acquisition scanning with overlapping of slices that compensates for potential cardiac motion data at all phases of the cardiac cycle to assess ejection fraction valves and wall motion. [2, 3]. This results in more patient radiation exposure in comparison with CTA with prospective-gated acquisition (originally used with the electron beam tomography scanner) [4]. Studies estimate radiation exposure for 16-row at 8.8 mSv for a 16 × 0.75 mm scan protocol with a pitch of 0.28 and power of 370 mA [5], and 13 and 18 mSv (for men and women, respectively) with 64-row MDCT [6]. In a recent study, Pugliese et al. [7] reported radiation doses in the order of 15:20 mSv (male:female), respectively. These doses for retrospective imaging are significantly higher than those seen with invasive diagnostic coronary angiography where the dose is between 2.1 and 7 mSv [8, 9], but lower than the effective radiation dose for a technetium Tc 99m sestamibi myocardial perfusion stress test (9–18 mSv, increasing to over 20 mSv with dual-isotope scanning) [10, 11].
Methods to minimize dose
Multiple different radiation reductions methods have been reported, all of which can result in complementary reductions in radiation exposure. This paper outlines reported and potential methods, as well as discusses the potential for future radiation reduction techniques. While it is logical and in keeping with the principles of ALARA (as low as reasonably achievable) to use the minimum radiation dose necessary in cardiac CT examinations, there are little data testing the effect of specific radiation sparing approaches on diagnostic quality. Cardiac CTA imaging protocols are also not as widely standardized, and thus maintaining diagnostic accuracy is important. An examination which is non-diagnostic, of course, exposes the patient to radiation without benefit. However, experience dictates that through attention to detail of the CT acquisition, excellent image quality can be maintained at a lower radiation exposure.
Dose related issues
Limiting craniocaudal coverage length
The region of the patient’s body that undergoes radiation exposure in the craniocaudal, or z-direction is the largest contributor to radiation absorption (via changes in the dose length product [DLP]). For cardiac CT examinations, proper attention to scan length should be made. For example, rather than rigidly scanning from carina to diaphragm as is frequently recommended by scanner manufacturers, the operator could potentially use the non-contrast images for calcium scoring, if necessary to ascertain the most cranial and caudal position of the coronary arteries.
Using the scout image, the field of view usually extends from ~1 cm below the carina to just below the diaphragm to ensure complete coronary imaging (Fig. 1). The scout film is highly inaccurate for determining the precise origin of the coronary arteries [12]. An alternative is to use images from the coronary calcium scoring to set the upper limit above the apex of the left anterior descending artery (the most superior artery) and the lower limit inferior to the posterior descending artery, leaving sufficient but not excessive margins, to allow for movement. Calcium scoring with MDCT delivers ~1.2 mSv when acquired with prospective ECG gating, and potentially lower if 100 kVp is used for this acquisition [4]. A 30% reduction in radiation dose has been shown by employing a calcium score to determine the superior and interior borders of the field of view, with the superior border at 1 cm above the visualized top of the coronary arteries and the inferior border at 1 cm below the posterior descending artery [13]. The savings in radiation dose was 4 mSv offsetting the dose delivered by calcium scoring (1.2 mSv). The average percentage reduction in radiation was 30% (SD ± 10) with a median of 22%. This method of dose reduction is incremental to dose modulation as well as reducing the mA or kV during image acquisition. Multipurpose examinations of the chest have long Z axis acquisitions and thus higher radiation dose. Multipurpose examinations of the chest tend to have long Z axis acquisitions and very high radiation exposures. For example, to do a “triple rule out” examination, both scan length and scan time are affected, and there is a consequent marked increase in radiation exposure. Thus, this protocol should be clinically indicated to justify the additional radiation.
A scout film (left panel) used to identify the upper and the lower limits of the region of interest for the subsequent CTA. The white arrow shows the level of the carina, which is a landmark for determining the top of the coronary tree. Middle panel An axial slice of a non-contrast coronary calcium scan showing the left main artery (black arrow). The exact level in which the superior most coronary arteries arise is most effectively visualized with axial data such as the calcium scan. Right panel An axial slice of a non-contrast coronary calcium scan defining the lower limits of the heart, demonstrating the level just below the posterior descending artery. There are no coronary arteries on this image, so concluding the CTA just below this would safely allow for complete imaging of the coronary tree
Tube amperage
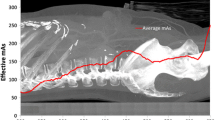
Tube mA is a variable setting that affects the number of photons generated and directly affects image signal to noise ratio. Radiation dose is approximately proportional to mA, and customarily, amperage can be adjusted for body mass and configuration [3]. Patients with higher mass will experience higher photon scatter and higher noise, while thinner patient will have images of good quality using lower amperage. Failure to adjust mA downward for thin patients will result in unnecessary radiation. It is important to reduce mA to the lowest necessary to make diagnostic images. Typical doses are 350 mA for small patients, 450 mA for medium sized patients and 550 or higher for large patients (scaled to maximum mA of the X-ray tube for the heaviest patients). Typical settings from the manufacturers are pre-set higher, to optimize image quality at the expense of radiation dose to the patient.
