Abstract
Methane is produced microbially in vast quantities in sediments throughout the world’s oceans. However, anaerobic oxidation of methane (AOM) provides a near-quantitative sink for the produced methane and is primarily responsible for preventing methane emissions from the oceans to the atmosphere. AOM is a complex microbial process that involves several different microbial groups and metabolic pathways. The role of different electron acceptors in AOM has been studied for decades, yet large uncertainties remain, especially in terms of understanding the processes in natural settings. This study reports whole-core incubation methane oxidation rates along an estuarine gradient ranging from near fresh water to brackish conditions, and investigates the potential role of different electron acceptors in AOM. Microbial community structure involved in different methane processes is also studied in the same estuarine system using high throughput sequencing tools. Methane oxidation in the sediments was active in three distinct depth layers throughout the studied transect, with total oxidation rates increasing seawards. We find extensive evidence of non-sulphate AOM throughout the transect. The highest absolute AOM rates were observed below the sulphate-methane transition zone (SMTZ), strongly implicating the role of alternative electron acceptors (most likely iron and manganese oxides). However, oxidation rates were ultimately limited by methane availability. ANME-2a/b were the most abundant microbial phyla associated with AOM throughout the study sites, followed by ANME-2d in much lower abundances. Similarly to oxidation rates, highest abundances of microbial groups commonly associated with AOM were found well below the SMTZ, further reinforcing the importance of non-sulphate AOM in this system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methane (CH4) is a powerful greenhouse gas affecting the global climate. Its atmospheric concentrations have more than doubled since the industrial revolution, due to anthropogenic activities (IPCC 2014). In aquatic systems, production of CH4 primarily takes place in sediments through methanogenesis, which is the final step in anaerobic breakdown of organic matter that occurs when other electron acceptors (EA) have been depleted and carbon dioxide (CO2) is the only viable electron acceptor remaining (Thauer 1998). The exact sediment depth of the primary methanogenic zone depends on the organic matter loading of the system, spanning from a few centimeters in productive coastal systems to several meters in the oligotrophic open ocean seabed (Jørgensen et al. 2001). Methanogenesis is also typically more active in freshwater sediments than in marine sediments, due to the presence of sulphate (SO42−) in seawater (Capone and Kiene 1988), which provides a more energetically favorable pathway for anaerobic remineralization. Eutrophication is expected to increase methanogenesis globally due to enhanced carbon loading (Beaulieu et al. 2019).
Although CH4 is produced in sediments in large quantities, as a highly reduced compound it is susceptible to microbial oxidation. These oxidative processes create a “filter” which prevent CH4 from escaping to the atmosphere (Knittel and Boetius 2009). In aquatic systems, CH4 is oxidized through both oxic and anoxic processes. Of these, aerobic oxidation of methane (MOX) is typically more prevalent in fresh waters (de Angelis and Scranton 1993). It is typically most prominent at steep oxyclines, such as the pycnocline of a stratified water column (Schmale et al. 2010; Jakobs et al. 2013) or the sediment–water interface (Fenchel et al. 1995).
Unlike MOX, anaerobic oxidation of methane (AOM) tends to be more efficient at higher salinities, due to increased availability of SO42− in seawater (Knittel and Boetius 2009). Of these two processes, AOM is thus the major sink of CH4 in marine systems, though extreme variability in process rates exists. Globally, AOM has been shown to be a near-quantitative sink for CH4 produced by sedimentary methanogenesis (Egger et al. 2018). It was originally thought that due to its SO42−-dependence, AOM is an exclusively marine process, but it has since been shown to be active also in lakes (Eller et al. 2005; Sivan et al. 2011; Martinez-Cruz et al. 2018).
SO42− mediated AOM (S-AOM) is mainly performed by anaerobic methanotrophic (ANME) archaea with sulfate reducing bacteria (SRB) via direct interspecies electron transfer (McGlynn et al. 2015). It is the most important AOM reaction in marine systems (Knittel and Boetius 2009) and the stoichiometry has been formulated as follows (Hoehler et al. 1994):
It has later been established that at least nitrite/nitrate (Ettwig et al. 2010; Timmers et al. 2017), as well as oxides of iron (Fe, Egger et al. 2015; Ettwig et al. 2016) and manganese (Mn, Beal et al. 2009) can also be used as alternatives to SO42− as terminal electron acceptors by the different ANME clades.
Iron mediated AOM (Fe-AOM):
Manganese mediated AOM (Mn-AOM):
Fe-AOM requires the concurrent presence of CH4 and reducible iron (i.e. Fe(OH)3) below the sulphate-methane transition zone (SMTZ). This is possible either under sedimentation regimes with high Fe(OH)3 deposition or recent shoaling of the SMTZ due to carbon loading, both of which are typical in eutrophied coastal systems (Rooze et al. 2016; Jilbert et al. 2018). Most microbes capable of Fe reduction can also reduce Mn and may do so preferentially due to the higher energy yield of the process (Lovley and Phillips 1988). Beal et al. (2009) showed that though Mn-AOM exhibits slower process rates compared to SO42−-AOM, energetically Mn-AOM can be up to 10 times more favorable. Mn-AOM and Fe-AOM will be collectively referred to as Me-AOM henceforth in this paper.
Many methanotrophic archaea are capable of AOM. These have been divided into three main groups and a number of subclusters: ANME-1 (subgroups a and b), ANME-2 (subgroups a, b, c and d, of which the two latter are distinct from the two former, and also from each other) and ANME-3 (Haroon et al. 2013; Ettwig et al. 2016; Timmers et al. 2017). Outside the ANME, a group of bacteria called Candidatus Methylomirablis (in phylum NC10) are capable of AOM (Ettwig et al. 2010). It has been suggested that the main biochemical pathway of CH4 oxidation in ANME is the enzymatic reversal of methanogenesis (McGlynn 2017). However, complexity arises from the fact that several of the ANME clades have been shown to have the capacity also for methanogenesis (Ding et al. 2016).
Understanding AOM, and the role of different compounds as electron acceptors is important, not only because of the global warming potential of CH4, but also because of the influence of AOM on other biogeochemical cycles, such as those of phosphorus (P), sulphur (S), Fe and Mn. As an example, Fe-AOM and Mn-AOM could potentially lead to increases in sediment release and subsequent lateral transfer (“shuttling”) of Fe, Mn and P from the coastal zone to offshore regions in systems such as the Baltic Sea (Jilbert and Slomp 2013; Reed et al. 2016; Rooze et al. 2016). In this study our aim was to investigate the factors controlling sedimentary AOM rates along an estuarine gradient in the northern Baltic Sea. To do this, we conducted full-core incubations on sediments from three sites in the estuary, spanning large vertical and lateral gradients in potential electron acceptor availability, thus allowing us to investigate the influence of electron acceptors on methane oxidation rates. In addition to the oxidation rate measurements, we make use of sediment microbial community compositional data from a parallel study in the same estuarine system, in order to investigate the key microbial groups involved in CH4 cycling. We hypothesize that the estuarine gradient is characterized by increasing availability of sulfate offshore, and that this in turn determines the depth of the SMTZ and vertical distribution of methane oxidation zones and related microbial communities in the sediments.
Materials and methods
Study area
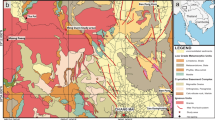
Samples were collected along a transect in the Pojo Bay estuary, located in Southwestern Finland, from four sites: A, C, D and J (Fig. 1). The non-sequential lettering for the sites is used to maintain compatibility with a companion paper detailing the wider CH4 dynamics in the same system (Myllykangas et al. 2020). The sites span a water column salinity gradient of 0–7.
Site A is located in the river mouth of River Karjaa, which is the primary freshwater source of the estuary. Site A is permanently oxic, with bottom water salinity 0–2, and receives organic carbon of primarily terrestrial origin (Jilbert et al. 2018). Due to its proximity to rivermouth flocculation of Fe, from both natural and anthropogenic sources, site A also has strongly elevated concentrations of Fe in the sediments (up to 10–20% by weight). Approximately half of the sedimentary Fe is expected to be in reducible oxide forms as determined by sequential extraction (Jilbert et al. 2018).
