Abstract
Estimating abundance of wild animal populations is crucial for their management and conservation. While spatial capture-recapture models are becoming increasingly common to assess the densities of elusive species, recent studies have indicated potential bias that can be introduced by unaccounted spatial variation of detectability. We used camera-trapping data collected in collaboration with local hunters from a transnational population survey of the Eurasian lynx (Lynx lynx) in Slovenia and Croatia, to provide the first density estimate for the threatened Eurasian lynx population in the Northern Dinaric Mountains. Population density was 0.83 (95% CI: 0.60–1.16) lynx/100 km2, which is comparable to other reintroduced Eurasian lynx populations in Europe. Furthermore, we showed that baseline detection rate was influenced by the type of site used, as well as by sex of the individual and local behavioural response. Scent-marking sites had on average a 1.6- and 2.5-times higher baseline detection rate compared to roads and other locations, respectively. Scent-marking behaviour is common for several mammals, and selecting sites that attracts the targeted species is used to increase detection rates, especially for rare and cryptic species. But we show that the use of different location types for camera trapping can bias density estimates if not homogenously distributed across the surveyed area. This highlights the importance of incorporating not only individual characteristics (e.g., sex), but also information on the type of site used in camera trapping surveys into estimates of population densities.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Estimating abundance and density of wild animal populations is crucial for their management and conservation (Goldsmith 1991). Whether it is a hunted game species or a protected species of conservation concern, suitable monitoring programs need to be implemented to evaluate the impact of management measures (Banasiak et al. 2021). For populations of rare and protected species, it is vital to continuously assess the basic population parameters to detect any changes in density or distribution, which enables decision-makers to take appropriate conservation interventions and ensure that species status improves or remains favourable. However, population estimates for elusive species can be difficult to obtain and therefore even baseline data is often lacking (Obbard et al. 2010).
Camera-trapping surveys are a widespread method for estimating population size and distribution, especially when individuals can be identified by e.g., distinct pelage patterns, creating detection records of individual animals at each camera site (Rovero and Zimmermann 2016). Spatial capture-recapture (SCR) is an analytical framework incorporating the spatial and temporal aspects of individual detections, in combination with species ecology, to estimate density of the targeted species. SCR models consist of two parts: i) the density model, describing how individuals are distributed in space, and ii) the spatial model of detection, describing how baseline detection rate of an individual decreasing with distance between home range centre and the camera site, i.e., the spatial scale parameter (Efford et al. 2004; Royle et al. 2014). The number of studies reporting population-level estimates of SCR-derived densities have increased substantially over the last years (Tourani 2022), providing population size estimates for rare and cryptic species, such as the snow leopard (Panthera uncia) (Oberosler et al. 2021), tiger (Panthera tigris) (Harihar et al. 2020), jaguar (Panthera onca) (Harmsen et al. 2020), leopard (Panthera pardus) (Allen et al. 2020), or Eurasian lynx (Lynx lynx) (Palmero et al. 2021).
Ecological studies often aim at assessing the variation of density of a target species within their study area, by incorporating covariates describing the variation in e.g. habitat, human impact or even interspecific interactions (Barrueto et al. 2020; Allen et al. 2020; Iosif et al. 2022). However, the need to account for spatial variation in detectability has recently been highlighted, as it reduces the bias of SCR parameter estimates (Barrueto et al. 2020; Fleming et al. 2021; Hofmeester et al. 2021; Oberosler et al. 2021), specifically for abundance estimates (Moqanaki et al. 2021). Population estimates are optimized by maximizing the number of detected individuals and the number of their spatial recaptures (Sollmann et al. 2012). Thus, camera-traps are often placed at locations with the highest presumed detection rate. Studies report cameras being placed at forest paths or roads which are commonly used for animal movement (Sollmann et al. 2011; Pesenti and Zimmermann 2013; Di Bitetti et al. 2014), or at locations that attract the target species, such as baited stations (Du Preez et al. 2014) or scent-marking sites (Brassine and Parker 2015). Marking sites represent important intraspecific communication hubs, but there can be considerable individual variation in the frequency of visits to such sites, often related to sex or/and life stage of an individual (Vogt et al. 2014; Allen et al. 2014; Melzheimer et al. 2020). Thus, placing camera-traps at marking sites can be an effective way to increase the number of individuals detected, however, using sites which attract the target species in combination with sites that do not can induce bias in abundance estimates (Di Bitetti et al. 2014; Du Preez et al. 2014).
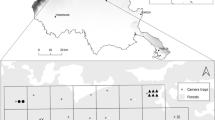
Eurasian lynx is the largest felid in Europe, an elusive apex predator of European forest ecosystems characterized by territorial and solitary lifestyle. It is the most threatened of all large carnivores in European temperate forests and it occurs in 11 distinct populations across the continent, including six that were reintroduced after extermination by human persecution in the beginning of the twentieth century (Chapron et al. 2014; Breitenmoser-Würsten and Breitenmoser 2021). The Eurasian lynx population in the Dinaric Mountains originates from a reintroduction project in Slovenia in 1973 (Čop 1990). However, the Dinaric lynx population started to decrease in the 2000s (Kos et al. 2012; Huber et al. 2013), largely due to inbreeding depression (Sindičić et al. 2013), and is today among the most endangered Eurasian lynx populations (von Arx et al. 2021). But so far, we lacked reliable estimates of lynx abundance in this population. In 2018, a population reinforcement program started with translocations of lynx from the Carpathian mountains to the Dinaric population (Fležar et al. 2021). Furthermore, a transnational population-level camera-monitoring program was initiated, to assess the current and future status of the population (Fležar et al. 2019a). The monitoring program is conducted in tight collaboration with local hunters and protected area rangers (Krofel et al. 2021), covering over 10.000 km2 area in Slovenia and Croatia (Fig. 1). Due to regional differences in available funding, manpower, and knowledge of lynx space use in the area, the effort, density of camera-traps, as well as the sites used for camera-trapping location type vary within the area (i.e., marking sites, road/trails and other sites; Fig. 1, Table 1). Consequently, in order to obtain estimates of density and abundance from the camera-monitoring program, there is a need to evaluate the potential impact of the regional variation in the location types used for camera-trapping sites.
