Abstract
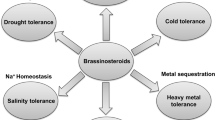
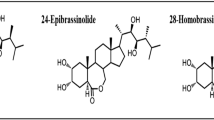
Brassinosteroids (BRs) are plant hormones that were isolated for the first time in the 1970s. This group currently includes more than 70 compounds that differ in their structure and physiological activity. BRs are present in plants in a free form or in the form of conjugates. BRs are known as plant growth regulators, but they also play a role in the plant response to environmental stresses. In the case of plants that are exposed to low/high temperature, exogenous BRs can counteract growth inhibition and reduce biomass losses as well as increase plant survival. BRs show a multidirectional activity in regulating the metabolism of plants exposed to extreme temperatures. The following BRs actions can be distinguished: changes in membrane physicochemical properties, regulation of the expression of selected genes (including stress-responsive genes), as well as indirect effects on metabolism through other hormones or signalling molecules (such as hydrogen peroxide). This review summarizes the current knowledge about the effects of BRs on the physiological and biochemical processes that occur in plants during exposure to low or high temperatures.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Abbreviations
- ABA:
-
abscisic acid
- APX:
-
ascorbate peroxidase
- AsA:
-
ascorbic acid
- BAK1:
-
BRI1 associated receptor kinase1
- BES1:
-
bri1-EMS-supressor1
- BIN2:
-
brassinosteroid insensitive2 kinase
- BR:
-
brassinosteroid
- BRI1:
-
cell surface receptor kinase
- Brz:
-
brassinazole
- BSK1:
-
brassinosteroid-signalling kinase1
- BZR1:
-
brassinazole resistant1
- CAT:
-
catalase
- CBFs:
-
C-repeat/dehydration responsive element binding factors
- CI:
-
chilling injury
- COR:
-
cold-responsive proteins
- DHAR:
-
dehydroascorbate reductase
- E:
-
transpiration rate
- ETR:
-
electron transport rate
- Fv/Fm :
-
maximum quantum efficiency of PS II photochemistry
- Fv’/Fm’ :
-
efficiency of open reaction centres in light
- gs:
-
stomatal conductance
- GR:
-
glutathione reductase
- GSH:
-
reduced glutathione
- HSP:
-
heat shock protein
- MDA:
-
malondialdehyde
- MDAR:
-
monodehydroascorbate reductase
- PN :
-
net photosynthetic rate
- POD:
-
peroxidase
- qP:
-
photochemical quenching coefficient
- ROS:
-
reactive oxygen species
- Rubisco:
-
ribulose—1,5-bis-phosphate carboxylase/oxygenase
- SOD:
-
superoxide dismutase
- ΦPSII :
-
effective quantum yield of PS II photochemistry
References
Aghdam, M.S., Asghari, M., Farmani, B., Mohayeji, M., Moradbeygi, H.: Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. - Sci. Hort. 144: 116–120, 2012.
Ashraf, M., Foolad, M.R.: Roles of glycine betaine and proline in improving plant abiotic stress resistance. - Environ. exp. Bot. 59: 206–216, 2007.
Bajguz, A.: Brassinosteroid enhanced the level of abscisic acid in Chlorella vulgaris subjected to short-term heat stress. - J. Plant Physiol. 166: 882–886, 2009.
Bajguz, A., Tretyn, A.: The chemical characteristic and distribution of brassinosteroids in plants. - Phytochemistry 62: 1027–1046, 2003.
Clouse, S.D.: A history of brassinosteroid research from 1970 through 2005: thirty-five years of phytochemistry, physiology, genes and mutants. - J. Plant Growth Regul. 34: 828–844, 2015.
Cui, L., Zou, Z., Zhang, J., Zhao, Y., Yan, F.: 24-Epibrassinoslide enhances plant tolerance to stress from low temperatures and poor light intensities in tomato (Lycopersicon esculentum Mill.). - Funct. integr. Genomics 16: 29–35, 2016.