Sites C and D are in the main depression of the inner bay (bottom water salinity 3–5), which is separated from the connecting archipelago by a narrow sill close to the city of Ekenäs. These sites experience seasonal hypoxia and sediment focusing, and are subject to relatively intense carbon accumulation from both terrestrial and phytoplankton sources (Jilbert et al. 2018). These sites have intermediate sedimentary Fe concentrations, but are strongly enriched in Mn due to redox shuttling of Mn-oxides during seasonal hypoxia in the inner bay (Tiihonen 2016).
Site J is seawards in the archipelago (bottom water salinity 6–7) along the main channel of the estuary. It is typically fully oxic and receives primarily phytoplankton-derived organic carbon (Jilbert et al. 2018). The sediments contain lower concentrations of Fe and Mn than those at sites A, C and D, but concentrations are nevertheless significantly elevated (e.g. Fe = 6–7%) with respect to sites offshore in the Baltic Sea (Jilbert et al. 2018).
In coastal sediments of the Gulf of Finland generally, bioturbation is known to be important at oxic sites but restricted at sites showing seasonal hypoxia (Gammal et al. 2017; Kauppi et al. 2018). Hence, of our sites, A and J are expected to show intense bioturbation, while sites C and D are expected to be relatively undisturbed by benthic fauna.
Sediment sampling for incubation experiments and pore water profiling
Sediment was retrieved using a GEMAX™—twin corer in August 2017 from sites A, C, and J on board R/V Saduria. Four acrylic minicores (26 ID × 400 mm) were taken from each site for incubation (three replicates and one control). During transport, minicores were capped from both ends and stored upright submersed in site bottom water at in situ temperature.
Samples for vertical porewater CH4 profiles were measured from a parallel core directly following retrieval. 10 ml of wet sediment was extracted at 2.5 cm intervals using cutoff plastic syringes. Sediment was immediately transferred into 65 mL glass bottles pre-loaded with supersaturated NaCl solution, which were immediately capped with butyl rubber septa and screw caps and stored upside down. Within 24 h, 10 mL of N2 headspace was injected by needle through the stopper, and an equivalent volume of sediment slurry removed through a second needle. After equilibration, two 1 mL subsamples of the headspace were analyzed with a gas chromatograph equipped with a flame ionization detector (Agilent Technologies 7890B). Sediment volume in the original sample was calculated from complementary porosity profiles.
One additional GEMAX-core was retrieved per site and sampled at 1 cm intervals with Rhizons™ for porewater extraction and later analyzed for Fe, Mn, S and Ca with inductively coupled plasma—optical emission spectrometry (ICP-OES). The samples were acidified with 10 µL of 65% HNO3 per mL of porewater in order to keep metals in solution. ICP-OES-derived S is interpreted to represent SO42−, since H2S is lost upon the addition of HNO3. The analyzed metals are interpreted to be present as divalent ions. For full details see Jilbert et al. (2018).
Determination of methane oxidation rates
Incubation
Each of the minicores was pre-drilled with holes at 1 cm intervals on the side. The holes were plugged with silicone and taped from both sides with water resistant tape. In the lab, a 25 µL of 0.25 KBq activity 14CH4 (Biotrend Chemikalien) label (2.26 nmol) dissolved in oxygen-free artificial seawater was injected into the cores through the side ports with a gastight glass syringe (Hamilton). The needle was first pushed through the core horizontally and then simultaneously withdrawn during the injection, in order to ensure even distribution of the tracer. The cores were incubated without top caps in a temperature-controlled water bath at in situ temperature for 24 h.
The incubation was stopped and microbial activity was halted by sectioning the cores at 2 cm intervals (total volume per slice approximately 11 cm3) and funneling the slices into pre-weighed 100 mL glass bottles containing 40 mL of 2.5% NaOH. Following the NaOH addition, pH in the slurry was consistently > 12, ensuring that all DIC in the slurry was present as carbonate (CO32−). The bottles were immediately closed with a septum cap (PFTA, 5 mm) and stored upside down.
Analysis
The total amount of CH4 in the samples after incubation and storage was calculated from a 1 mL subsample taken from the headspace through the septum cap, which was subsequently analyzed with a gas chromatograph equipped with a flame ionization detector (Agilent Technologies 7890B). To capture residual 14CH4 tracer not converted to 14CO2 during incubation, the headspace of samples was purged with synthetic air through a glass tube containing copper oxide and combusted in a tube oven at 850 °C. The combusted 14CO2 was trapped in a 20 mL scintillation vial containing 10 mL of CO2-absorbant (2-phenylethylamine and 2-methoxyethanol, 1:7 v/v). After 15 min of purging, 10 mL of Ultima Gold™ scintillation cocktail (Perkin Elmer) was added and samples were subsequently analyzed using a liquid scintillation counter (Wallac® 1415). The bottles were weighed again to assess total sediment volume of each sample.
Following combustion, the slurry jars were left uncapped for 10 min to allow all residual CH4 to escape. Subsequently, a 20 mL glass scintillation vial containing CO2-absorbant (6 mL of 0.5 M NaOH and 2-phynyethylamine solution, 1:1 v/v) was gently inserted into the sediment slurry bottle. The slurry was then acidified with 12 mL of 6 M HCl injected to the bottom of the slurry and capped rapidly. The acidification procedure lowered the pH of the slurry to < 1, which caused the release of all DIC in the slurry as CO2, which was subsequently trapped in the amine solution in the headspace. After 12 h under gentle magnetic stirring, the scintillation vials were removed and wiped thoroughly with ethanol, after which 10 mL of scintillation liquid was added, and the samples were analyzed for 14CO2 with a liquid scintillation counter.
Controls
One full minicore from each site was dedicated as a control and treated the same way as the replicates, except that the tracer was added only after the core had been sliced and fixed in NaOH. Controls were analyzed from five different depths from each site. The volume of injected tracer was identical to that during the sample incubations.
Rate calculations
In situ rates of AOM were calculated from the fraction of produced 14CO2 in the total 14C pool using Eq. 1 (modified from Treude et al. 2005):
where 14CO2 and 14CH4 are the activities (disintegrations per minute, DPM) of microbially produced CO2 during incubation and the activity of the injected CH4 not incorporated into CO2, respectively, CH4 is the total amount of CH4 in the sample in nmol at time zero, t is the incubation time in days and v volume of slices in cm3. Average of DPM values from the blanks were subtracted from the 14CO2 values, and only samples with activity more than 3 \(\times\) SD of the blanks were considered active. The DPM values of 14CH4were corrected with the ratio between T0 and post-incubation CH4 concentrations to account for losses of CH4 during storage, which are assumed to occur at the same rate for both 14CH4 and 12CH4 (Treude et al. 2005). Total 14C label recovered in both combustion and acidification steps was 85–95%.
Depth-integrated oxidation rates were calculated from the mean oxidation rates between 0 and 31 cm depths utilizing the trapezoid rule:
where f(x) is oxidation rate in mmol m−3 day−1, x is the sediment depth zone in cm.
Microbial communities
Analysis of bacterial and archaeal community composition
Samples for community composition analysis were collected in June 2015 from sites A and D with a GEMAX™ corer. This work was carried out as part of a parallel project, and data was made available to the current study. The presence of a well-defined shallow (5–15 cm) SMTZ at all muddy sites in the Pojo Bay system (Myllykangas et al. 2020) facilitates qualitative comparison of the vertical structure of the microbial communities and CH4 oxidation rates, regardless of spatial offset in sampling locations.
Sediment was sliced at 2.5 cm intervals and stored in 2 mL sterilized plastic vials at − 80 °C. DNA was extracted from frozen sediment samples (200–400 mg) using a previously published protocol based on bead-beating and phenol–chloroform extraction (Griffiths et al. 2000). DNA yields of extractions were determined with Qubit 3.0 Fluorometer and Qubit™ dsDNA HS Assay Kit for DNA (Thermo Fisher Scientific). DNA extractions were stored at − 20 °C before sequencing analyses.