The location of our study area in Europe with distribution of Eurasian lynx (left) (Kaczensky et al. 2021) and overview of the regions included in the study: Slovenia (S), Gorski Kotar, Croatia (G) and Velebit, Croatia (V). Solid and dashed black lines represent national and regional borders, respectively. The grey gradient shows the lynx habitat suitability (black – most suitable, white – non-suitable), according to Skrbinšek and Krofel (2008); blue is the Adriatic Sea. Distribution of camera trapping sites is showed according to their site types: marking sites (red), roads (blue) and other (grey). Sites which were excluded from the analysis are shown in yellow
In this study, we provide the first estimates of density and abundance for the threatened Dinaric lynx population in Slovenia and Croatia, which are essential for understanding current population status. They also represent an important baseline information for future evaluations of the impact of ongoing population reinforcement. Furthermore, we assess the effect of camera-trap location type (i.e. placing cameras at marking sites, roads/trails or other locations) and sex on the spatial model of detection and evaluate their potential impact on the density estimation. The baseline detection rate was expected to be highest at marking sites, due to their attractiveness for the lynx, compared to non-marking locations (Vogt et al. 2014; Allen et al. 2017). The baseline detection rate and the spatial scale parameter were expected to be higher for males than females as males were expected to exhibit more frequent visits to camera-traps (Pesenti and Zimmermann 2013; Goldberg et al. 2015), and to be exposed to more camera-trapping sites due to their larger home range size (Herfindal et al. 2005; Aronsson et al. 2016), respectively.
Methods
Study area
This study was conducted in the Northern Dinaric Mountains in Slovenia and Croatia (hereafter Dinaric Mountains). The Dinaric Mountains are covered by a continuum of mixed forests, mainly silver fir (Abies alba) and common beech (Fagus sylvatica), interspersed with small patches of cultivated land and small settlements. The area is characterized by high micro and macro ruggedness due to its karstic origin, with common landscape features such as cliffs, dolines, caves and other prominent carstic rock formations (Čonč et al. 2022). The altitude is ranging from sea level to 1800 m, with some isolated mountain peaks reaching above the tree line. The climate is a mix of influences from the Alps, the Adriatic sea, and the Pannonia basin, with average annual temperature of 7 °C (ranging between monthly averages of 18 °C in July and − 2 °C in January), and average annual precipitation of 1700 mm. Snow cover lasts from 50 days at lower altitudes and up to several months at higher elevations. The vegetative growth period lasts from late April to late October. The Dinaric fauna is well preserved, fostering several species of ungulates, meso- and small carnivores, as well as large carnivores: grey wolf (Canis lupus), brown bear (Ursus arctos) and Eurasian lynx (Skrbinšek et al. 2018; Fležar et al. 2019b; Bartol et al. 2021). A variety of human activities take place in the area, the main ones being regulated hunting, logging and recreation. Average human population density is between 28 (in Slovenian study area) and 8 (in Velebit) people/km2 with most settlements concentrated in the larger valleys.
We defined Slovenia, Gorski Kotar and Velebit as our three separate study regions, representing the regional differences in camera-monitoring design (Fig. 1, see Camera trapping for further details). The three study regions comprise the total study area, i.e. the Dinaric Mountains. International collaboration between Slovenia and Croatia aiming at coordinated long-term lynx monitoring in the Dinaric Mountains allowed for uninterrupted exchange of data, including identifying lynx with cross-border territories.
Camera trapping
Camera traps were set up exclusively in forested areas, which is the optimal habitat of lynx in the Dinaric Mountains (Skrbinšek and Krofel 2008). We classified the camera trapping sites in three distinct categories (hereafter ‘location type’), based on their main characteristics; 1) lynx scent-marking site; i.e. forest cabins, conspicuous rocks, rock shelters, caves and other similar objects (Allen et al. 2017) confirmed to be used by lynx for scent-marking in this region by data collected through snow-tracking and/or camera trapping in the past or present survey (Slijepčević et al. 2019), 2) forest road; unpaved forest roads and logging trails and 3) other; ridges, large rocks, rock walls and other prominent karstic landscape features but where no indication of lynx scent marking was observed (Fig. 2). The ‘forest road’ and ‘other’categories were non-marking sites. We used 174 camera-trapping sites with one camera and 54 camera-trapping sites with two cameras set, following the camera trapping guidelines from Stergar and Slijepčević (2017) to optimize lynx identification (see Sect. 3. for further details). We used cameras with white flash (CuddeBack X-Change Color Model 1279, Cuddeback, Green Bay, Wisconsin) at sites where we expected lynx to pass by, and cameras of the same model with black (940 nm light wave) or regular infrared light (850 nm light wave) (StealthCam STC-G42NG, Stealth Cam, Irving; Moultrie M40-i, PRADCO Outdoor brands, Birmingham; LTL Acorn models Ltl-6310WMG and Ltl-6511WMC) at sites where we expected lynx to stop for scent marking to avoid disturbing it with flash (Stergar and Slijepčević 2017).
In Slovenia, camera traps were set at all three types of locations, while camera traps were primarily set at marking sites in Gorski Kotar, and at non-marking sites in Velebit. Moreover, a higher density of camera trapping sites was set up in Slovenia compared to Croatia (mean 2.5 and 5.8 km between sites, respectively, see Table 1 for further details). To meet the minimum requirement of SCR analysis for ensuring a non-zero capture probability, we selected at least one camera trapping site in a potential home range of a studied population (Royle et al. 2014), the reported 95% MCP home-range size ranging between 60 and 343 km2 (Krofel et al. 2021). Initially, we set up 297 camera trapping sites, however, for the purpose of this study we excluded 69 camera trapping sites that might be biased according to Iosif et al. (2022), i.e. sites in areas where we did not have past information about lynx presence or where data collected was limited due to camera malfunctions, theft or other hardware issues. The majority of these sites were situated at the southernmost and northernmost range of the camera trapping array (Fig. 1) and yielded no or poor capture histories, which if included in the analysis would inflate the effective sampling area and potentially bias the overall density estimates produced by the SCR models (Moqanaki et al. 2021). Mean trap spacing and spatial scale parameter calculated by the models (see Results) confirmed that designs in all regions and the entire study area fit the standard recommendation of 2σ (Efford and Fewster 2013; Dupont et al. 2021).
For this study, we used data collected from August 15th 2019 to February 15th 2020, yielding records from a total of 185 sampling occasions and 22,748 camera trapping days in total. The survey period was designed to end before estimated start of the mating season and also before the peak in dispersal (Breitenmoser and Breitenmoser-Würsten 2008).