Deng, Z., Zhang, X., Tang, W., Oses-Prieto J.A., Suzuki, N., Gendron, J. M., Chen, H., Guan, S., Chalkley, R.J., Peterman, T.K., Burlingame, A.L., Wang, Z.-Y.: A proteomic study of brassinosteroid response in Arabidopsis. - Mol. Cell Proteomics 6: 2058–2071, 2007.
Dhaubhadel, S., Browning, K.S., Gallie, D.R., Krishna, P.: Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. - Plant J. 29: 681–691, 2002.
Dhaubhadel, S., Chaudhary, S., Dobinson, K. F., Krishna, P.: Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. - Plant mol. Biol. 40: 333–342, 1999.
Divi, U.K., Rahman, T., Krishna, P.: Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. - Plant Biotechnol. J. 14: 419–432, 2016.
Dockter, C., Gruszka, D., Braumann, I., Druka, A., Druka, I., Franckowiak, J., Gough, S.P., Janeczko, A., Kurowska, M., Lundqvist, J., Lundqvist, U., Marzec, M., Matyszczak, I., Müller, A. H., Okleštková, J., Schulz, B., Zakhrabekova, S., Hansson, M.: Induced variations in brassinosteroid genes define barley height and sturdiness, and expand the “Green Revolution" genetic toolkit. - Plant Physiol. 166: 1912–1927, 2014.
Eremina, M., Unterholzner, S.J., Rathnayake, A.I., Castellanos, M., Khan, M., Kugler, K.G., May, S.T., Mayer, K.F.X., Rozhon, W., Poppenberger, B.: Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. - Proc. nat. Acad. Sci. USA 113: 5982–5991, 2016.
Fahad, S., Hussain, S., Saud, S., Khan, F., Hassan, S., Amanullah, N.W., Arif, M., Wang, F., Huang, J.: Exogenously applied plant growth regulators affect heatstressed rice pollens. - J. Agron Crop Sci. 202: 139–150, 2016.
Fariduddin, Q., Yusuf, M., Chalkoo, S., Hayat, S., Ahmad A.: 28-homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. - Photosynthetica 49: 55–64, 2011.
Filek, M., Rudolphi-Skórska, E., Sieprawska, A., Kvasnica, M., Janeczko, A.: Regulation of the membrane structure by brassinosteroids and progesterone in winter wheat seedlings exposed to low temperature. - Steroids 128: 37–45, 2017.
Fujioka, S., Yokota, T.: Biosynthesis and metabolism of brassinosteroids. - Physiol. Plant. 100: 710–715, 1997.
Grove, M.D., Spencer, G.F., Rohwedder, W.K., Mandava, N., Worley, J.F., Warthen, J.D., Jr., Steffens, G.L., Flippen-Anderson, J.L., Cook, J.C., Jr.: Brassinolide, a plant growthpromoting steroid isolated from Brassica napus pollen. - Nature 281: 216–217, 1979.
Hayat, S., Hayat, Q., Alyemeni, M.N., Wani, A.S., Pichtel, J., Ahmad, A.: Role of proline under changing environments: a review. - Plant Signal Behav. 7: 1456–1466, 2012.
He, R.-Y., Wang, G.-J., Wang, X.-S.: Effects of brassinolide on growth and chilling resistance of maize seedlings. - In: Cutler, G.H., Yokota, T, Adam, G. (ed.): Brassinosteroids. Vol. 19. Pp. 220–230. American Chemical Society, Washington 1991.
Henry, R.P.: Multiple roles of carbonic anhydrase in cellular transport and metabolism. - Annu. Rev. Plant Physiol. 58: 523–538, 1996.
Hodges, D. M., DeLong, J.M., Forney, C.F., Prange, R.K.: Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. - Planta 207: 604–611, 1999.
Hörtensteiner, S., Kräutler, B.: Chlorophyll breakdown in higher plants. - BBA Bioenergetics 1807: 977–988, 2011.