PCR of 16S rRNA genes and amplicon sequencing took place commercially at FISABIO (Valencia, Spain; https://fisabio.san.gva.es/en/inicio). For each sample, V3-V4 region of the bacterial and archaeal 16S rRNA genes were simultaneously targeted using primer pair Pro341F (5′-CCTACGGGNBGCASCAG-3′)/Pro805R (5′-GACTACNVGGGTATCTAATCC-3′) (Takahashi et al. 2014). Archaea were also specifically studied from each sample by targeting the V3-V4 region of the archaeal 16S rRNA genes using primer pair 340F (5′-CCCTAYGGGGYGCASCAG-3′)/806R (5′-GGACTACVSGGGTATCTAAT-3′) (Takai and Horikoshi 2000; Gantner et al. 2011). PCR mixtures (total volume of 25 µL) included approximately 25 ng of template DNA, 12 µL of 2 × KAPA HiFi HotStart ReadyMix (KK2602, Roche) and 5 µL of each primer (1 µmol L−1). DNA amplicon libraries were generated using a limited cycle PCR: initial denaturation at 95 ºC for 3 min, followed by 25 cycles of annealing (95 ºC for 30 s, 55 ºC for 30 s, 72 ºC for 30 s) extension at 72 ºC for 5 min. Thereafter, Illumina sequencing adaptors and dual-index barcodes (Nextera XT index kit v2, FC-131-2001) were added to the amplicons. Libraries were purified with AMPure XP after each amplification. Their DNA content was measured using a Qubit 2.0 Fluorometer and a dsDNA HS Assay Kit (Thermo Fisher). Libraries were normalized and pooled in equal amounts prior to sequencing. The pool containing the indexed amplicons was then loaded onto the MiSeq reagent cartridge v3 (MS-102–3003) and 4 pM of this pool was spiked with 25% PhiX control to improve base calling during sequencing, as recommended by Illumina for amplicon sequencing. Sequencing was conducted using a paired-end 2 × 300 bp cycle run on an Illumina MiSeq sequencing system.
Bioinformatic analyses
Trimming of the raw data (parameters, min_length: 50; trim_qual_right: 30; trim_qual_type: mean; trim_qual_window: 20) was done using prinseq-lite (Schmieder and Edwards 2011). The paired end reads, R1 and R2, from Illumina sequencing were joined using FLASH program applying default parameters (Magoč and Salzberg 2011).
Mothur (Schloss et al. 2009) was used in subsequent sequence analyses. The sequences were aligned using Silva reference alignment (Release 123). Chimeric sequences, identified using UCHIME (Edgar et al. 2011), were removed from each library and a preclustering algorithm (Huse et al. 2010) was used to reduce the effect of sequencing errors. Sequences were assigned taxonomies with a naïve Bayesian classifier (bootstrap cut-off value 75%) (Wang et al. 2007), using the Silva database (Release 128). Thereafter, sequences classified as chloroplast, mitochondria and eukaryota were removed from each library, while in addition, bacterial sequences were removed from the archaeal V3-V4 library (340F/806R).
Sequences were divided into operational taxonomic units (OTUs) at a 97% similarity level. Singleton OTUs (OTUs with only one sequence) were removed, and the data were then normalized by subsampling to the same size, which was 20,755 for prokaryotic (i.e. bacterial and archaeal) and 63,673 for archaeal libraries. Good´s coverage was over 0.88 and 0.99 in each prokaryotic and archaeal library, respectively, confirming that sequence variation was well covered (data not shown). This study focused specifically on OTUs assigned to known aerobic and anaerobic methanotrophic taxa and on known methanogenic taxa (Nazaries et al. 2013). In addition, as sulfate reducing bacteria are involved in S-AOM and as we also generally wanted to reveal genetic potential for anaerobic sulfate and Fe3+ respiration, we also focused on known sulfate and Fe3+ reducing taxa (Kuever et al. 2005; Youssef et al. 2009; Kuever 2013; Lovley 2013; Rabus et al. 2013).
Sequence accession numbers
Raw sequencing data has been submitted to National Center for Biotechnology Information’s (NCBI) Short Read Archive (SRA) under accession number PRJNA574569.
Results
Porewater biogeochemistry
Profiles of SO42− were broadly similar between the three sites (Figs. 2 and 3), though there was a seawards increase in maximum SO42− values in the top sediment from 2.9 to 5.0 mmol L−1, from site A to J. Similar to SO42−, CH4 profiles displayed similarity between sites, with key differences (Fig. 3). Lowest concentrations of 0.01 mmol L−1 were found at site A surface sediments and highest of 5 mmol L−1 at site J below 30 cm depth. At site A, CH4 was largely absent in the upper sediments, whereas in contrast C was the only site studied with clearly discernible amounts of CH4 near the sediment–water interface. Overall, CH4 concentrations displayed a seaward increase, with both absolute concentrations and inventories increasing markedly from site A to J. Site A was the only station that showed a steep decrease in CH4 concentration below 33 cm depth. All sites display a clear SMTZ, defined as the depth of equal porewater CH4 and SO42− concentrations. The depth of the SMTZ varied between the sites, being located at 13.0, 7.6 and 10.5 cm depth at sites A, C and J, respectively (Fig. 2).
Vertical sediment profiles of methane oxidation rates from all sites (three replicates per site shown with data points, mean rate at each depth interval shown with the red line), coupled with dissolved porewater CH4 and SO42− concentrations from the same sites. The grey line denotes the depth of the SMTZ, as defined by the depth of equivalent CH4 and SO42− concentration. Note the different x axes
Other biogeochemical parameters were similarly variable between the sites. Porewater Fe concentrations exhibited large variation between the study sites (Fig. 2). Highest concentrations were found at site A, nearest to the river mouth. Surface sediments at site A contained 898 µmol L−1 of Fe in porewaters. Concentrations declined rapidly towards the SMTZ, down to 253 µmol L−1, after which they increased again reaching 655 µmol L−1 at 24 cm depth. Site C showed a slight elevation of Fe in the surface sediment (169 µmol L−1), but there, too, concentrations decreased rapidly towards the SMTZ and were largely below the detection limit below it. Below 20 cm depth, the iron concentrations started increasing again. Site J Fe concentrations were low throughout, with only a small elevation of 22 and 23 µmol L−1 found at 2 cm depth and below 26 cm depth, respectively.
Porewater profiles of Mn were similarly highly variable between sites (Fig. 2). Site A displayed a mostly flat profile, with slightly lower concentrations near the sediment surface, but ca. 50 µmol L−1 concentrations throughout the rest of the sediment column. Site C contained a small peak near the sediment surface, followed by a rapid decrease towards the SMTZ, below which Mn increased again, reaching 196 µmol L−1 at 30 cm depth. Site J displayed a similar pattern, except that overall the concentrations were lower, reaching only 69 µmol L−1 at 30 cm depth, and the increase did not begin until clearly below the SMTZ.
Calcium profiles were included as a proxy for porewater salinity. Accordingly, the average porewater calcium concentrations increased with distance offshore (Fig. 2). At site A, porewater calcium concentration increased slightly with sediment depth from 1593 to 1715 µmol L−1. At site D concentrations varied between 1775 and 2011 µmol L−1, decreasing slightly with depth. At site J, concentrations increased slightly with depth and varied between 2392 to 2656 µmol L−1.
Methane oxidation rates
CH4 oxidation activity showed clear and distinct vertical zonation at all three sites (Fig. 3). The activity zones could be divided into three layers: 1. surface-sediment (above the SMTZ), 2. close to the SMTZ, and 3. below the SMTZ. CH4 oxidation in the surface sediment layer was most evident at site C with a rate of 29.52 ± 7.21 nmol cm−3 day−1. Site A featured a distinct peak in oxidation activity between the SMTZ and the sediment surface, while at site J oxidation above the SMTZ was low. Oxidation activity near and around the SMTZ was most evident at site J, with a peak of 32.09 ± 1.69 nmol cm−3 day−1 found at 11 cm depth, which was also the highest recorded rate throughout the study transect. Site C also featured a very apparent, albeit smaller, increase in oxidation activity near the SMTZ. Clearly elevated oxidation rates were found below the SMTZ at site J, with the highest rate of 13.89 ± 7.34 nmol cm−3 day−1 found from 31 cm depth. Site A also showed oxidation activity below the SMTZ, though in lesser degree, whereas at site C oxidation rates drop to near zero in the deepest parts of the core. Standard deviation between replicate oxidation rates was 4.1–93.9%, with an average of 33.97%.