Data preparation
Lynx records were annotated with sex, age and individual identity. Sex was determined if genital area was clearly visible or if kittens were present with females, thus individuals were marked as females, males or unknown sex. Each record of lynx was subjected to identification based on the animal’s unique pelage pattern (Topličanec et al. 2022) by trained observers (n = 3), i.e. observers which have identified lynx during the previous (pilot) surveys, following the identification guidelines published by Choo et al. (2020). In case of poor-quality records, at least one additional observer attempted to identify the lynx independently until full consensus was reached, including annotating the individual as ‘unidentified’ if the observers could not agree on the identity of an animal. All records where consensus could not be reached and all records of lynx with only one flank of the body recorded were discarded from the analyses. Juveniles, i.e. kittens detected with their mother were also excluded from the analysis because of the high mortality of kittens (Andrén et al. 2006; Duľa et al. 2021). Thus, only data about individually identified adult lynx originating from high quality records where the pattern was clearly visible from both flanks of the body were used to build capture histories. We recorded the number of spatial recaptures in each region to check that our data met the recommendation of at least 20 spatial recaptures for accurate and precise estimation of the spatial scale parameter (Efford et al. 2004). Data about lynx and trap deployment were exported directly from Camelot software (Hendry and Mann 2017), which was used for camera trapping data annotation, and reorganized to fit SCR analysis.
SCR modelling
Lynx density, baseline detection rate and spatial scale parameter were estimated with maximum likelihood spatial capture-recapture models (Royle et al. 2014) using oSCR package (Sutherland et al. 2019) in R software v. 4.1.0 (R Core Team 2021). The distribution of individual activity centres was defined as Bernoulli random trials (Royle et al. 2014) and the spatial model of detection followed a half-normal detection function (Efford and Schofield 2020). We assumed homogeneous distribution of the individuals across space. We ran separate SCR models for the two study regions from which the recommendations on 20 spatial recaptures were met (Efford et al. 2004) (Slovenia and Gorski Kotar; Table 1), and for the entire transboundary study area (Dinaric Mountains). The low number of spatial recaptures in Velebit region (Table 1) would yield density estimates of poor precision (Efford et al. 2004) so the data from Velebit was only used in the models for the entire study area. We first ran null models and standard SCR models including local behavioural response (‘b’) to assess the individual-level variation in baseline detection rate at specific camera trapping sites (Royle et al. 2011), following the findings of Iosif et al. (2022). Next, we included the additive effect of sex on baseline detection rate and the spatial scale parameter, as suggested by Goldberg et al. (2015). Sex was included as a binary covariate (female as reference category). For the Slovenia and the Dinaric Mountains models, we also included the additive effect of location type as a categorical three-level (i.e., marking site, road and other) or two-level (i.e., marking site vs. road and other pooled as non-marking site) factorial covariate, with marking site as reference category (Table 1 in Supplementary file1).
For each of the models (Slovenia, Gorski Kotar and the Dinaric Mountains), we defined the extent of the effective sampling area, i.e. the “state space”, multiplying the respective σ values by 3 to buffer the camera trapping grid and using a resolution of buffer cells at most the full value of the same parameter (Royle et al. 2014). We set the buffer width to 15, 10 and 20 km for Slovenia, Gorski Kotar and Dinaric Mountains, respectively. The resolution of buffer cells was set to 2.5 × 2.5 for Slovenia and Gorski Kotar and 4 × 4 km for Dinaric Mountains. We restricted the state space by excluding all cells located in non-lynx-habitat defined by the habitat suitability model for lynx in the Dinaric Mountains (Skrbinšek and Krofel 2008), i.e. the Adriatic sea, urbanized or highly agricultural land (Fig. 1 in Supplementary file1). We ranked candidate models based on Akaike Information Criterion (AIC), with models having ΔAIC ≤ 2 considered having substantial support (Burnham and Anderson 2004). Among the highest-ranking models, the one with the best fit (lowest AIC value) and the highest predictive power (highest AIC weight) was used to calculate the abundance of lynx at the level of the population, i.e. the Dinaric Mountains and at the regional level, e.g. for Slovenia and Gorski Kotar.
Results
In total, we detected lynx at 171 independent occasions during the survey period (85 in Slovenia, 57 in Gorski Kotar, and 29 in Velebit), identifying in total 44 individuals (Table 1). Lynx could not be identified in 13% of the obtained photos and all but one animal had a coat pattern with big spots, which seems to be a prevailing pattern in the Dinaric lynx population (Topličanec et al. 2022). One individual male lynx in Slovenia was recorded 19 times, while 14 lynx were recorded only once (Fig. 2 in Supplementary file1). The overall maximum mean of recaptures and mean of spatial recaptures (Sutherland et al. 2019), as well as the number of camera-trapping sites and trapping occasions was highest in Slovenia and lowest in Velebit (Table 1).
Dinaric lynx population density
The density of the Dinaric lynx population was estimated to an average of 0.83 (95% CI: 0.60–1.16) lynx/100 km2 corresponding to the population abundance of 96 (69–133) individuals in a state space encompassing 11,536 km2. Based on the models with the best fit, the estimated density in Slovenia averaged on 0.51 (95% CI: 0.30–0.88) lynx/100 km2 corresponding to the abundance of 25 (15–43) individuals in a state space of 4938 km2. Despite a smaller effective sampling area (2956 km2) the estimated density in Gorski Kotar (average of 1.13 (95% CI: 0.63–2.04) lynx/100 km2) was more than double the density in Slovenia.
The effect of camera-trap location type
The highest-ranked models for the Slovenia and Gorski Kotar study regions, as well as the Dinaric Mountains, included the standard local trap response for baseline detection rate (Table 2). For the Dinaric Mountains, there was an additive effect of a 3-level location type on baseline detection rate (Table 2). In line with our expectations, baseline detection rate was highest at marking sites followed by roads and other locations (Fig. 3). When allowing baseline detection rate to vary between camera-trap location types, the estimated density in the Dinaric mountains was 0.83 (95% CI: 0.60–1.16) lynx/100 km2, compared to 0.72 (95% CI: 0.52–1.01) lynx/100 km2 without considering this effect (Table 2, in Supplementary file1). When only considering the Slovenia study region, the highest-ranked model included the 2-level location type covariate, indicating a higher baseline detection rate at marking sites also for Slovenia (Fig. 3).