Horváth, I., Glatz, A., Nakamoto, H., Mishkind, M.L., Munnik, T., Saidi, Y., Goloubinoff, P., Harwood, J.L., Vigh, L.: Heat shock response in photosynthetic organisms: membrane and lipid connections. - Progr. Lipid Res. 51: 208–220, 2012.
Hu, W.H., Wu, Y., Zeng, J.Z., He, L., Zeng, Q.M.: Chillinduced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. - Photosynthetica 48: 537–544, 2010.
Janeczko, A. (ed): Wystepowanie, transport i wybrane aspekty aktywnosci fizjologicznej brasinosteroidów w roslinach uprawnych z rodzin Poaceae I Fabaceae. [Presence, Transport and PhysiologicalAactivity of Brassinosteroids in Crop Plants from Poaceae and Fabaceae family]. - Polish Academy of Sciences, Krakow 2016. [In Polish.]
Janeczko, A., Gruszka, D., Pociecha, E., Dziurka, M., Filek, M., Jurczyk, B., Kalaji, H.M., Kocurek, M., Waligórski, P.: Physiological and biochemical characterisation of watered and drought-stressed barley mutants in the HvDWARF gene encoding C6-oxidase involved in brassinosteroid biosynthesis. - Plant Physiol. Biochem. 99: 126–141, 2016.
Janeczko, A., Gullner, G., Skoczowski, A., Dubert, F., Barna, B.: Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. - Biol. Plant. 51: 355–358, 2007.
Janeczko, A., Hura, K., Skoczowski, A., Idzik, I., Biesaga-Koscielniak, J., Niemczyk, E.: Temperature-dependent impact of 24-epibrassinolide on the fatty acid composition and sugar content in winter oilseed rape callus. - Acta Physiol Plant. 31: 71–79, 2009.
Janeczko, A., Okleštková, J., Pociecha, E., Koscielniak, J., Mirek, M.: Physiological effects and transport of 24-epibrassinolide in heat-stressed barley. - Acta Physiol. Plant. 33: 1249–1259, 2011.
Jiang, Y.-P., Huang, L.-F., Cheng, F., Zhou, Y.-H., Xia, X.-J., Mao, W.-H., Shi, K., Yu, J.-Q.: Brassinosteroids accelerate recovery of photosynthetic apparatus from cold stress by balancing the electron partitioning, carboxylation and redox homeostasis in cucumber. - Physiol Plant. 148: 133–145, 2013.
Jin, S.H., Li, X.Q., Wang, G.G., Zhu, X.T.: Brassinosteroids alleviate high-temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. - AoB Plants 7: plv009, 2015.
Johnson, G., Williams, J.P.: Effect of growth temperature on the biosynthesis of chloroplastic galactosyldiacylglycerol molecular species in Brassica napus leaves. - Plant Physiol. 91: 924–929, 1989.
Kagale, S., Divi, U.K., Krochko, J.E., Keller, W.A., Krishna, P.: Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. - Planta 225: 353–364, 2007.
Kim, T.-W., Chang, S.C.,. Lee, J.S, Takatsuto, S., Yokota, T., Kim, S.-K.: Novel biosynthetic pathway of castasterone from cholesterol in tomato. - Plant Physiol. 135: 1231–1242, 2004.
Kim, Y.-S., Joo, S.-H., Hwang, J.-Y., Park, C.H., Kim, S.-K.: Characterization of C29-brassinosteroids and their biosynthetic precursors in immature seeds of Phaseolus vulgaris. - Bull. korean chem. Soc. 27: 1117–1118, 2006.
Kreslavski, V.D., Los, D.A., Allakhverdiev, S.I., Kuznetsov, V.V.: Signaling role of reactive oxygen species in plants under stress.–Russ. J Plant Physiol. 59: 141–154, 2012.
Krishna, P.: Brassinosteroid-mediated stress responses. - J. Plant Growth Regul. 22: 289–297, 2003.