To quantify the lateral (between stations) and vertical differences in CH4 oxidation rates systematically, we divided each profile into three sections of equal thickness, and calculated depth-integrated rates for each. Full-core integrated rates displayed an overall seaward increase from 2.32 to 3.51 mmol m−2 day−1 from site A to J (Table 1). At site A, the highest integrated oxidation rate of 0.94 mmol m−2 day−1 was found at the 1–10 cm depth interval, with a decrease in the 11–20 cm depth interval and a slight increase in the deepest section. In contrast, site C displayed a downwards trend in the integrated rates, decreasing from 0.87 mmol m−2 day−1 in the topmost section, down to 0.46 mmol m−2 day−1 in the bottom section. While site J featured the lowest integrated rates of the whole study in the surface section (0.38 mmol m−2 day−1), in contrast the highest (2.00 mmol m−2 day−1) and second highest (1.13 mmol m−2 day−1) integrated rates of the whole study were found from the 11–20 cm and 21–31 cm depth zones respectively.
Microbial community structure
A clear vertical structure was observed in the microbial community composition at sites A and D as determined by relative abundances of 16S ribosomal RNA genes (Figs. 4 and 5, Supplementary Figs. 1 and 2). The dominant archaeal taxa in both sites were Methanomicrobia and Thermoplasmata (within Euryarchaeota) as well as Bathyarcheota, Thaumarcheota and Woesearcheota (Supplementary Fig. 1). The bacterial community was more diverse than the archaeal community. The most dominant bacterial taxa in both sites included e.g. Deltaproteobacteria, Gammaproteobacteria and Betaproteobacteria (within Proteobacteria) as well as Bacteroidetes, Chloroflexi and Verrucomicrobia (Supplementary Fig. 2 ). In the following, we report the relative abundance of microbial groups associated with specific CH4-related processes and with sulfate and Fe3+ reduction within this vertical structure, as well as differences between sites A and D. Since the microbial data is derived from cores sampled in June 2015, we report the depth of the SMTZ as determined from these cores by Jilbert et al. (2018), rather than from the 2017 profiles shown in Figs. 2 and 3.
Relative abundances of different microbial groups (AOM = clades associated with AOM, MOG = clades of methanogens, MOX = clades of bacteria capable of MOX, TOTAL = groups summed up) at sites A and D in June 2015. Note the different scales on the x-axes for archaea (columns I–II) and prokaryotes (III–IV). Abundances of archaea are reported as a fraction of total archaea and abundances of prokaryotes reported as a fraction of total prokaryotes (archaea + bacteria). The grey line represents the depth of the SMTZ in June 2015 (Jilbert et al. 2018)
Vertical structure of methane-related as well as sulfate and Fe3+ reducing microbial communities
The relative abundances of taxa associated with AOM, MOX and MOG showed a clear vertical zonation at both sites (Fig. 4k–n). MOX-related taxa dominate in the surface sediments, while AOM and MOG taxa became more dominant with increasing depth in the sediment column. The contrasting depth of the SMTZ at these two sites is reflected in the depth of the switch from MOX-to AOM-dominance of the CH4 oxidizer community, which occurs deeper at site D (Fig. 4m–n). Below the SMTZ, AOM-related groups show a similar abundance to methanogens at site D, while at site A, methanogens dominate over AOM-related groups throughout the deeper sediments (Fig. 4k–l). Putative sulfate and Fe3+ reducing bacteria were present all through the sediment columns at both sites (Fig. 5). The vertical variations in the total relative abundance of Fe3+ reducers were quite minor at both sites, yet at site A they were highest in the surface above SMTZ and in the bottom layers, while multiple minor peaks in their relative abundance were observed along the sediment column in site D (Fig. 5). In contrast, sulfate reducers had lower relative abundance at the surface layers and at SMTZ than deeper in the sediment column at site A, while at site D they had highest relative abundance at the surface layers above the SMTZ and decreased drastically in deeper layers especially from 30 to 40 cm depth (Fig. 5).
Groups associated with AOM
From the microbial groups commonly associated with AOM, ANME-2a/b was the most abundant at both sites. The relative abundance of ANME-2a/b generally increased with depth in the sediment column (Fig. 4a–d). When reported as a fraction of total prokaryotes, relative abundances of this group were thus highest below the SMTZ at both sites (Fig. 4c–d). This effect was most pronounced at site D, where ANME-2a/b also dominated AOM-related groups (Fig. 4d). At site A, high relative abundances of ANME-2a/b were also observed in the uppermost 10 cm, broadly within the SMTZ at this site (Fig. 4a). ANME-2d contributed an additional important group at site A, with a clear increase below the SMTZ (Fig. 4a, c). At site D, ANME-2d was present in small numbers (0.01–0.32%) in the archaeal community throughout the sediment column (Fig. 4b). ANME-1 was present in even smaller numbers at throughout the sediment column at site A (0.002–0.009% of archaeal 16S rRNA genes) but entirely absent at site D (Fig. 4a-b). Ca. Methylomirabilis (NC10 phylum) was also present in very small numbers (0.002–0.005% of 16S prokaryotic rRNA genes at 4–12 cm depth) and only at site A, with the highest abundance of 0.010% found from 20 cm depth (Fig. 4c).
Groups associated with MOX
Methylococcales (Type I) were the most abundant group of MOB in surface sediment at both sites, remaining more abundant deeper in the sediment at site A (Fig. 4i). However, their abundance decreased rapidly below the SMTZ. Methylocystaceae (Type II) MOB showed two peaks in abundance below the SMTZ at site A, but were largely absent from site D (Fig. 4i-j). MOB belonging to the phylum Verrucomicrobia, i.e. Ca. Methylacidiphilum sp., were present in small numbers in the surface sediment of both sites.
Groups associated with methanogenesis (MOG)
Methanomicrobiales, Methanosaetaceae and Methanosarcinaceae were the most abundant methanogen taxa at both sites, while other methanogens, i.e. Methermicoccaceae, Methanobacteria, Methanocellales and Methanomassiliicoccus sp. were scarce (Fig. 4). Methanomicrobiales, which consisted dominantly of Methanoregulaceae, were the most abundant at site A and made up to 20% of all archaea at 35 cm depth (Fig. 4e), while the MOG community at site D was less abundant but more varied in distribution between Methanomicrobiales, Methanosaetaceae and Methanosarcinaceae (Fig. 4f). At both sites, the relative abundance of all the dominant taxa of methanogens generally increased below the SMTZ. However, at site D the largest increase was observed deeper in the sediment column (Fig. 4h).
Groups associated with sulfate and Fe3+ reduction
The relative abundance of sulfate reducers was higher at site D than A. Sulfate reducers were also more abundant than Fe3+ reducers at both sites (Fig. 5). The putative sulfate reducing community in both sites consisted mostly of Desulfobacterales and Desulfarculaceae with minor contribution also from Desulfobacca sp., Desulfomonile sp, Desulfosporosinus sp. and Desulfovibrionales (Fig. 5). Desulfobacterales and Desulfarculaceae were generally of similar relative abundance at site A, except at surface layers and at SMTZ, where Desulfobacterales were at their highest and Desulfarculaceae at their lowest levels (Fig. 5). In contrast, in site D, Desulfobacterales significantly outnumbered Desulfarculaceae at all layers and generally followed the same depth pattern as total sulfate reducing community, while the relative abundance of Desulfarculaceae was quite stable through the sediment column (Fig. 5).
The putative Fe3+ reducing bacterial community in both sites consisted mostly of Deferribacteres, Geobacteraceae and Thiobacillus with minor contribution also from Geothrix sp., Pseudomonas sp., Thermotogae, Bacillus sp., Desulfuromonas sp., Shewanella sp., Ferribacterium sp. and Aeromonas sp. (Fig. 5). The dominant taxa differed in their vertical distribution patterns. Geobacteraceae generally had highest relative abundance at surface layers above SMTZ, whereas Deferribacteres were more abundant at deeper layers (Fig. 5).