Estimates for baseline detection rate (mean with 95% confidence interval) from null models and the best candidate models for (a) Slovenia, (b) Gorski Kotar, and the (c) entire study area (Dinaric Mountains). Numbering of facets (null, m1, m2, m3) for each study region correspond to model ID assigned to a specific model structure in Table 2
The effect of sex
In line with our expectations, baseline detection rate was higher for males compared to females in both the Slovenia study region and in the Dinaric Mountains (Fig. 3). For Gorski Kotar, where camera traps were set almost exclusively on marking sites, the highest-ranked model includes an effect of sex on baseline detection rate (Table 2), however, contrary to the other regions, the effect of sex indicated a higher baseline detection rate for females compared to males (Fig. 3). Contrary to our expectation, sex did not affect the spatial scale parameter for the Slovenia study region nor for the Dinaric Mountains. For Gorski Kotar, the highest-ranked model indicated higher value for the spatial scale parameter for males compared to females (resulting in an increased difference between sex-specific densities; Table 2).
Discussion
In this study, we combine camera-trapping data from the large-scale lynx monitoring program, covering 11,536 km2 in Slovenia and Croatia, to provide the first transnational estimates of lynx density and abundance in the Northern Dinaric Mountains, one of the most threatened Eurasian lynx populations in Europe. We incorporate the regional variation in monitoring design, showing that both sex and the behaviour of the targeted species in relation to the camera-trap location characteristics, as well as the spatial variation in placement and distribution of camera-traps, influence the spatial model of detection and hence the resulting density estimates. Specifically, we showed that for Eurasian lynx, a territorial felid, baseline detection rate can be related to the attractiveness of the location type used for camera traps, with the highest baseline detection rate for scent-marking sites. Furthermore, our results emphasise the importance of accounting for sex-specific baseline detection rates, and adds to the growing knowledge that local behavioural response informs the baseline detection rate (Gardner et al. 2010; Royle et al. 2011; Barrueto et al. 2020; Iosif et al. 2022).
As lynx in the Dinaric Mountains are not limited by national borders, transnational monitoring is essential for assessing the population status. Our study is contributing to the few available studies reporting SCR applications at such a wide geographic scale using dataset shared by two neighbouring countries (Tourani 2022; but see Bischof et al. 2020). Moreover, it represents a good example of how hunters can be involved in monitoring activities of a large carnivore, adding to findings of Cretois et al. (2020). As the Dinaric lynx population is strictly protected and non-hunted, their collaboration is a continuation of their stewardship of lynx in the region, which started with the reintroduction in 1973 (Čop 1990), as well as an action of their nature conservation advocacy (e.g. https://www.biodiversitymanifesto.com/). Understanding the baseline status of the lynx in the Dinaric Mountains through direct involvement of the crucial stakeholders may help building trust in the conservation efforts, improving the acceptability of the species and governing its long-term persistence.
The density of Eurasian lynx in the Dinaric Mountains (95% CI: 0.60–1.16 lynx/100 km2) is comparable to densities of other reintroduced Eurasian lynx populations in Europe, i.e. French Jura and Vosges mountains (0.24–0.91 lynx/100 km2; Gimenez et al. 2019), Swiss Alps (1.04–1.47 lynx/100 km2; Pesenti and Zimmermann 2013) and Bavarian-Bohemian forest (0.69–1.33 lynx/100km2; Palmero et al. 2021). Given high inbreeding levels (Sindičić et al. 2013; Krofel et al. 2021), considerable reduction of the distribution range and effective population size that was observed in Dinaric lynx population in the past decades (Kos et al. 2012; Krofel et al. 2021), the densities are expectedly lower compared to the source lynx populations in Southern Carpathians (1.7 ± 0.38 SE lynx/100 km2; Iosif et al. 2022).
While we observed differences in density estimates between study regions, we did not attempt to explain the potential effect of habitat characteristics on density at the regional or entire study area (Dinaric Mountains) scale. It has been shown that the SCR models are robust to misspecification of the density model, or even perform worse when including density covariates in monitoring designs with low effort for populations with inhomogeneous densities (Dupont et al. 2021). In our case, the camera-trapping effort varied between regions; the spacing of traps, as well as average spatial recaptures were two times larger in the Slovenia region compared to the Gorski Kotar and the Velebit regions. Thus, landscape covariates extracted from a sampling area with variable effort was not expected to reliably represent the underlying landscape effect (Dupont et al. 2021). Moreover, locations for camera-traps were limited to the most suitable habitat for lynx in the Dinaric Mountains, as defined by Skrbinšek & Krofel (2008) (Fig. 1). Consequently, to provide information on population-level density of the threatened lynx population, we focused on testing covariates in the spatial model of detection, which on the contrary to habitat characteristics explaining within area density variation, can result in changes of the overall estimation of density.
Spatial variation in detection probability
Placing camera traps at sites attracting the targeted species may be an easy choice to increase the detection rates (Breitenmoser et al. 2006; Karnaukhov et al. 2020), which is often the main goal of opportunistic surveys aiming at confirming presence or absence of species in a certain area. This is also a practice recommended by several monitoring guidelines, including Eurasian lynx (e.g. Breitenmoser et al. 2006; Karnaukhov et al. 2020). In line with our expectations, our results showed that lynx baseline detection rate was highest at sites used for scent marking; in fact, scent-marking sites had a 1.6- and 2.5-times higher mean baseline detection rate compared to roads and other locations (i.e. other landscape characteristics beside marking sites or roads; Fig. 2), respectively. In this context it should be noted that felids, including Eurasian lynx, increase their scent-marking frequency when walking along the roads (Krofel et al. 2017), therefore higher detection on roads could also be linked with marking behaviour, even if the section of the road with camera trap does not include an active marking site.