Lafuente, M.T., Zacarias, L., Martinez-Téllez, M.A., Sanchez-Ballesta, M.T., Granell, A.: Phenylalanine ammonia-lyase and ethylene in relation to chilling injury as affected by fruit age in citrus. - Postharvest Biol. Technol. 29: 308–317, 2003.
Li, B., Zhang, C., Cao, B., Qin, G., Wang, W., Tian, S.: Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. - Amino Acids 43: 2469–2480, 2012.
Li, J.M., Jin, H.: The regulation of brassinosteroids signaling. - Trends Plant Sci. 12: 37–41, 2007.
Li, H., Ye, K., Shi, Y., Cheng, J., Zhang, X., Yang, S.: BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. - Mol. Plants 10: 545–559, 2017.
Liu, Y., Jiang, H., Zhao, Z., An, L.: Abscisic acid is involved in brassinosteroids-induced chilling tolerance in the suspension cultured cells from Chorispora bungeana. - J. Plant Physiol. 168: 853–862, 2011.
Liu, Y., Zhao, Z., Si, J., Di, C., Han, J., An, L.: Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. - Plant Growth Regul. 25: 207–214, 2009.
Mazorra, L.M., Holton, N., Bishop, G.J., Núñez, M.: Heat shock response in tomato brassinosteroid mutants indicates that thermotolerance is independent of brassinosteroid homeostasis. - Plant Physiol. Biochem. 49: 1420–1428, 2011.
Micheli, F.: Pectin methylesterases: cell wall enzymes with important roles in plant physiology. - Trends Plant Sci. 6: 414–419, 2001.
Mitchell, J.W., Mandava, N., Worley, J.F., Plimmer, J.R., Smith, M.V.: Brassins - a new family of plant hormones from rape pollen. - Nature 225: 1065–1066, 1970.
Morillon, R., Catterou, M., Sangwan, R.S., Sangwan, B.S., Lassalles, J.P.: Brassinolide may control aquaporin activities in Arabidopsis thaliana. - Planta 212: 199–204, 2001.
Parthier, B.: The role of phytohormones (cytokinins). III Chloroplast development. - Biochem. Physiol. Pflanz. 174: 173–214, 1979.
Park, S.C., Kim, T.-W., Kim, S.-K.: Identification of brassinosteroids with 24R-methyl in immature seeds of Phaseolus vulgaris. - Bull. korean chem. Soc. 21: 1274–1276, 2000.
Pociecha, E., Dziurka, M., Oklestkova, J., Janeczko, A.: Brassinosteroids increase winter survival of winter rye (Secale cereale L.) by affecting photosynthetic capacity and carbohydrate metabolism during the cold acclimation process. - Plant Growth Regul. 80: 127–135, 2016.
Pociecha, E., Dziurka, M., Waligórski, P., Krepski, T., Janeczko, A.: 24-Epibrassinolide pre-treatment modifies cold-induced photosynthetic acclimation mechanisms and phytohormone response of perennial ryegrass in cultivardependent manner. - J. Plant Growth. Regul. 36: 618–628, 2017.
Qu, T., Liu, R., Wang, W., An, L., Chen, T., Liu, G., Zhao, Z.: Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress. - Cryobiology 63: 111–117, 2011.
Samakovli, D., Margaritopoulou, T., Prassinos, C., Milioni, D., Hatzopoulos, P.: Brassinosteroid nuclear signaling recruits HSP90 activity. - New Phytol. 203: 743–757, 2014.
Sakuraba, Y., Lee, S.H., Kim, Y.S., Park, O.K., Hörtensteiner, S., Paek, N.C.: Delayed degradation of chlorophylls and photosynthetic proteins in Arabidopsis autophagy mutants during stress-induced leaf yellowing. - J. exp. Bot. 65: 3915–3925, 2014.
Schaller, H.: The role of sterols in plant growth and development. - Progr. Lipid Res. 42: 163–175, 2003.