Discussion
Vertical structure of methane oxidation rates in the context of porewater data
The oxidation rates measured in this study (Fig. 3) are broadly comparable to those of previous studies in the Baltic Sea, e.g. Thang et al. (2013) reported maximum rates of 16 nmol cm−3 day−1 from Sweden (Himmerfjärden bay) and Treude et al. (2005) reported rates of 1–14 nmol cm−3 day−1 in the upper 20 cm of the sediment in Germany (Eckernförde bay). However, while the rate profiles of Thang et al. and Treude et al. typically contained only a single clear peak of oxidation near the SMTZ, the oxidation rate profiles in this study can broadly be divided into three distinct layers: 1. surface-sediment (above the SMTZ), 2. close to the SMTZ, and 3. below the SMTZ. Oxidation in each layer was most likely mediated by different EAs. In layer 1, above the SMTZ, and especially close to the sediment surface, the observed oxidation was most likely related to MOX (Osudar et al. 2015; Steinle et al. 2017). In layer 2, the steep converging CH4 and SO42− gradients, forming a well-defined SMTZ at all three sites, strongly suggest S-AOM at this depth (Iversen and Jørgensen 1985). Indeed, the most conspicuous peaks in oxidation at sites C and J are observed at the SMTZ (Fig. 3). However, we also measured oxidation activity in layer 3 at all sites, well below the SMTZ, at depths where porewater SO42− was completely depleted. Therefore, we must attribute this activity to AOM mediated by other EAs than SO42−, most likely by oxides of either Fe (Egger et al. 2015; Rooze et al. 2016) or Mn (Beal et al. 2009). The porewater profiles show evidence for reduction of Fe and Mn oxides below the SMTZ at all sites (Fig. 2). Accumulation of porewater Fe is most apparent at site A, while accumulation of Mn is most apparent at site C, likely reflecting the contrasting availability of Fe and Mn oxides at these two sites.
Lateral variability in methane oxidation along transect
One key finding of the study is that the maximum depth-integrated rates of CH4 oxidation at any site were observed at site J. Moreover, this site also showed higher rates of oxidation in layer 3 (below the SMTZ), with respect to the other sites (Table 1). This observation suggests that non-SO42− AOM may be an important biogeochemical process in the archipelago regions of the northern Baltic Sea, and apparently more prevalent in the archipelago sediments than in the low-salinity estuaries that drain into these areas. This conclusion appears surprising, since the availability of Fe and Mn oxides is greater in the inner estuarine areas (sites A and C in this study), and the porewater data from these sites suggest more active reduction of oxides. However, we note that there are several reasons why the accumulation of porewater Fe2+ and Mn2+ may not directly reflect rates of Fe- and Mn-AOM. Principally, dissimilatory reduction of oxides coupled to organic matter respiration may also generate these dissolved species (e.g. Egger et al. 2015). Also, the presence of excess porewater H2S at the SMTZ will draw down their concentrations through precipitation of sulphide minerals (e.g. Reed et al. 2011). Jilbert et al. (2018) showed that site J is characterized by a strongly developed H2S maximum at the SMTZ, which could explain the relatively low porewater Fe and Mn accumulation at this site despite high rates of AOM.
We therefore suggest that rates of non-SO42− AOM are not controlled by EA availability in our study transect. Apparently, all the studied sites have sufficient Fe and/or Mn oxides in the sediments to facilitate AOM below the depth of SO42− penetration. Rather, the availability of CH4 (i.e. the rate of methanogenesis) controls the observed rate of non-SO42− AOM at any given site. Depth-integrated oxidation rates below the SMTZ were highest at site J (Fig. 3), which is also characterized by the highest porewater CH4 concentrations (Fig. 2). The reason for the high porewater CH4 at site J is likely related to the high accumulation rate of degradable organic material at this location. Rates of CH4 production in the 12–20 cm interval of the sediment column at site J have been estimated from porewater profiles as 8–10 nmol cm−3 day−1 (Jilbert et al. 2018). That study showed that the sediments of the archipelago are dominated by phytoplankton-derived organic carbon, while the inner estuarine sites have a mixed phytoplankton-terrestrial organic matter composition. Hence, for an equivalent total accumulation rate of carbon, archipelago areas are likely to have higher overall rates of remineralization reactions—including methanogenesis—due to the relative lability of the carbon source (Arndt et al. 2013).
Site-specific characteristics in the oxidation rate profiles
Due to the presence of oxygen in bottom waters, we expected to find evidence of MOX in the surface sediments at all sites (Fenchel et al. 1995). In addition, we expected to find the surface sediment at the nearly freshwater site A to contain the highest oxidation rates of the three sites. This is motivated by previous observations that MOX has a strong inverse relationship with salinity (de Angelis and Scranton 1993), likely caused by changes in the relative availability of oxygen, sulfate and methane along salinity gradients. However, while the integrated rates in the surface sediment layer were indeed highest at site A (Table 1), it lacked the distinct oxidation peak seen especially at site C and to a lesser degree at site J (Fig. 3).
We suggest that the differences in oxidation activity in the surface sediments can again be explained partly by CH4 availability: site C was the only site to contain appreciable amounts of porewater CH4 close to the sediment–water interface, and accordingly displayed the shallowest SMTZ of the three sites (Fig. 2). Also, given that sites A and J are consistently oxic, strong bioturbation is expected (Vaquer-Sunyer and Duarte 2008). This may blur the diagenetic zones, allowing MOX to co-occur with S-AOM in the vicinity of burrows and hence to obscure the development of a distinct double-peak structure in the oxidation rate profile.
All three sites featured distinct oxidation maxima near the depth of SMTZ, interpreted as S-AOM, though the peak became more pronounced offshore (Fig. 2). Integrated oxidation rates in layer 2 (close to the SMTZ) increased considerably offshore and highest integrated rates of the whole study were found at site J in this layer (Table 1). The increased rates of S-AOM offshore are likely a reflection of dual substrate availability. S-AOM is dependent on both the availability of CH4 and SO42− (Valentine 2002). As outlined above, CH4 concentrations are highest at site J due to organic matter degradability. Moreover, due to higher bottom water salinity, SO42− availability is also greater at this site.
Microbial communities at the study sites
The sediment archaeal and bacterial communities at sites A and D generally consisted of the same taxa as in coastal and offshore sites of another northern Baltic Sea area, the Bothnian Sea (Rasigraf et al. 2019) (Supplementary Figs. 1 and 2). Furthermore, the same dominant methanogen taxa, Methanomicrobiales (Methanoregulaceae), Methanosaetaceae and Methanosarcinaceae, were present both at our study sites and in Bothnian Sea sediments (Rasigraf et al. 2019) (Fig. 4). Although Rasigraf et al. (2019) were able to construct a metagenome-assembled-genome of Methanomassiliicoccaceae thriving in Bothnian Sea sediments, this family, alongside other methanogenic taxa, were at very low levels both at ours and their study sites. Methanomicrobiales and Methanosaetaceae are considered to drive hydrogenotrophic and acetoclastic methanogenic pathways, respectively, while both acetoclastic and methylotrophic pathways have been suggested for Methanosarcinaceae (Nazaries et al. 2013). However, in environments with Fe3+ reducing bacteria and conductive minerals such as in iron-rich sediments of our study sites, Methanosaetaceae and Methanosarcinaceae could also drive CO2 – reduction (to CH4) coupled with either direct or indirect (via conductive materials) electron transfer from iron-reducing bacteria, such as Geobacter (Rotaru et al. 2014, 2018). Some metagenomic datasets also indicate Bathyarchaeota, which were dominant archaea both at our study sites and Bothnian Sea sediments (Rasigraf et al. 2019) (Supplementary Fig. 1), can be both methanogenic and methanotrophic (Evans et al. 2015). Shotgun metagenomic data would be needed to assess genetic potential of Bathyarchaeota at our study sites. However, the metagenomes of Rasigraf et al. (2019) suggest a non-methanogenic/non-methanotrophic fermentative lifestyle for Bathyarchaeota in Bothnian Sea sediments.
Genetic potential for sulfate and Fe3+ reduction was present throughout the sediment columns based on distribution of sulfate and Fe3+ reducing bacteria (Fig. 5). Because many of the Fe3+ reducing bacteria can also reduce Mn4+, genetic potential for Mn4+ reduction was also present (Lovley 2013). The higher relative abundance of sulfate reducers at site D very likely represented higher marine influence, thus higher availability of sulfate (Fig. 5). Quite surprisingly, except for Desulfobacterales at site A, the relative abundance of sulfate reducers was not highest at the SMTZ. In addition, sulfate reducers were present in significant relative abundance also in deeper sulfate-depleted layers (Fig. 5). These deviations can be explained by sulfate reducing bacteria having also alternative metabolism strategies. For example, many sulfate reducers can switch to fermentation if sulfate is depleted (Rabus et al. 2013). We acknowledge that the deviations can also represent a bias in functional grouping, since this was based mostly on data from isolated bacteria. Modern metagenomic studies have shown that potential for both sulfate and Fe3+ reduction is spread also beyond the taxa from which isolates exist (Rasigraf et al. 2019; Garber et al. 2020).