Dose modulation (electrocardiographic pulsing)
Current generation scanners are capable of varying tube current output (mA) in synchrony to the patient’s electrocardiogram. This is done to reduce radiation during phases in the cardiac cycle when the heart is moving more dynamically (ventricular systole and end diastole [which correlates with atrial systole]). The ideal “pulsing window” (when current becomes maximal) is as short as possible, typically focused around the 70% phase of the cardiac cycle. This becomes a complex decision as there is a trade-off between pulse window width, heart rate and scanner type. All current-generation cardiac-capable scanners have built-in software that either varies pulsing window width with heart rate or allows the operator to customize these protocols. The use of ECG pulsing can decrease radiation dose by 50% or more and is generally recommended unless other parameters threaten the image quality (such as irregular heart rate). The time of least motion occurs between 40 and 80% of the R–R interval (mid diastole) [14, 15]. With dose modulation, the dose is reduced by 18–47% depending on the patient’s heart rate [16–18]. Slower heart rates will lead to more effective use of dose-modulation and lower radiation doses (with the exception of the dual source scanner, where slower heart rates will increase radiation exposure). The multicenter 64-MDCT ACCURACY trial utilized dose modulation and still reported negative predictive values of 99% [1]. While EKG-regulated dose modulation could be implemented in a majority of patients, sometimes it cannot be utilized due to scanning conditions requiring additional image reconstructions during different phases of the cardiac cycle (such as irregular heart rhythms and fast heart beats). Although the radiation burden of cardiac CT studies can be efficiently reduced by dose modulation, a further decrease in radiation without compromising diagnostic image quality would be indeed very desirable [19].
Scanner type
In general, increasing the number of detector-rows and reducing detector size tends to increase the radiation dose due to the increasing surface area of lead collimators (which can only be so thin while still being effective) in comparison to detector area. The more lateral detectors that are present lead to less efficient delivery of dose, as the lateral detectors (those not directly across from the X-ray tube) require more radiation exposure due to scatter. Thus, four row scanners had lower patient radiation doses than 16 slice scanners, despite longer radiation times. Similarly 16 row scanners impart lower doses than 64, and subsequently, doses from 64 will be lower than 256 and 320 row scanners (for similar protocols). In addition, complex effects are also produced by dual source scanners which have two X-ray sources and detector rings operating during the scan time, but have a reduced scan time and heart-rate variable pitch. Theoretically, dual source scanners reduce the scan duration because their faster temporal resolution allows imaging faster heart rates thereby reducing diastolic time, which balances the double dose of radiation being emitted, leading to similar doses as 64 row MDCT scanners and lower doses than 256 and 320 row scanners.
Reducing tube voltage
Another methodology to reduce the dose is to reduce tube voltage. Tube kVp affects the peak photon energy and affects image contrast. In general, kVp has been used to frequently adjust for body mass. New studies suggest that that protocols utilizing 100 or 80 kVp may be effective in thin patients for reducing dosage in coronary calcium measurements or coronary CT angiography, without degrading diagnostic accuracy [20]. Tube voltage has a more dramatic effect on radiation dosage, which varies approximately with the square of the kVp. Budoff et al. [21] demonstrated use of 100 kVp reduced radiation dose 42% using prospective triggering and 40% using retrospective imaging, as compared to 120 kVp (P < 0.001). Hausleiter et al. [22] showed a 53–64% reduction in estimated radiation dose using both reduced kVp and dose modulation. As noted above, this benefit is independent and incremental to dose modulation radiation savings and other savings listed above (Table 1).