Besides increasing the baseline detection rate, an advantage of placing camera traps at scent-marking sites was that it increased number of clear images of coat pattern for individual identification (see also Oberosler et al. 2021). Because lynx often stopped and remained at the site for sniffing and/or scent-marking, we were often able to obtain a series of high-quality images from both sides of the animal. This helped us to later identify the same individual from photos of only one side when passing by at sites with a single-camera setup. Such possibility to use single-camera setup can increase the sampling area with the available equipment, often resulting in more spatial recaptures (Suryawanshi et al. 2019). Furthermore, marking sites have been recommended for camera traps in large-scale surveys of remote and hardly accessible areas (Augugliaro and Rovero 2015) or where they represent the only plausible option for detecting elusive species (Brassine and Parker 2015). Scent-marking behaviour is similar across most of the felid species (Allen et al. 2016) and widespread among mammals in general (Gosling & Roberts 2001). Felids select sites in order to prolong the persistence of the scent mark, and increase its detectability by conspecifics (Mohorovoć & Krofel 2020, Allen et al. 2017). Thus, an individual may increase its success in defending its territory and finding mates (Gosling & Roberts 2001). However, previous studies have found that the use of such sites varies between sexes, among individuals, their status and age, and seasonally (Vogt et al. 2014; Krofel et al. 2017). Our results highlight the importance of incorporating the auxiliary information on scent-marking behaviour at camera-trapping sites and test for its potential effect on detection rate and incorporate this in density estimates. When allowing baseline detection rate to vary between camera trap location types, the estimated density in the Dinaric mountains was 0.83 (95% CI: 0.60–1.16) lynx/100 km2, compared to 0.72 (95% CI: 0.52–1.01) lynx/100 km2 without this effect (Table 2, Table S2).
Importantly, the effect of location type on baseline detection rate for the entire Dinaric Mountain study area was not as evident when only considering the Slovenia study region. In addition to covering only approximately one fourth of the entire survey area, the Slovenia study region was characterized by a higher camera trapping effort with higher density of camera traps and a mixed placement at the different location types (Fig. 1). The use of different location types in the three regions within the Dinaric Mountains (i.e., all types in Slovenia, primarily marking sites in Gorski Kotar, and primarily roads and other in Velebit) results in a spatial variation in detection rate. According to the simulation study by Moqanaki et al. (2021), autocorrelated spatial heterogeneity in detection probability can result in biased population estimates. Hence, the Dinaric Mountains illustrates a situation where it is important to account for the effect of location type on baseline detection rate resulting in spatial variation of detection rates, as situation likely to be occurring in other camera trapping studies. In the Slovenia region on the other hand, the distribution of camera trap at the different location types reflects a situation where the spatial variation in detection probability is random, which does not represent a major source of bias for density estimation (Bischof et al. 2017; Efford and Fewster 2013; Sollmann et al. 2012; Moqanaki et al 2021).
Sex-specific density estimates
Our expectations regarding differences between the sexes were partly supported. Baseline detection rate was higher for males compared to females in the Slovenian region and the Dinaric Mountains model, but sex did not inform the spatial scale parameter. Surveys of species with a similar social structure recognized the importance of accounting for sex-specific differences in detectability and sex was shown to affect the baseline detection rate in combination with spatial scale parameter (Sollmann et al. 2011; Goldberg et al. 2015) or to affect the spatial scale parameter only (Augustine et al. 2020; Barrueto et al. 2020; Oberosler et al. 2021). For Eurasian lynx, previous camera-trapping studies did not find differences in baseline detection rate between males and females (Palmero et al. 2021), or did not include sex as a covariate due to difficulty in identifying the sex of all lynx at camera traps (Gimenez et al. 2019; Iosif et al. 2022). By including the additive effect of sex in our study, the average female lynx density estimates increased from 0.19 (0.09–0.42) to 0.27 (0.12–0.62) lynx/100 km2 in Slovenia and from 0.37 (0.24–0.57) to 0.43 (0.27–0.68) lynx/100 km2 in the Dinaric Mountains (Table 2 in Supplementary file1). Even though confidence intervals for the different estimates overlap, it indicates that there is a risk to underestimate female density, which is important to consider in surveys where the focal demographic group are reproductive females. Thus, our results highlight both the importance of assessing the potential effect of sex as this might differ between studies, and the need to design camera traps to increase the chance to determine sex of the individuals. While sex can hardly be observed from lateral records of lynx (e.g. obtained at roads), sex determination is generally easy from the records at marking sites because the animal spends more time there (providing more footage in general) and especially because genitals can be easily observed during urine spraying, which is among the most common scent-marking behaviour in felids (Allen et al. 2016). This is another advantage of using scent-marking sites for camera-trapping surveys.
We expected sex to affect also the spatial scale parameter similar to the findings of Sollmann et al. (2011), as the lynx is characterized by smaller home ranges in females compared to males (Herfindal et al. 2005; Aronsson et al. 2016), and differences in home range size between males and females were also observed in telemetry study in our study area (Fležar et al. 2023). However, our results from Slovenia and the Dinaric Mountains did not indicate any differences in the distance at which females or males were detected from their presumed home range centre (i.e. activity centre). Instead, the higher baseline detection rate for males indicates that females move less than males, but over a similar area. Slovenia represents the northernmost edge of the Dinaric lynx distribution, which may suffer from shrinking distribution range during the past two decades (Koren et al. 2006; Kos et al. 2012; Krofel et al. 2021). Our results show the lowest lynx density in Slovenia, and very similar densities for the sexes (Table 2). Thus, the lack of difference in the spatial scale parameter can be partly explained by a less saturated population with a similar number of males and females, which might reduce the differences in area use between the sexes. Moreover, data used in this study were restricted to non-mating period (until mid-February), thus excluding the large part of the mating season when males increase their ranging behaviour substantially. That behaviour can, in turn, increase the overall home range size reported in telemetry studies (e.g. Jȩdrzejewski et al. 2002) but remain undetected in seasonal camera trapping studies. On the other hand, in Gorski Kotar, where lynx density was higher, we did find an additive effect of sex on the spatial scale parameter, estimating lower activity range for females, which is expected for solitary felids where females have smaller home ranges than males (Sollmann et al. 2011). In turn, the effect of sex on the spatial scale parameter also increased the differences in sex-specific density estimates for this region compared to in Slovenia, with female density being almost double the density of males (Table 2). Furthermore, as long as sex was included as a covariate for the spatial scale parameter, the slightly higher detection rate for females compared to males, indicated by the highest ranked model, did not influence the sex specific density estimates (Table 2). This suggests a possible compensatory effect of variation in baseline detection rate on spatial scale parameter (Efford and Mowat 2014).
