Abstract
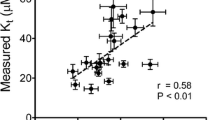
Two experiments were conducted to investigate the kinetics of manganese (Mn) transport in Caco-2 cell monolayers and the gene expressions of Mn transport carriers in apical (AP) and basolateral (BL) membranes. In experiment 1, the cells were treated with the medium containing 146 μmol/L of Mn (MnSO4·H2O). Both the uptake and transport of Mn from AP–BL or from BL–AP at different time-points were assessed to determine the optimal time for kinetics of Mn transport. The transport of Mn increased linearly with higher efficiency values in AP–BL than in BL–AP direction, however, the uptake of Mn revealed an asymptotic pattern within 120 min. In experiment 2, the kinetics of Mn transport in AP–BL was determined with media containing Mn concentrations from 0 to 2,500 μmol/L at 40 and 120 min, respectively, and mRNA levels of divalent metal transporter 1 (DMT1) and ferroportin (FPN1) were determined in Caco-2 cells treated with the medium containing 0 or 800 μmol/L of Mn for 120 min. The kinetics of Mn transport showed a carrier-mediated process when Mn concentrations were lower than 1,000 μmol/L and a linear increment when Mn concentrations exceeded 1,000 μmol/L at either 40 or 120 min. Mn treatment decreased (P < 0.01) DMT1 mRNA level and increased (P < 0.01) FPN1 mRNA level. The results from the present study suggested that Mn transport in AP–BL fit both carrier-mediated saturable and non-saturable diffusion processes, and Mn transport carriers DMT1 and FPN1 mediate the apical uptake and basolateral exit of Mn in Caco-2 cells.





Similar content being viewed by others
References
Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275:19906–19912
Alvarez-Hernandez X, Smith M, Glass J (1994) Regulation of iron uptake and transport by transferring in Caco-2 cells, an intestinal cell line. Biochim Biophys Acta 1192:215–222
AOAC International (2000) Official methods of analysis of AOAC International, 17th edn. AOAC Int., Gaithersburg
Artursson P, Ungell AL, Lofroth JE (1993) Selective paracellular permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments. Pharm Res 10:1123–1129
Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD (2009) Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139(3):434–438
Bai SP, Lu L, Luo XG, Liu B (2008) Kinetics of manganese absorption in ligated small intestinal segments of broilers. Poult Sci 87:2596–2604
Bai SP, Lu L, Wang RL, Xi L, Zhang LY, Luo XG (2012) Manganese source affects manganese transport and gene expression of divalent metal transporter 1 in the small intestine of broilers. Br J Nutr 108:267–276
Barr WH, Riegelman S (1970) Intestinal drug absorption and metabolism I: comparison of methods and models to study physiological factors of in vitro and in vivo intestinal absorption. J Pharm Sci 59:154–163
Chantret I, Barbat A, Dussaulx E, Brattain MG, Zweibaum A (1988) Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res 48:1936–1942
Chua AC, Morgan EH (1997) Manganese metabolism is impaired in the Belgrade laboratory rat. J Comp Physiol 167:361–369
Condomina J, Zornoza-Sabina T, Granero L, Polache A (2002) Kinetics of zinc transport in vitro in rat small intestine and colon: interaction with copper. Eur J Pharm Sci 16:289–295
Conrad ME, Umbreit JN, Moore EG, Hainsworth LN, Porubcin M, Simovich MJ, Nakada MT, Dolan K, Garrick MD (2000) Separate pathways for cellular uptake of ferric and ferrous iron. Am J Physiol Gastrointest Liver Physiol 279:G767–G774
Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Dreier A, Barut B, Zapata A, Law TC, Bruqnara C, Lux SE, Pinkus GS, Pinkus JL, Kinqsley PD, Palis J, Fleming MD, Andrews NC, Zon LI (2000) Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature (Lond) 403:776–781
Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC (2005) The iron exporter ferroportin/Sc40a1 is essential for iron homeostasis. Cell Metab 1:191–200