Microbial community data in the context of oxidation rate data
Our theory that availability of CH4 was the key factor controlling the observed oxidation rates is supported by the microbial data. At both stations sampled for microbial communities, MOG generally occur concurrently with ANME (Fig. 4k–n), implying that AOM co-occurs with methanogenesis regardless of depth in the sediment column. Importantly, both MOG and ANME taxa increase in abundance below the SMTZ at both sites (Fig. 4m–n). There is also significant overlap in the presence of MOB and ANME, especially at site A, and MOB are found in much greater abundances deeper in the sediment than at site D (Fig. 4m–n). This also supports our previous conclusions regarding the potential effects of bioturbation and the blurring of diagenetic zones above the SMTZ.
Of the AOM related clades included in this study, ANME-1 is generally considered the least flexible in terms of EAs and is mainly associated with S-AOM (Timmers et al. 2017). By contrast, ANME-2a/b have been shown to be capable of both S-AOM and Me-AOM (Timmers et al. 2017), and ANME-2d is suggested to be capable of at least S-AOM, N-AOM, Fe-AOM and Mn-AOM (Haroon et al. 2013; Ettwig et al. 2016; Timmers et al. 2017). The presence of sulfate and Fe3+ reducing (and Mn4+ reducing) bacteria throughout the sediment columns further support the role of sulfate reducers as bacterial partners in S-AOM and also more generally the role of SO42− and Fe3+ (and Mn4+) as electron acceptors in anaerobic processes. The dominance of ANME 2a/b in our data likely reflects the mixed-EA environment of the estuarine system in general, with relatively low SO42− and high oxide availability when compared to fully marine settings. Treude et al. (2005) and Rasigraf et al. (2019) also found that in Eckernförde bay (southern Baltic) and Bothnian Sea (northern Baltic), respectively, which also feature relatively shallow SMTZs, ANME-2 were the dominant clade. ANME-2 have also been shown to dominate other shallow SMTZ sediments in the ocean (Losekann et al. 2007).
ANME-2d has previously been found the most abundant AOM clade in some lakes (Weber et al. 2017; Rissanen et al. 2017). We also observed the highest abundances of ANME-2d at the river mouth-site, while at the slightly more saline site D, ANME-2d was still present, but in much lower abundances (Fig. 4a–d). Thus, despite multiple EAs that ANME-2d could potentially utilize (Timmers et al. 2017), it seems that salinity strongly inhibits the presence of ANME-2d, reinforcing previous interpretations of this as a primarily freshwater clade (Timmers et al. 2017).
The anaerobic taxa Ca. Methylomirablis (NC10 phyla), also capable of N-AOM (Welte et al. 2016), was also present in our study, but only at site A (Fig. 4c, d). These taxa has previously been found from both freshwater and marine environments (Welte et al. 2016). Its environmental significance remains unknown, as some studies have found no significant contributions from bacteria of the NC10 phyla to CH4 oxidation (Beck et al. 2013; Martinez-Cruz et al. 2018), while others have shown the clade capable of very active nitrite N-AOM in lakes, though only in the first few millimeters of the sediment surface (Deutzmann et al. 2014). In our study it was most abundant relatively deep (20 cm) in the sediment, though we have no way of showing whether it was active at those depths. Nevertheless, it is possible that bioirrigation may introduce nitrite deeper in the sediment and enable N-AOM to occur there intermittently. This, too, could further contribute to the blurring of the diagenetic zones as discussed previously, as Deutzmann et al. (2014) also note that N-AOM by the NC10 bacteria could potentially be confused with MOX.
The MOB present in the surface sediment of site A were predominantly type I, which was to be expected as they are considered to be most common in estuaries (Dean et al. 2018). However, there was also a clear enrichment of type II MOB below the SMTZ (Fig. 4i). The co-occurrence of aerobic and anaerobic methanotrophs has previously been observed in lakes (Deutzmann et al. 2014; Martinez-Cruz et al. 2018). While it is possible that MOX occurs below the SMTZ at site A due to bioturbation, it is unlikely. More likely, the presence of type II MOB deeper in the sediments is the combined result of burial due to intensive sedimentation and selective survival (Rissanen et al. 2019). Indeed, Type II MOB have been shown to be able to survive for extended periods in a dormant state and recover CH4 oxidation capability even after decades of anoxic conditions (Roslev and King 1994).
Alternative interpretations of oxidation pathways
Here we present a number of alternative potential biogeochemical pathways that could lead to the observed diagenetic zonation, and assess the likelihood that they may play a role in our study system.
All the sites studied here have been previously shown to accumulate porewater Fe2+ below the SMTZ, which we interpret to be linked to Fe-AOM and organoclastic Fe reduction (Jilbert et al. 2018). However, such accumulation has also been postulated to reflect the “cryptic sulphur cycle” (Holmkvist et al. 2011). While it is possible that this process is taking place in this location, it is most likely confined to the vicinity of the SMTZ or immediately below. H2S is required to sustain the cryptic sulphur cycle, and this is rapidly depleted below the SMTZ at these sites (Jilbert et al. 2018).
AOM coupled to denitrification (N-AOM) has been shown to be potentially an even more energetically favorable pathway than Me-AOM (Raghoebarsing et al. 2006; Ettwig et al. 2010). This process is expected to be confined to the oxic/anoxic interface in the sediment, which in our study area is so close to the sediment surface that the sampling resolution used is insufficient to capture it. However, as indicated above, given the potential for bioturbation and the presence of Ca. Methylomirablis (NC10 phyla) and ANME-2d, it is possible that N-AOM is also taking place at least at site A.
Methanogens are capable of also oxidizing CH4, while on the other hand methanotrophs are capable of reducing CO2 to CH4 (Timmers et al. 2017). This potentially creates a “cryptic CH4” cycle, in which CH4 is being oxidized and produced concurrently. These processes may be mediated partly by the same microbes or via syntrophic couplings, both within the SMTZ (Beulig et al. 2019) and shallower in the sediment column (Maltby et al. 2016; Xiao et al. 2017). While we cannot rule out these processes being present from the SMTZ upwards, no evidence yet exists of such processes deeper in the sediments in conjunction with Me-AOM.
Implications and conclusions
Our estimates for CH4 oxidation rates are broadly similar in magnitude to rates of CH4 production in sediments from the Pojo Bay estuary (Jilbert et al. 2018; Myllykangas et al. 2020). Thus, our results suggest that sedimentary CH4 oxidation filters in this estuary and its connected archipelago are functioning efficiently and removing the vast majority of the produced CH4 in situ, preventing its escape to the water column. This is supported by similarities in the abundances of methanogenic and methanotrophic microbial taxa in sediment profiles, indicating strong co-occurrence of these processes.
We identify three distinct layers of oxidation activity each contributing to the function of the oxidative filter; MOX, S-AOM and Me-AOM. Me-AOM below the SMTZ is clearly very active throughout the transect, with maximum rates observed at site J. Similarly, S-AOM rates are highest in this site. Overall it is likely the increased availability of CH4 caused the total integrated rates of both processes to increase offshore. Similarly to the oxidation rates, in the microbial community structure, ANME-2a/b were relatively most abundant below the SMTZ, especially in higher salinity conditions whereas MOB and methanogens were more abundant in the lower salinity.
In many coastal systems, rates of both methanogenesis and methanotrophy have increased as a consequence of anthropogenic eutrophication (Borges et al. 2018). As well as potential impacts on CH4 fluxes to the atmosphere, the links between CH4 processes and other elemental cycles may lead to broader impacts on coastal sediment biogeochemistry. Our study shows that non-SO42− AOM is important in coastal sediments of the northern Baltic Sea, highlighting in particular the coupling between CH4 and Fe cycling. The Fe-AOM model of Rooze et al. (2016) found that only 9% of all CH4 oxidation was performed by Fe-AOM. However, according to their results Fe-AOM had a significant role in Fe cycling, accounting for 46% of all Fe(OH)3 reduction. Our study suggests that in coastal areas, the role of Fe-AOM may be even greater, leading to even stronger impacts on Fe cycling. This could have further downstream effects on other biogeochemical cycles, such as phosphorus transport to deep basins of the Baltic (Jilbert and Slomp 2013).