Limiting field of view (Fov)
An important technique that is currently underused, which will both minimize radiation and afford better image quality, is to restrict the xy field of view. A smaller xy field of view will improve image quality, as the FOV divided by 512 is the resolution in the X–Y axis (to an optimal resolution of about 0.3–0.35 mm for current scanners). The bowtie filter allows for smaller radiation exposure by limiting the scatter of the X-ray towards the detectors. Intrinsically, it is more efficient to irradiate detectors directly across from the X-ray tube and thus, requires less photons. Thus, the bowtie filter allows less X-rays out of the central part of the X-ray tube, as less are required to expose the detectors. As one moves laterally from the center of the detector array, the number of photons needed to create an image is higher (less efficiency and thus, the relative dose increases for scanners with higher numbers of detectors (see #4 above). The bowtie filter allows more photons to go toward the lateral detectors, thus looking like a bowtie, with a small center and large lateral edges. These filters are optimized for patient size, and the smallest bowtie filters, only allow the lateral tissue to be exposed to 32 cm, the medium bowtie to 36 cm and the largest bowtie to over 40 cm. Since all hearts (including bypass patients) fit in a small bowtie filter, the use of larger bow-ties would only be necessary when larger field of views are needed. In a study of radiation doses (measured radiation exposures using the LightSpeed VCT 64-MDCT scanner (GE Healthcare, Waukesha, WI, USA), the dose using the small cardiac bowtie (can only be post-processed up to 32 cm field of view), resulted in a dose of 8 mSv (retrospective imaging, as low as 1 mSv with prospective imaging) [23]. A medium bowtie filter for a retrospectively acquired cardiac CT angiogram using the same protocol affords 13.4 mSv of radiation. This is a 40% dose reduction by simply using an appropriate sized bowtie filter for the study required (cardiac CT requires at most 25 cm of view). Larger patients may require a medium bowtie filter due to optimization protocols, but for cardiac applications, a large bowtie filter would never be necessary. The routine use of small bowtie filters (and small FOV) will both reduce radiation dose and limit incidental findings related to non-cardiac disease (primarily non-cancerous lung nodules). There is no evidence yet to support that discovering these incidental findings will lead to improvement in outcomes. There is data available that these incidental findings increase surgical rates, costs and patient anxiety [24]. For recommended bowtie filters based on body habitus for the GE VCT Scanner, see Table 2. These findings need to be validated for other scanners and manufacturers.
Sequential (prospective) scanning versus retrospective gating
Until recently, all studies were done with retrospective gating, using continuous X-ray beam exposure to create thousands of images, then selecting a subset of those at the optimal phase of the cardiac cycle for interpretation. An alternative imaging mode is the axial step-and-shoot acquisition (prospective gating). This was first used with electron beam CT angiography as early as 1995, and with calcium scoring with MDCT since 1998. Approximately 2 years ago, prospective gating was introduced for MDCT angiography. This is by far the most significant dose reduction technique, as it turns off the X-ray tube except for a short exposure period centered around the ideal imaging phase (typically mid-diastole). In patients with slow and very steady heart rates, extremely low radiation doses can be achieved by sequential scanning with tube output only during a narrow ECG window (Table 3). The X-ray beam is triggered by the ECG and is turned on only during the required phase of the cardiac cycle (usually 75% of the cardiac cycle) and turned off during the rest of the cardiac cycle. Retrospective ECG gating can be done with or without ‘padding’. Padding provides additional phase information to account for variations in heart rate by adding time before and after the center phase of the acquisition (e.g. adding 100 ms before and after the 75% phase thus providing additional phase information). Using the GE technology, padding is prescribed in the range of 0–200 ms and is added to both sides of the center of the acquisition. This allows more phases of data to be available to the reader and is generally used when heart rates are >60 bpm or when heart rate variability is noted. The padding should be wider with more rapid heart rates [25]. When padding was used, it is added in a heart rate dependent fashion as follows: 30–39 bpm, 175 ms padding; 40–49 bpm, 150 ms padding; 50–59 bpm, 125 ms padding and ≥60 bpm, 100 ms padding. Padding default is set to approximately ±10% of the patient’s R–R cycle. Patients with very stable and slow heart rates underwent no padding to ensure the lowest possible radiation dose. The prescribed PA window is defined as center phase ± milliseconds padding (Fig. 2).