Conclusions
This study demonstrates that transnational camera-trapping surveys based on active hunter involvement can be an efficient approach for obtaining datasets for robust population density assessment of an elusive large carnivore. We also show that heterogeneity of camera trapping sites resulting from an alternative field design, including highly attractive scent-marking sites, should be accounted for in the SCR modelling as it importantly informs the density estimation. For the threatened lynx in the Dinaric Mountains, assessment of the population status provided in this study represents a key reference point, which will enable objective evaluation of the impact of ongoing conservation efforts in the near future.
Data availability
All records of lynx with annotations are available to registered users at an international large carnivore database (www.portal.mbase.org).
References
Allen ML, Wittmer HU, Wilmers CC (2014) Puma communication behaviours: Understanding functional use and variation among sex and age classes. Behaviour 151:819–840. https://doi.org/10.1163/1568539X-00003173
Allen ML, Wittmer HU, Setiawan E et al (2016) Scent marking in Sunda clouded leopards (Neofelis diardi): Novel observations close a key gap in understanding felid communication behaviours. Sci Rep 6:1–9. https://doi.org/10.1038/srep35433
Allen ML, Hočevar L, de Groot M, Krofel M (2017) Where to leave a message? The selection and adaptive significance of scent-marking sites for Eurasian lynx. Behav Ecol Sociobiol 71:136. https://doi.org/10.1007/s00265-017-2366-5
Allen ML, Wang S, Olson LO et al (2020) Counting cats for conservation: seasonal estimates of leopard density and drivers of distribution in the Serengeti. Biodivers Conserv 29:3591–3608. https://doi.org/10.1007/s10531-020-02039-w
Andrén H, Linnell JDC, Liberg O et al (2006) Survival rates and causes of mortality in Eurasian lynx (Lynx lynx) in multi-use landscapes. Biol Conserv 131:23–32. https://doi.org/10.1016/j.biocon.2006.01.025
Aronsson M, Low M, López-Bao JV et al (2016) Intensity of space use reveals conditional sex-specific effects of prey and conspecific density on home range size. Ecol Evol 6(9):2957–2967. https://doi.org/10.1002/ece3.2032
Augugliaro C, Rovero F (2015) Searching for the ghost of the mountains Report of a camera trapping study of the snow leopard and other mammals in the Altai Mountains of NW Mongolia
Augustine BC, Kéry M, Olano Marin J et al (2020) Sex-specific population dynamics and demography of capercaillie (Tetrao urogallus L.) in a patchy environment. Popul Ecol 62:80–90. https://doi.org/10.1002/1438-390X.12031
Banasiak NM, Hayward MW, Kerley GIH (2021) Ten years on: have large carnivore reintroductions to the Eastern Cape Province, South Africa, Worked? African J Wildl Res 51:111–116. https://doi.org/10.3957/056.051.0111
Barrueto M, Sawaya MA, Clevenger AP (2020) Low wolverine (Gulo gulo) density in a national park complex of the canadian rocky mountains. Can J Zool 98:287–298. https://doi.org/10.1139/cjz-2019-0165
Bartol M, Černe R, Črtalič J, et al (2021) Spremljanje stanja ohranjenosti volkov v Sloveniji v sezoni 2020–2021, končno poročilo. Ljubljana, oktober 2021
Bischof R, Steyaert SM, Kindberg J (2017) Caught in the mesh: roads and their network-scale impediment to animal movement. Ecography 40(12):1369–1380
Bischof R, Milleret C, Dupont P et al (2020) Estimating and forcasting spatial population dynamics of apex predators using transnational genetic monitoring. PNAS 117(48):30531–30538. https://doi.org/10.1073/pnas.2011383117
Brassine E, Parker D (2015) Trapping elusive cats: using intensive camera trapping to estimate the density of a rare African Felid. PLoS One 10:e0142508. https://doi.org/10.1371/journal.pone.0142508
Breitenmoser U, Breitenmoser-Würsten C (2008) Der Luchs – Ein Großraubtier in der Kulturlandschaft. Salm Verlag, Wohlen/Bern, Schweiz
Breitenmoser U, Breitenmoser-Würsten C, Von Arx M, et al (2006) Guidelines for the Monitoring of Lynx. KORA 33 e, 32 pp
Breitenmoser-Würsten C, Breitenmoser U (2021) The Eurasian lynx in Continental Europe. Cat News. Special Issue no. 14, 87 pp
Burnham KP, Anderson DR (2004) Multimodel Inference Sociol Methods Res 33:261–304. https://doi.org/10.1177/0049124104268644
Chapron G, Kaczensky P, Linnell JDC et al (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346:1517–1519. https://doi.org/10.1126/science.1256620
Choo YR, Kudavidanage EP, Amarasinghe TR et al (2020) Best practices for reporting individual identification using camera trap photographs. Glob Ecol Conserv. https://doi.org/10.1016/j.gecco.2020.e01294
Čonč Š, Oliveira T, Portas R et al (2022) Dolines and cats: remote detection of karst depressions and their application to study wild felid ecology. Remote Sens 14:656. https://doi.org/10.3390/rs14030656
Čop J (1990) Review of the resettlement of lynx (Lynx lynx L.) in Slovenia (YU) 1973–1990. Ljubljana
Cretois B, Linnell JDC, Grainger M et al (2020) Hunters as citizen scientists: contributions to biodiversity monitoring in Europe. Glob Ecol Conserv 23:e01077. https://doi.org/10.1016/j.gecco.2020.e01077
Di Bitetti MS, Paviolo A, De Angelo C (2014) Camera trap photographic rates on roads vs. off roads: location does matter. Mastozoología Neotrop 21:37–46
Du Preez BD, Loveridge AJ, Macdonald DW (2014) To bait or not to bait: A comparison of camera-trapping methods for estimating leopard Panthera pardus density. Biol Conserv 176:153–161. https://doi.org/10.1016/j.biocon.2014.05.021
Duľa M, Bojda M, Chabanne DBH et al (2021) Multi-seasonal systematic camera-trapping reveals fluctuating densities and high turnover rates of Carpathian lynx on the western edge of its native range. Sci Rep 11:1–12. https://doi.org/10.1038/s41598-021-88348-8
Dupont G, Royle JA, Nawaz MA et al (2021) Optimal sampling design for spatial capture–recapture. Ecology 102:e03262. https://doi.org/10.1002/ecy.3262
Efford MG, Fewster RM (2013) Estimating population size by spatially explicit capture-recapture. Oikos 122:918–928. https://doi.org/10.1111/j.1600-0706.2012.20440.x
Efford MG, Mowat G (2014) Compensatory heterogeneity in spatially explicit capture-recapture data. Ecology 95:1341–1348
Efford MG, Schofield MR (2020) A spatial open-population capture-recapture model. Biometrics 76:392–402. https://doi.org/10.1111/biom.13150
Efford MG, Dawson DK, Robbins CS (2004) DENSITY: Software for analysing capture-recapture data from passive detector arrays. Anim Biodivers Conserv 27:217–228