Erikson KM, Aschner M (2003) Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int 43:475–480
Erikson KM, Syversen T, Aschner JL, Aschner M (2005) Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ Toxicol Pharmacol 19:415–421
Ferruzza S, Rossi C, Scarino ML, Sambuy Y (2012) A protocol for differentiation of human intestinal Caco-2 cells in asymmetric serum-containing medium. Toxicol In Vitro. doi:10.1016/j.tiv.2012.01.008
Finley JW, Patrick M (1997) Mn absorption: the use of Caco-2 cells as a model of the intestinal epithelia. J Nutr Biochem 8:92–101
Forbes JR, Gros P (2003) Iron, manganese, and cobalt transport by Nramp1 (SLC11a1) and Nramp2 (SLC11a2) expressed at the plasma membrane. Blood 102:1884–1892
Gagne P, Dayton CM (2002) Best regression model using information criteria. J Mod Appl Stat Methods 2:479–488
Garcia-Aranda JA, Wapnip RA, Lifshitz F (1983) In vivo intestional absorption of manganese in the rat. J Nutr 113:2601–2607
Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482–488
Halleux C, Schneider YJ (1991) Iron absorption by intestinal epithelial cells: 1. Caco 2 cells cultivated in serum-free medium, on polyethylenetere-phthalate microporous membranes, as an in vitro model. In Vitro Cell Dev Biol 27A:293–302
Halpin KM, Chausow DG, Baker DH (1986) Efficiency of manganese absorption in chicks fed corn-soy and casein diets. J Nutr 116:1747–1751
He WL (2009) Difference and mechanism of iron bioavailability in grain of different rice genotypes. Dissertation, Zhejiang University, pp 42–44
He WL, Feng Y, Li XL, Yang XE (2008) Comparison of iron uptake from reduced iron powder and FeSO4 using the Caco-2 cell model: effects of ascorbic acid, phytic acid, and pH. J Agric Food Chem 56:2637–2642
Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749
Hildago IJ, Borchardt RT (1990) Transport of bile acids in a human intestinal epithelial cell line, Caco-2. Biochim Biophys Acta 1035:97–103
Ji F, Luo XG, Lu L, Liu B, Yu SY (2006a) Effects of manganese source and calcium on manganese uptake by in vitro everted gut sacs of broilers’ intestinal segments. Poult Sci 85:1217–1225
Ji F, Luo XG, Lu L, Liu B, Yu SY (2006b) Effects of manganese source on manganese absorption by the intestine of broilers. Poult Sci 85:1947–1952
Leblondel G, Allain P (1999) Manganese transport by Caco-2 cells. Biol Trace Elem Res 67:13–27
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 25:402–408
Louvard D, Kedinger M, Hauri HP (1992) The differentiating intestinal epithelial-cell establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Dev Biol 8:157–195
Madara JL, Trier JS (1982) Structure and permeability of goblet cell tight junctions in rat small intestine. J Membr Biol 66:145–157
Marcial MA, Carlson SL, Madara JI (1984) Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membr Biol 80:59–79
McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5:299–309
Montosi G, Paglia P, Garuti CA, Guzman CA, Bastin JM, Colombo MP, Pietrangelo A (2000) Wild-type HFE protein normalizes transferrin iron accumulation in macrophages from subjects with hereditary hemochromatosis. Blood 96:1125–1129
Pitelka DR, Taggart BN, Hamamoto ST (1983) Effect of extracellular calcium depletion on membrane topography and occluding junctions of mammary epithelial cell in culture. J Cell Biol 96:613–624
Ranaldi G, Islam K, Sambuy Y (1992) Epithelial cells in culture as a model for the intestinal transport of antimicrobial agents. Antimicrob Agents Chemother 36:1374–1381
Reeves PG, Briske-Anderson M, Newman SM Jr (1996) High zinc concentrations in culture media affect copper uptake and transport in differentiated human colon adenocarcinoma cells. J Nutr 126:1701–1712
Reeves PG, Briske-Anderson M, Johnson L (1998) Physiologic concentrations of zinc affect the kinetics of copper uptake and transport in the human intestinal cell model, Caco-2. J Nutr 128:1794–1801