References
Arndt S, Jørgensen BB, LaRowe DE et al (2013) Quantifying the degradation of organic matter in marine sediments: a review and synthesis. Earth Sci Rev 123:53–86. https://doi.org/10.1016/j.earscirev.2013.02.008
Beal EJ, House CH, Orphan VJ (2009) Manganese- and iron-dependent marine methane oxidation. Science 325:184–187. https://doi.org/10.1126/science.1169984
Beaulieu JJ, DelSontro T, Downing JA (2019) Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nat Commun 10:1375. https://doi.org/10.1038/s41467-019-09100-5
Beck DAC, Kalyuzhnaya MG, Malfatti S et al (2013) A metagenomic insight into freshwater methane-utilizing communities and evidence for cooperation between the Methylococcaceae and the Methylophilaceae. PeerJ 1:e23. https://doi.org/10.7717/peerj.23
Beulig F, Røy H, McGlynn SE, Jørgensen BB (4) Cryptic CH4 cycling in the sulfate–methane transition of marine sediments apparently mediated by ANME-1 archaea. ISME J 13:250–262. https://doi.org/10.1038/s41396-018-0273-z
Borges AV, Speeckaert G, Champenois W et al (2018) Productivity and temperature as drivers of seasonal and spatial variations of dissolved methane in the Southern Bight of the North Sea. Ecosystems 21:583–599. https://doi.org/10.1007/s10021-017-0171-7
Capone DG, Kiene RP (1988) Comparison of microbial dynamics in marine and freshwater sediments: contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33:725–749. https://doi.org/10.4319/lo.1988.33.4part2.0725
de Angelis MA, Scranton MI (1993) Fate of methane in the Hudson River and Estuary. Glob Biogeochem Cycles 7:509–523. https://doi.org/10.1029/93GB01636
Dean JF, Middelburg JJ, Röckmann T et al (2018) Methane feedbacks to the global climate system in a warmer world. Rev Geophys 56:207–250. https://doi.org/10.1002/2017RG000559
Deutzmann JS, Stief P, Brandes J, Schink B (2014) Anaerobic methane oxidation coupled to denitrification is the dominant methane sink in a deep lake. Proc Natl Acad Sci 111:18273–18278. https://doi.org/10.1073/pnas.1411617111
Ding J, Fu L, Ding ZW et al (2016) Experimental evaluation of the metabolic reversibility of ANME-2d between anaerobic methane oxidation and methanogenesis. Appl Microbiol Biotechnol 100:6481–6490. https://doi.org/10.1007/s00253-016-7475-y
Edgar RC, Haas BJ, Clemente JC et al (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Egger M, Rasigraf O, Sapart CJ et al (2015) Iron-mediated anaerobic oxidation of methane in brackish coastal sediments. Environ Sci Technol 49:277–283. https://doi.org/10.1021/es503663z
Egger M, Riedinger N, Mogollón JM, Jørgensen BB (2018) Global diffusive fluxes of methane in marine sediments. Nat Geosci 11:421–425. https://doi.org/10.1038/s41561-018-0122-8
Eller G, Kanel L, Krüger M (2005) Cooccurrence of aerobic and anaerobic methane oxidation in the water column of Lake Plu see. Appl Environ Microbiol 71:8925–8928. https://doi.org/10.1128/AEM.71.12.8925-8928.2005
Ettwig KF, Butler MK, Le Paslier D et al (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548. https://doi.org/10.1038/nature08883
Ettwig KF, Zhu B, Speth D et al (2016) Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc Natl Acad Sci 113:12792–12796. https://doi.org/10.1073/pnas.1609534113
Evans PN, Parks DH, Chadwick GL, Robbins SJ, Orphan VJ, Golding SD, Tyson GW (2015) Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 350:434–438. https://doi.org/10.1126/science.aac7745
Fenchel T, Bernard C, Esteban G et al (1995) Microbial diversity and activity in a Danish Fjord with anoxic deep water. Ophelia 43:45–100. https://doi.org/10.1080/00785326.1995.10430576
Gammal J, Norkko J, Pilditch CA, Norkko A (2017) Coastal hypoxia and the importance of benthic macrofauna communities for ecosystem functioning. Estuaries Coasts 40:457–468. https://doi.org/10.1007/s12237-016-0152-7
Garber AI, Nealson KH, Okamoto A, McAllister SM, Chan CS, Barco RA, Merino N (2020) FeGenie: a comprehensive tool for the identification of iron genes and iron gene neighborhoods in genome and metagenome assemblies. Front Microbiol 11:37. https://doi.org/10.3389/fmicb.2020.00037
Gantner S, Andersson AF, Alonso-Sáez L, Bertilsson S (2011) Novel primers for 16S rRNA-based archaeal community analyses in environmental samples. J Microbiol Methods 84:12–18. https://doi.org/10.1016/j.mimet.2010.10.001
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491. https://doi.org/10.1128/AEM.66.12.5488-5491.2000
Haroon MF, Hu S, Shi Y et al (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500:567–570. https://doi.org/10.1038/nature12375
Hoehler TM, Alperin MJ, Albert DB, Martens CS (1994) Field and laboratory studies of methane oxidation in an anoxic marine sediments: evidence for methanogen-sulphate reducer consortium. Glob Biochem Cycles 8:451–463
Holmkvist L, Ferdelman TG, Jørgensen BB (2011) A cryptic sulfur cycle driven by iron in the methane zone of marine sediment (Aarhus Bay, Denmark). Geochim Cosmochim Acta 75:3581–3599. https://doi.org/10.1016/j.gca.2011.03.033
Huse SM, Welch DM, Morrison HG, Sogin ML (2010) Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol 12:1889–1898. https://doi.org/10.1111/j.1462-2920.2010.02193.x
IPCC (2014) Climate change 2013—the physical science basis. Cambridge University Press, Cambridge
Iversen N, Jørgensen B (1985) Anaerobic methane oxidation rates at the sulphate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark). Limnol Oceanogr 30:944–955
Jakobs G, Rehder G, Jost G et al (2013) Comparative studies of pelagic microbial methane oxidation within the redox zones of the Gotland Deep and Landsort Deep (central Baltic Sea). Biogeosciences 10:7863–7875. https://doi.org/10.5194/bg-10-7863-2013
Jilbert T, Asmala E, Schröder C et al (2018) Impacts of flocculation on the distribution and diagenesis of iron in boreal estuarine sediments. Biogeosciences 15:1243–1271. https://doi.org/10.5194/bg-15-1243-2018
Jilbert T, Slomp CP (2013) Iron and manganese shuttles control the formation of authigenic phosphorus minerals in the euxinic basins of the Baltic Sea. Geochim Cosmochim Acta 107:155–169. https://doi.org/10.1016/j.gca.2013.01.005
Jørgensen BB, Weber A, Zopfi J (2001) Sulfate reduction and anaerobic methane oxidation in Black Sea sediments. Deep Sea Res Part I 48:2097–2120. https://doi.org/10.1016/S0967-0637(01)00007-3
Kauppi L, Bernard G, Bastrop R et al (2018) Increasing densities of an invasive polychaete enhance bioturbation with variable effects on solute fluxes. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-25989-2
Knittel K, Boetius A (2009) Anaerobic oxidation of methane: progress with an unknown process. Annu Rev Microbiol 63:311–334. https://doi.org/10.1146/annurev.micro.61.080706.093130
Kuever J, Rainey FA, Widdel F (2005) Order III. Desulfobacterales ord. nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM (eds) Bergey's manual of systematic bacteriology, 2nd edn, vol. 2 (The Proteobacteria), part C (The Alpha-, Beta-, Delta-, and Epsilonproteobacteria). Springer, New York
Kuever J (2013) The Family Desulfarculaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The Prokaryotes. Springer, Berlin
Losekann T, Knittel K, Nadalig T et al (2007) Diversity and abundance of aerobic and anaerobic methane oxidizers at the Haakon Mosby Mud Volcano, Barents Sea. Appl Environ Microbiol 73:3348–3362. https://doi.org/10.1128/AEM.00016-07
Lovley DR, Phillips EJP (1988) Manganese inhibition of microbial iron reduction in anaerobic sediments. Geomicrobiol J 6:145–155. https://doi.org/10.1080/01490458809377834
Lovley D (2013) Dissimilatory Fe(III) and Mn(IV)-reducing prokaryotes. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The Prokaryotes. Springer, Berlin
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Maltby J, Sommer S, Dale AW, Treude T (2016) Microbial methanogenesis in the sulfate-reducing zone of surface sediments traversing the Peruvian margin. Biogeosciences 13:283–299. https://doi.org/10.5194/bg-13-283-2016