Fleming J, Grant EHC, Sterrett SC et al (2021) Experimental evaluation of spatial capture–recapture study design. Ecol Appl 31:1–11. https://doi.org/10.1002/eap.2419
Fležar U, Pičulin A, Bartol M et al (2019a) Eurasian lynx (Lynx lynx) monitoring with camera traps in Slovenia in 2018–2019. Ljubljana
Fležar U, Costa B, Bordjan D et al (2019b) Free food for everyone: artificial feeding of brown bears provides food for many non-target species. Eur J Wildl Res. https://doi.org/10.1007/s10344-018-1237-3
Fležar U, Pičulin A, Bartol M et al (2021) Eurasian lynx in the Dinaric Mountains and the south- eastern Alps, and the need for population reinforcement. Cat News Special Issue 14:21–24
Fležar U, Hočevar L, Sindičić M et al (2023) Surveillance of the reinforcement process of the Dinaric - SE Alpine lynx population in the lynx monitoring year 2021–2022. LIFE Lynx technical report. Ljubljana
Gardner B, Royle JA, Wegan MT et al (2010) Estimating black bear density using DNA data from hair snares. J Wildl Manage 74:318–325. https://doi.org/10.2193/2009-101
Gimenez O, Gatti S, Duchamp C et al (2019) Spatial density estimates of Eurasian lynx (Lynx lynx) in the French Jura and Vosges Mountains. Ecol Evol 9:11707–11715. https://doi.org/10.1002/ece3.5668
Goldberg JF, Tempa T, Norbu N et al (2015) Examining temporal sample scale and model choice with spatial capture-recapture models in the common leopard Panthera pardus. PLoS ONE 10:1–19. https://doi.org/10.1371/journal.pone.0140757
Goldsmith FB (1991) Monitoring for Conservation and Ecology. Springer Science & Business Media, Dordrecht
Gosling LM, Roberts SC (2001) Scent-marking by male mammals: Cheat-proof signals to competitors and mates. Adv Study Behav 30:169–217
Harihar A, Lahkar D, Singh A et al (2020) Estimating leopard (Panthera pardus) densities when populations comprise polymorphic phenotypes: An application of spatial mark-resight models. bioRxiv. https://doi.org/10.1101/2020.06.08.129197
Harmsen BJ, Foster RJ, Sanchez E et al (2017) Long term monitoring of jaguars in the Cockscomb Basin Wildlife Sanctuary, Belize. Implications for camera trap studies of carnivores. PLoS One 12:e0179505. https://doi.org/10.1371/journal.pone.0179505
Harmsen BJ, Foster RJ, Quigley H (2020) Spatially explicit capture recapture density estimates: Robustness, accuracy and precision in a long-term study of jaguars (Panthera onca). PLoS One 15:e0227468. https://doi.org/10.1371/journal.pone.0227468
Hendry H, Mann C (2017) Camelot - Intuitive Software for Camera Trap Data Management. bioRxiv 203216. https://doi.org/10.1101/203216
Herfindal I, Linnell JDC, Odden J et al (2005) Prey density, environmental productivity and home-range size in the Eurasian lynx (Lynx lynx). J Zool 265:63–71. https://doi.org/10.1017/S0952836904006053
Hofmeester TR, Thorsen HN, Cromsigt JPGM et al (2021) Effects of camera-trap placement and number on detection of members of a mammalian assemblage. Ecosphere 12:e03662 https://doi.org/10.1002/ecs2.3662
Huber Đ, Kusak J, Sindičić M, et al (2013) Izvješće o stanju populacije risa u Hrvatskoj za razdoblje 2011. i 2012. godine. Izvješće o stanju Popul risa u Hrvat za Razdob 2011 i 2012 God 74. https://doi.org/10.13140/2.1.4075.9363
Iosif R, Popescu VD, Ungureanu L et al (2022) Eurasian lynx density and habitat use in one of Europe’s strongholds, the Romanian Carpathians. J Mammal 103:415–424. https://doi.org/10.1093/jmammal/gyab157
Jȩdrzejewski W, Schmidt K, Okarma H, Kowalczyk R (2002) Movement pattern and home range use by the Eurasian lynx in Białowieża Primeval Forest (Poland). Ann Zool Fennici 39:29–41
Kaczensky P, Linnell JDC, Huber Đ et al (2021) Dryad Data - Distribution of large carnivores in Europe 2012 - 2016: Distribution maps for Brown bear, Eurasian lynx, Grey wolf, and Wolverine. In: Dryad, Dataset. https://datadryad.org/stash/dataset/doi:10.5061/dryad.pc866t1p3. Accessed 21 Jan 2022
Karnaukhov AS, Korablev MP, Kuksin AN et al (2020) Snow leopard population monitoring guidebook. Krasnoyarsk
Koren I, Jonozovič M, Kos I (2006) Status and distribution of the Eurasian lynx (Lynx lynx L.) in Slovenia in 2000–2004 and comparison with the years 1995–1999. Acta Biol Slov 49:27–41
Kos I, Koren I, Potočnik H, Krofel M (2012) Stanje in razširjenost evrazijskega risa (Lynx lynx) v Sloveniji v obdobju 2005–2009. Acta Biol Slov 55:49–63
Krofel M, Hočevar L, Allen ML (2017) Does human infrastructure shape scent marking in a solitary felid? Mamm Biol 87:36–39. https://doi.org/10.1016/j.mambio.2017.05.003
Krofel M, Fležar U, Hočevar L, et al (2021) Surveillance of the reinforcement process of the Dinaric - SE Alpine lynx population in the lynx-monitoring year 2019–2020. LIFE Lynx technical report. Ljubljana
Melzheimer J, Heinrich SK, Wasiolka B et al (2020) Communication hubs of an asocial cat are the source of a human–carnivore conflict and key to its solution. Proc Natl Acad Sci USA 117:33325–33333. https://doi.org/10.1073/PNAS.2002487117
Moqanaki EM, Milleret C, Tourani M et al (2021) Consequences of ignoring variable and spatially autocorrelated detection probability in spatial capture-recapture. Landsc Ecol 36:2879–2895. https://doi.org/10.1007/s10980-021-01283-x
Obbard ME, Howe EJ, Kyle CJ (2010) Empirical comparison of density estimators for large carnivores. J Appl Ecol 47:76–84. https://doi.org/10.1111/j.1365-2664.2009.01758.x
Oberosler V, Tenan S, Groff C, Krofel M (2021) First spatially - explicit density estimate for a snow leopard population in the Altai Mountains. Biodivers Conserv 1–15. https://doi.org/10.1007/s10531-021-02333-1
Palmero S, Belotti E, Bufka L et al (2021) Demography of a Eurasian lynx (Lynx lynx) population within a strictly protected area in Central Europe. Sci Rep 11:1–12. https://doi.org/10.1038/s41598-021-99337-2
Pesenti E, Zimmermann F (2013) Density estimations of the Eurasian lynx (Lynx lynx) in the Swiss Alps. J Mammal 94:73–81. https://doi.org/10.1644/11-MAMM-A-322.1
R Core Team (2021) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Rovero F, Zimmermann F (2016) Camera trapping for wildlife research. Pelagic Publishing, UK, Exter
Royle JA, Magoun AJ, Gardner B et al (2011) Density estimation in a wolverine population using spatial capture-recapture models. J Wildl Manage 75:604–611. https://doi.org/10.1002/jwmg.79
Royle JA, Chandler RB, Sollmann R, Gardner B (2014) Spatial Capture-Recapture: First Edition. Elsevier, Inc.