Shows stenosis in left anterior descending from patient who underwent both cardiac gated helical (RS-OHA) and SnapShot Pulse acquisitions (PA) within 1 week. Images courtesy of Cardiovascular Medical Group, Beverly Hills, CA. Left panel is retrospective image, middle panel is prospective image and right panel is corresponding cardiac catheterization
Multiple studies have demonstrated similar or superior image quality with prospective imaging (LightSpeed VCT 64-MDCT scanner), and resulted in average doses of 1–3 mSv [26–28]. Superior image quality with prospective imaging is most likely due to lack of ‘Z axis’ motion, as there is no movement of the table (zero pitch) during image acquisition. Multiple studies have reported mean patient radiation doses 77–80% lower for prospective gating than for retrospective gating, without compromising image quality or diagnostic accuracy. Budoff et al. [21] showed a 90% reduction in estimated radiation dose using a combination of reduced kVp (100) and prospective triggering together. In a majority of patients, patients can undergo prospective triggering with marked radiation dose reduction, if ejection fraction information or multiple phase acquisition is not necessary. This removes one of the biggest barriers to more widespread implementation of cardiac CT [29].
Future horizons for dose reduction
The newly released High Definition CT (CT750 HD, GE Healthcare, Waukesha, WI, USA) uses the first new detector material in 20 years. This detector material, by changing the molecular structure of real garnets, a scintillator that is proposed to deliver images 100 times faster, with up to 33% greater detail through the body and up to 47% greater detail in the heart. This also allows up to 2,496 views per rotation (a 2.5× increase) to deliver improved spatial resolution and improved image quality across the entire field of view. While validation studies are now underway, the vendor reports a reduced dose by as much as 83% for cardiac CT. The exact application and overlap with other dose reduction techniques remains to be studied, but the use of more efficient detectors may reduce signal to noise ratios without requiring the typical increase in radiation dose. The High Definition CT should improve visualization of stents and other small structures currently limited by spatial resolution in current 64–320 scanners (Fig. 3).
Left image Resolution of stent impaired with current 64-multi-detector computed tomography. Middle image Improved visualization of in-stent restenosis by High Definition CT, with confirmation of in-stent restenosis by invasive angiography. The arrows represent the stent borders on angiography. Images courtesy of Dr. J. L. Sablayrolles, Centre Cardiologique du Nord, Saint Denis, France
Summary
Cardiac CT imaging protocols should be constructed to minimize the radiation exposure to the patient while providing the best possible information for an accurate diagnosis. Diagnostic imaging most likely saves thousands of lives each year by providing medical information to the physician for better medical management of the patient for the overall outcome of the patient’s health. While there may be a finite risk associated with a diagnostic study using low level ionizing radiation, the typical patient presents a higher risk by not having the imaging study performed. Using the techniques outlined for adequate patients (based upon body habitus, heart rate and cardiac rhythm), dose reductions up to 70–90% can be achieved when compared to earlier acquisition strategies for cardiac CT.
References
Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK (2008) Diagnostic performance of 64-detector row coronary computed tomographic angiography of individuals undergoing invasive coronary prospective multicenter ACCURACY (assessment by coronary computed individuals without known coronary artery disease: results from the tomographic angiography for evaluation of coronary artery stenosis in angiography) trial. J Am Coll Cardiol 52(21):1724–1732
Cademartiri F, Malagutti P, Belgrano M et al (2005) Non-invasive coronary angiography with 64-slice computed tomography. Minerva Cardioangiol 53(5):465–472
Budoff MF, Gopal A, Gopalakrishnan D (2006) Cardiac computed tomography: diagnostic utility and integration in clinical practice. Clin Card 29(9 Suppl 1):I4–I14
Morin RL, Gerber TC, McCollough CH (2003) Radiation dose in computed tomography of the heart. Circulation 107(6):917–922
Bae KT, Hong C, Whiting BR (2004) Radiation dose in multidetector row computed tomography cardiac imaging. J Magn Reson Imaging 19:859–863
Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA (2005) Spiral computed tomography diagnostic accuracy of noninvasive coronary angiography using 64-slice. J Am Coll Cardiol 46:552–557
Pugliese F, Mollet NR, Runza G et al (2006) Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol 16(3):575–582
Mollet NR, Cademartiri F, van Mieghem CA, Runza G, McFadden EP, Baks T, Serruys PW, Krestin GP, de Feyter PJ (2005) High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation 112:2318–2323
Kocinaj D, Cioppa A, Ambrosini G, Tesorio T, Salemme L, Sorropago G, Rubino P, Picano E (2006) Radiation dose exposure during cardiac and peripheral arteries catheterisation. Int J Cardiol 113(2):283–284
Thompson R, Cullom S (2006) Issues regarding radiation dosage of cardiac nuclear and radiography procedures. J Nucl Cardiol 13:19–23
Clarke EA, Notghi A, Harding LK (1997) Are MIBI/tetrofosmin heart studies a potential radiation hazard to technologists? Nucl Med Commun 18:574–577
Bakhsheshi H, Mao SS, Budoff MJ, Lu B, Brundage BH (2000) Preview method for electron beam scanning of the coronary arteries. Acad Radiol 7:620–626
Gopal A, Budoff MJ (2007) A new method to reduce radiation exposure during multi-row detector cardiac computed tomographic angiography. Int J Cardiol. doi:10.1016/j.ijcard.2007.08.072
Lu B, Mao SS, Zhuang N et al (2001) Coronary artery motion during the cardiac cycle and optimal ECG triggering for coronary artery imaging. Invest Radiol 36(5):250–256
Mao S, Lu B, Oudiz RJ et al (2000) Coronary artery motion in electron beam tomography. J Comput Assist Tomogr 24(2):253–258
Flohr TG, Schoepf UJ, Kuettner A et al (2003) Advances in cardiac imaging with 16-section CT systems. Acad Radiol 10(4):386–401
Jakobs TF, Becker CR, Ohnesorge B et al (2002) Multislice helical CT of the heart with retrospective EKG gating: reduction of radiation exposure by EKG-controlled tube current modulation. Eur Radiol 12(5):1081–1086
Abada HT, Larchez C, Daoud B, Sigal-Cinqualbre A, Paul JF (2006) MDCT of the coronary arteries: feasibility of low-dose CT with EKG-pulsed tube current modulation to reduce radiation dose. AJR Am J Roentgenol 186(6 Suppl 2):S387–S390
Budoff MJ (2008) Cardiac CT principles. Chapter 1. In: Budoff MJ, Shinbane J (eds) Handbook of cardiovascular CT, essentials for clinical practice, 1st edn. Springer, London
Gopal A, Mao SS, Karlsberg D, Young E, Waggoner J, Ahmadi N, Pal RS, Leal J, Karlsberg RP, Budoff MJ (2008) Radiation reduction with prospective ECG-triggering acquisition using 64-multidetector computed tomographic angiography. Int J Cardiovasc Imaging. doi:10.1007/s10554-008-9396-z
Budoff MJ, Waggoner J, Ahmadi N, Pal RS, Sarlak B, Honoris L, Carson S, Child J, Mao SS, Gopalakrishnan D, Gopal A (2008) Substantial radiation dose reduction in 64-multidetector cardiac computed tomographic angiography by using lower X-ray energy during scanning. J Am Coll Cardiol 51:A148
Hausleiter J, Meyer T, Hadamitzky M, Huber E, Zankl M, Martinoff S, Kastrati A, Schomig A (2006) Radiation dose estimates from cardiac multislice computed tomography in daily practice. Circulation 113:1305–1310
Budoff MJ (2008) Ethical issues related to lung nodules on cardiac CT. Am J Roentg
Budoff MJ, Gopal A (2007) Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr 1:97–105
Steigner ML, Otero HJ, Cai T, Mitsouras D, Nallamshetty L, Whitmore AG et al (2008) Narrowing the phase window width in prospectively ECG-gated single heart beat 320-detector row coronary CT angiography. Int J Cardiovasc Imaging. 25(1):85–90. doi:10.1007/s10554-008-9347-8
Shuman WP, Branch KR, May JM, Mitsumori LM, Lockhart DW, Dubinsky TJ, Warren BH, Caldwell JH (2008) Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: comparison of image quality and patient radiation dose. Radiology 248(2):431–437
Hirai N, Horiguchi J, Fujioka C, Kiguchi M, Yamamoto H, Matsuura N, Kitagawa T, Teragawa H, Kohno N, Ito K (2008) Prospective versus retrospective ECG-gated 64-detector coronary CT angiography: assessment of image quality, stenosis, and radiation dose. Radiology 248(2):424–430
Earls JP, Berman EL, Urban BA, Curry CA, Lane JL, Jennings RS, McCulloch CC, Hsieh J, Londt JH (2008) Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology 246(3):742–753
Kalra MK, Brady TJ (2008) Current status and future directions in technical developments of cardiac computed tomography. J Cardiovasc Comput Tomogr 2:71–80
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Budoff, M.J. Maximizing dose reductions with cardiac CT. Int J Cardiovasc Imaging 25 (Suppl 2), 279–287 (2009). https://doi.org/10.1007/s10554-008-9405-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-008-9405-2