Martinez-Cruz K, Sepulveda-Jauregui A, Casper P et al (2018) Ubiquitous and significant anaerobic oxidation of methane in freshwater lake sediments. Water Res 144:332–340. https://doi.org/10.1016/j.watres.2018.07.053
McGlynn SE (2017) Energy metabolism during anaerobic methane oxidation in ANME archaea. Microbes Environ 32:5–13. https://doi.org/10.1264/jsme2.ME16166
McGlynn SE, Chadwick GL, Kempes CP, Orphan VJ (2015) Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 526:531–535. https://doi.org/10.1038/nature15512
Myllykangas J-P, Hietanen S, Jilbert T (2020) Legacy effects of eutrophication on modern methane dynamics in a boreal estuary. Estuaries Coasts 43:189–206. https://doi.org/10.1007/s12237-019-00677-0
Nazaries L, Murrell JC, Millard P, Baggs L, Singh BK (2013) Methane, microbes and models: fundamental understanding of the soil methane cycle for future predictions. Environ Microbiol 15:2395–2417. https://doi.org/10.1111/1462-2920.12149
Osudar R, Matoušů A, Alawi M et al (2015) Environmental factors affecting methane distribution and bacterial methane oxidation in the German Bight (North Sea). Estuar Coast Shelf Sci 160:10–21. https://doi.org/10.1016/j.ecss.2015.03.028
Rabus R, Hansen TA, Widdel F (2013) Dissimilatory sulfate- and sulfur-reducing prokaryotes. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The Prokaryotes. Springer, Berlin
Raghoebarsing AA, Pol A, van de Pas-Schoonen KT et al (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440:918–921. https://doi.org/10.1038/nature04617
Rasigraf O, Helmond NAGM, Frank J, Lenstra WK, Egger M, Slomp CP, Jetten MSM (2019) Microbial community composition and functional potential in Bothnian Sea sediments is linked to Fe and S dynamics and the quality of organic matter. Limnol Oceanogr 65(S1):S113–S133. https://doi.org/10.1002/lno.11371
Reed DC, Gustafssson BG, Slomp CP (2016) Shelf-to-basin iron shuttling enhances vivianite formation in deep Baltic Sea sediments. Earth Planet Sci Lett 434:241–251. https://doi.org/10.1016/j.epsl.2015.11.033
Reed DC, Slomp CP, Gustafsson BG (2011) Sedimentary phosphorus dynamics and the evolution of bottom-water hypoxia: a coupled benthic-pelagic model of a coastal system. Limnol Oceanogr 56:1075–1092. https://doi.org/10.4319/lo.2011.56.3.1075
Rissanen AJ, Karvinen A, Nykänen H et al (2017) Effects of alternative electron acceptors on the activity and community structure of methane-producing and consuming microbes in the sediments of two shallow boreal lakes. FEMS Microbiol Ecol 93:1–16. https://doi.org/10.1093/femsec/fix078
Rissanen AJ, Peura S, Mpamah PA et al (2019) Vertical stratification of bacteria and archaea in sediments of a small boreal humic lake. FEMS Microbiol Lett 366:1–11. https://doi.org/10.1093/femsle/fnz044
Rooze J, Egger M, Tsandev I, Slomp CP (2016) Iron-dependent anaerobic oxidation of methane in coastal surface sediments: Potential controls and impact. Limnol Oceanogr 61:S267–S282. https://doi.org/10.1002/lno.10275
Roslev P, King GM (1994) Survival and recovery of methanotrophic bacteria starved under oxic and anoxic conditions. Appl Environ Microbiol 60:2602–2608
Rotaru A-E, Shrestha PM, Liu F, Shrestha M, Shrestha D, Embree M, Zengler K, Wardman C, Nevin KP, Lovley DR (2014) A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci 7:408–415. https://doi.org/10.1039/C3EE42189A
Rotaru A-E, Calabrese F, Stryhanyuk H, Musat F, Shrestha PM, Weber HS, Snoeyenbos-West OLO, Hall POJ, Richnow HH, Musat N, Thamdrup B (2018) Conductive particles enable syntrophic acetate oxidation between Geobacter and Methanosarcina from coastal sediments. mBio 9:e00226–e318. https://doi.org/10.1128/mBio.00226-18
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Schmale O, Schneider V, Deimling J, Gülzow W et al (2010) Distribution of methane in the water column of the Baltic Sea. Geophys Res Lett 37:1–5. https://doi.org/10.1029/2010GL043115
Schmieder R, Edwards R (2011) Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. https://doi.org/10.1093/bioinformatics/btr026
Sivan O, Adler M, Pearson A et al (2011) Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol Oceanogr 56:1536–1544. https://doi.org/10.4319/lo.2011.56.4.1536
Steinle L, Maltby J, Treude T et al (2017) Effects of low oxygen concentrations on aerobic methane oxidation in seasonally hypoxic coastal waters. Biogeosciences 14:1631–1645. https://doi.org/10.5194/bg-14-1631-2017
Takahashi S, Tomita J, Nishioka K et al (2014) Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 9:e105592. https://doi.org/10.1371/journal.pone.0105592
Takai K, Horikoshi K (2000) Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66:5066–5072. https://doi.org/10.1128/AEM.66.11.5066-5072.2000
Thang NM, Brüchert V, Formolo M et al (2013) The impact of sediment and carbon fluxes on the biogeochemistry of methane and sulfur in littoral Baltic Sea sediments (Himmerfjärden, Sweden). Estuaries Coasts 36:98–115. https://doi.org/10.1007/s12237-012-9557-0
Thauer RK (1998) Biochemistry of methanogensis: a tribute to Marjory Stephenson. Microbiology 144:2377–2407
Tiihonen R (2016) The distribution of iron and manganese in coastal sediments of the Gulf of Finland. University of Helsinki
Timmers PHA, Welte CU, Koehorst JJ et al (2017) Reverse methanogenesis and respiration in methanotrophic archaea. Archaea 2017:1–22. https://doi.org/10.1155/2017/1654237
Treude T, Krüger M, Boetius A, Jørgensen B (2005) Environmental control on anaerobic oxidation of methane in the gassy sediments of Eckernfoerde Bay(German Baltic). Limnol Oceanogr 50:1771–1786. https://doi.org/10.4319/lo.2005.50.6.1771
Valentine DL (2002) Biogeochemistry and microbial ecology of methane oxidation in anoxic environments: a review. Antonie Van Leeuwenhoek 81:271–282. https://doi.org/10.1023/A:1020587206351
Vaquer-Sunyer R, Duarte CM (2008) Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci 105:15452–15457. https://doi.org/10.1073/pnas.0803833105
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Weber HS, Habicht KS, Thamdrup B (2017) Anaerobic methanotrophic archaea of the ANME-2d cluster are active in a low-sulfate, iron-rich freshwater sediment. Front Microbiol 8:1–13. https://doi.org/10.3389/fmicb.2017.00619
Welte CU, Rasigraf O, Vaksmaa A et al (2016) Nitrate- and nitrite-dependent anaerobic oxidation of methane. Environ Microbiol Rep 8:941–955. https://doi.org/10.1111/1758-2229.12487
Xiao Q, Zhang M, Hu Z et al (2017) Spatial variations of methane emission in a large shallow eutrophic lake in subtropical climate. J Geophys Res Biogeosci 122:1597–1614. https://doi.org/10.1002/2017JG003805
Youssef N, Elshahed MS, McInerney MJ (2009) Microbial processes in oil fields: culprits, problems, and opportunities. Adv Appl Microbiol 66:141–251. https://doi.org/10.1016/S0065-2164(08)00806-X
Acknowledgements
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. This project was funded by the Academy of Finland project 267112 to SH and 286642 to AJR, and the foundation of Onni Talas (personal grant to JPM). We extend our gratitude to Tina Treude for her invaluable insights and comments on the design of the radiotracer experiments. We also wish to thank the staff and field crew at the Tvärminne Zoological station for providing field and laboratory work infrastructure. Finally, we wish to thank Kaj-Roger Hurme for his invaluable assistance in the radiotracer analysis. We thank two anonymous reviewers for their comments, which greatly improved the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: R. Kelman Wieder
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Myllykangas, JP., Rissanen, A.J., Hietanen, S. et al. Influence of electron acceptor availability and microbial community structure on sedimentary methane oxidation in a boreal estuary. Biogeochemistry 148, 291–309 (2020). https://doi.org/10.1007/s10533-020-00660-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00660-z