Sindičić M, Sinanović N, Majić Skrbinšek A et al (2010) Legal status and management of the Dinaric lynx population. Veterinaria 58:229–238
Sindičić M, Polanc P, Gomerčić T et al (2013) Genetic data confirm critical status of the reintroduced Dinaric population of Eurasian lynx. Conserv Genet 14:1009–1018. https://doi.org/10.1007/s10592-013-0491-x
Skrbinšek T, Krofel M (2008) Analiza kvalitete habitata, hrana in kompeticija. Dinaris final report. Ljubljana
Skrbinšek T, Konec K, Bragalanti N, et al (2018) 2018 Annual Population Status Report for Brown Bears in Northern Dinaric Mountains and Central-Eastern Alps. LIFE DinAlp Bear technical report. Ljubljana.
Slijepčević V, Fležar U, Konec M, et al (2019) Baseline demographic status of SE Alpine and Dinaric lynx population. LIFE Lynx technical report. Zagreb
Sollmann R, Furtado MM, Gardner B et al (2011) Improving density estimates for elusive carnivores: accounting for sex-specific detection and movements using spatial capture-recapture models for jaguars in central Brazil. Biol Conserv 144:1017–1024. https://doi.org/10.1016/j.biocon.2010.12.011
Sollmann R, Gardner B, Belant JL (2012) How does spatial study design influence density estimates from spatial capture-recapture models? PLoS ONE 7:e34575. https://doi.org/10.1371/journal.pone.0034575
Stergar M, Slijepčević V (2017) Lynx camera trapping guidelines. LIFE Lynx technical report. Ljubljana
Suryawanshi KR, Khanyari M, Sharma K (2019) Sampling bias in snow leopard population estimation studies. Popul Ecol 61:268–276. https://doi.org/10.1002/1438-390X.1027
Sutherland C, Royle JA, Linden DW (2019) oSCR: A spatial capture–recapture R package for inference about spatial ecological processes. Ecography (Cop) 42:1459–1469. https://doi.org/10.1111/ecog.04551
Topličanec I, Gomerčić T, Spajić T, Sindičić M (2022) Big spots in a small population: Analyzing characteristics and temporality of coat patterns in Croatian lynx population. Zool Anz 297:79–84. https://doi.org/10.1016/j.jcz.2022.02.003
Tourani M (2022) A review of spatial capture–recapture: Ecological insights, limitations, and prospects. Ecol Evol 12:1–13. https://doi.org/10.1002/ece3.8468
Vogt K, Zimmermann F, Kölliker M, Breitenmoser U (2014) Scent-marking behaviour and social dynamics in a wild population of Eurasian lynx Lynx lynx. Behav Processes 106:98–106. https://doi.org/10.1016/j.beproc.2014.04.017
von Arx M, Kaczensky P, Linnell J, Lanz T et al (2021) Conservation status of the Eurasian lynx in West and Central Europe. Cat News Special Issue 14:5–8
Acknowledgements
We are grateful to all the local hunters, professional hunting wardens, protected-area rangers and other volunteers from Slovenia and Croatia whose voluntary fieldwork generated majority of the camera-trapping data used for this study. Tadeja Virant and Tadej Murn annotated the camera trapping records in Camelot while Gregor Simčič and Roman Luštrik helped with preparing the data for the SCR analyses. Teresa Oliveira, Ruben Portas Perez, Laurie Bedouet and an anonymous reviewer provided many helpful comments on the earlier versions of the manuscript.
Funding
The study was co-funded by the LIFE program (project LIFE Lynx LIFE16 NAT/SI/000634), the Slovenian Ministry of Natural Resources and Spatial Planning, the Interreg Central Europe program (3Lynx project), Slovenian Research Agency (grants N1–0163 and P4–0059) and Pahernik foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: U.F.,M.A.,M.K.; Methodology: U.F.,M.A.; Formal analysis: U.F.,M.A.; Writing—original draft preparation: U.F.,M.K.; Writing—review and editing: U.F.,M.A.,M.K.,R.Č.,T.G.,M.S.,M.S.,I.T.,M.B.; Funding acquisition: R.Č.,M.K.,M.S.,M.S.,T.G.,V.S.,U.F.; Resources: A.P.,L.H.,M.S.,M.B.,A.R.,I.T.,V.S.,U.F.,M.K.; Supervision: M.K., M.A.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by David Hawksworth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fležar, U., Aronsson, M., Černe, R. et al. Using heterogeneous camera-trapping sites to obtain the first density estimates for the transboundary Eurasian lynx (Lynx lynx) population in the Dinaric Mountains. Biodivers Conserv 32, 3199–3216 (2023). https://doi.org/10.1007/s10531-023-02646-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-023-02646-3