Senaratna, T., Mackay, C.E., McKersie, B.D., Fletcher, R.A.: Uniconazole-induced chilling tolerance in tomato and its relationship to antioxidant content. - J. Plant Physiol. 133: 56–61, 1988.
Shu, H.M., Guo, S.Q., Gong, Y.Y., Jiang, J.W., Ni, W.C.: RNA-seq analysis reveals a key role of brassinolideregulated pathways in NaCl-stressed cotton. - Biol. Plant. 61: 667–674, 2017.
Singh I., Kumar U., Singh S.K., Gupta C., Singh M., Kushwaha S.R.: Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. - Physiol. mol. Biol. Plants 18: 229–236, 2012.
Singh, I., Shono, M.: Effect of 24-epibrassinolide on pollen viability during heat-stress in tomato. - Indian J. exp. Biol. 41: 174–176, 2003.
Song, Y.L., Dong, Y.J., Tian, X.Y., Kong, J., Bai, X.Y., Xu, L.L., He, Z.L.: Role of foliar application of 24-epibrassinolide in response of peanut seedlings to iron deficiency. - Biol. Plant. 60: 329–342, 2016.
Takatsuto, S., Kosuga, N., Abe, B-I., Noguchi, T., Fujioka, S., Yokota, T.: Occurrence of potential brassinosteroid precursor steroids in seeds of wheat and foxtail millet. - J. Plant Res. 112: 27–33, 1999.
Thussagunpanit, J., Jutamanee, K., Kaveeta, L., Chai-arree, W., Pankean, P., Homvisasevongsa, S., Suksamrarn, A.: Comparative effects of brassinosteroid and brassinosteroid mimic on improving photosynthesis, lipid peroxidation and rice seed set under heat stress. - J. Plant Growth Regul. 34: 320–331, 2015b.
Thussagunpanit, J., Jutamanee, K., Sonjaroon, W., Kaveeta, L., Chai-arree, W., Pankean, P., Suksamrarn, A.: Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. - Photosynthetica 53: 312–320, 2015a.
Tomashow, M.F.: Role of cold-responsive genes in plant freezing tolerance. - Plant Physiol. 118: 1–7, 1998.
Wang, Q., Ding, T., Gao, L., Pang, J., Yang, N.: Effect of brassinolide on chilling injury of green bell pepper in storage. - Sci. Hort. 144: 195–200, 2012.
Wang, W., Bai, M.Y., Wang, Z.Y.: The brassinosteroid signalling network - a paradigm of signal transduction. - Curr. Opin. Plant Biol. 21: 147–153, 2014.
Wang, Z.Y., Wang, Q.M., Chong, K., Wang, L., Bai. M.Y., Jia, C.G.: The brassinosteroid signal transduction pathway. - Cell Res. 16: 427–434, 2006.
Weiss, D., Ori, N.: Mechanisms of cross talk between gibberellin and other hormones. - Plant Physiol. 144: 1240–1246, 2007.
Wilen, R., Sacco, W., Gusta, M. L. V., Krishna, P.: Effects of 24-epibrassinolide on freezing and thermotolerance of bromegrass (Bromus inermis) cell cultures. - Physiol. Plant. 95: 195–202, 1995.
Winter, J., Schneider, B., Meyenburg, S., Strack, D., Adam, G.: Monitoring brassinosteroid biosynthetic enzymes by fluorescent tagging and HPLC analysis of their substrates and products. - Phytochemistry 51: 237–242, 1999.
Wu, X., Yao, X., Chen, J., Zhu, Z., Zhang, H., Zha, D.: Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. - Acta Physiol Plant. 36: 251–261, 2014b.
Wu, X.X., He, J., Zhu, Z.W., Yang, S.J., Zha, D.S.: Protection of photosynthesis and antioxidative system by 24-epibrassinolide in Solanum melongena under cold stress. - Biol. Plant. 58: 185–188, 2014a.