Riley SA, Warhust G, Crowe PT, Turnberg LA (1991) Active hexose transport across cultured human Caco-2 cells: characterization and influence of culture condition. Biochim Biophys Acta 1066:175–182
Roth JA, Garrick MD (2003) Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol 66:1–13
Roth JA, Horbinski C, Higgins D, Lein P, Garrick MD (2002) Mechanisms of manganese-induced rat pheochromocytoma (PC12) cell death and cell differentiation. Neurotoxicology 23:147–157
SAS Institute (2003) SAS user’s guide: statistics. Version 9.03. SAS Inst Inc, Cary
Settle EA, Mraz FR, Douglas CR, Bletner JK (1968) Effect of diet and manganese level on growth perosis and 54Mn uptake in chicks. J Nutr 97:141–146
Tchernitchko D, Bourgeois M, Martin ME, Beaumont C (2002) Expression of the two mRNA isoforms of the iron transporter Nramp2/DMT1 in mice and function of the iron responsive element. Biochem J 363:449–455
Testolin G, Ciappellano S, Alberio A, Piccinini F, Paracchini L, Jotti A (1993) Intestinal absorption of manganese: an in vitro study. Can Med Assoc J 126:503–505
Thomson ABR, Valberg LS (1972) Intestinal uptake of iron, cobalt, and manganese in the iron-deficient rat. Am J Physiol 223:1327–1329
Thomson ABR, Olatunbosun D, Valberg LS (1971) Interrelation of intestinal transport system for manganese and iron. J Lab Clin Med 78:642–655
Troadec MB, Ward DM, Lo E, Kaplan J, Domenico ID (2010) Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood 116:4657–4664
Underwood EJ (1981) Manganese. In: The mineral nutrition of livestock, 2nd edn. Commonw Agric Bureaux, Slough, pp 125–131
Vandesompele J, Katleen DP, Filip P, Bruce P, Nadine VR, Anne DP, Frank S (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control gene. Genome Biol 3(7):0034.1–0034.111
Walter E, Kissel T (1995) Heterogeneity in the human intestinal cell line Caco-2 leads to differences in transepithelial transport. Eur J Pharm Sci 3(4):215–230
Wang XQ, Li GJ, Zheng W (2006) Upregulation of DMT1 expression in choroidal epithelia of the blood-CSF barrier following manganese exposure in vitro. Brain Res 1097:1–10
Yin Z, Jiang H, Lee EY, Ni M, Erickson KM, Milatovic D, Bowman AB, Aschner M (2010) Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. Neurochemistry 112:1190–1198
Yu A, Weiner J, Hamel D, Lee F (1994) An animal model for investing manganese absorption at various regions of the gastrointestinal tract. Drug Dev Ind Pharm 20:1285–1293
Zeng H, Jackson MI, Cheng WH, Combs GFJ (2011) Chemical form of selenium affects its uptake, transport, and glutathione peroxidase activity in the human intestinal Caco-2 cell model. Biol Trace Elem Res 143:1209–1218
Zha LY, Xu ZR, Wang MQ, Gu LY (2008) Chromium nanoparticle exhibits higher absorption efficiency than chromium picolinate and chromium chloride in Caco-2 cell monolayers. J Anim Physiol Anim Nutr 92:131–140
Zhang AS, Xiong S, Tsukamoto H, Enns CA (2004) Localization of iron metabolismrelated mRNAs in the rat liver indicate that HFE is expressed predominantly in hepatocytes. Blood 103:1509–1514
Zodl B, Zeiner M, Paukovits P, Steffan I, Marktl W, Ekmekcioglu C (2005) Iron uptake and toxicity in Caco-2 cells. Microchem J 79:393–397
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (project no. 31272465; Beijing, P. R. China), the Key International Cooperation Program of the National Natural Science Foundation of China (project no. 31110103916; Beijing, P. R. China) and China Agriculture Research System (project no. CARS-42; Beijing, P. R. China).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, X., Xie, J., Lu, L. et al. Kinetics of manganese transport and gene expressions of manganese transport carriers in Caco-2 cell monolayers. Biometals 26, 941–953 (2013). https://doi.org/10.1007/s10534-013-9670-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9670-y