Xia, X.-J., Huang, L.F., Zhou, Y.H., Mao, W.H., Shi, K., Wu, J.X., Asami, T., Chen, Z., Yu, J.Q.: Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. - Planta 230: 1185–1196, 2009b.
Xia, X.-J., Wang, Y.-J., Zhou, Y.-H., Tao, Y., Mao, W.-H., Shi, K., Asami, T., Chen, Z., Yu, J.-Q.: Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. - Plant Physiol. 150: 801–814, 2009a.
Xia, X.-J., Zhou, Y.-H., Shi, K., Zhou, J., Foyer, C.H., Yu, J.-Q.: Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. - J. exp. Bot. 66: 2839–2856, 2015.
Xia, X.-J., Fang, P.-P., Guo, X., Qian, X.-J., Zhou, J., Shi, K., Zhou, Y.-H., Yu, J.-Q.: Brassinosteroid-mediated apoplastic H2O2-glutaredoxin 12/14 cascade regulates antioxidant capacity in response to chilling in tomato. - Plant Cell Environ. 2017: 1–13, 2017.
Yang, G, Komatsu, S.: Microarray and proteomic analysis of brassinosteroid - and gibberellin-regulated gene and protein expression in rice. - Genomics Proteomics Bioinfor. 2: 77–83, 2004.
Yang, C.-J., Zhang, C., Lu, Y.-N., Jin, J.-Q., Wang, X.-L.: The mechanisms of brassinosteroids’ action: from signal transduction to plant development. - Mol. Plants 4: 588–600, 2011.
Yang, S., Maeshima, M., Tanaka, Y., Komatsu, S.: Modulation of vacuolar H+ - pumps and aquaporin by phytohormones in rice seedling leaf sheaths. - Biol. pharmacol. Bull. 26: 88–92, 2003.
Yokota, T., Ogino, Y., Takahashi, N., Saimoto, H., Fujioka, S., Sakurai, A.: Brassinolide is biosynthesized from castasterone in Catharanthus roseus crown gall cells. - Agr. Biol. Chem. 54: 1107–1108, 1990.
Yoshizawa, E., Kaizuka, M., Yamagami, A., Higuchi-Takeuchi, M., Matsui, M., Kakei, Y., Shimada, Y., Sakuta, M., Osada, H., Asami, T., Nakano, T.: BPG3 is a novel chloroplast protein that involves the greening of leaves and related to brassinosteroid signaling. - Biosci. Biotechnol. Biochem. 78: 420–429, 2014.
Yu, J.Q., Huang, L.F., Hu, W.H., Zhou, Y.H., Mao, W.H., Ye, S.F., Nogués, S.: A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. - J. exp. Bot. 55: 1135–1143, 2004.
Yuan, L., Shu, S., Sun, J., Guo, S., Tezuka, T.: Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. - Photosynth. Res. 112: 205–214, 2012.
Zhang, S., Wei, Y., Lu, Y., Wang, X.: Mechanisms of brassinosteroids interacting with multiple hormones. - Plant Signal. Behav. 4: 1117–1120, 2009.
Zhang, Y.P., He, J., Yang, S.J., Chen, Y.Y.: Exogenous 24-epibrassinolide ameliorates high temperature-induced inhibition of growth and photosynthesis in Cucumis melo. - Biol. Plant. 58: 311–318, 2014.
Zhang, Y.P., Zhu, X.H., Ding, H.D., Yang, S.J., Chen, Y.Y.: Foliar application of 24-epibrassinolide alleviates hightemperature-induced inhibition of photosynthesis in seedlings of two melon cultivars. - Photosynthetica 51: 341–349, 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgment: The work was funded by the grant of the National Science Centre (2016-2018) No. 2015/17/B/NZ9/01695 (Poland).
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sadura, I., Janeczko, A. Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol Plant 62, 601–616 (2018). https://doi.org/10.1007/s10535-018-0805-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-018-0805-4