Abstract
Invasion of nonindigenous species is considered one of the most urgent problems affecting native ecosystems and agricultural systems. Mechanistic models that account for short-term population dynamics can improve prediction because they incorporate differing demographic processes that link the environmental conditions of a spatial location explicitly with the invasion process. Yet short-term population dynamics are rarely accounted for in spatial models of invasive species spread.
Accounting for transient (short-term) population dynamics that arise from the interaction of age structure and vital rates, we predict the stochastic population growth rate and establishment probability of wild pigs following introduction into any location in North America. Established ecological theory suggests that the rate of spatial spread is proportional to population growth rate. Using observed geographic distribution data for wild pigs we calculated geographic spread rates (watersheds/year) from 1982 to 2021. We investigated if observed spread rates increased in watersheds with higher stochastic population growth rates. Stochastic population growth rate and establishment probability of wild pigs increased with increasing initial population (propagule) size and length of establishment time. Areas along the Mississippi, Ohio, and lower portions of the Missouri river drainages had the highest probability of wild pig establishment with many regions having probabilities close to 1. Spread rates demonstrated strong spatial heterogeneity with the greatest rates of spread (5.8 watersheds/year) occurring from 2008 to 2013 prior to the establishment of a National wild pig control program in 2013. Spread rates declined 82% (0.57 watersheds/year) in the period from 2013 to 2021 compared to the period from 1982 to 2013. We found significant positive associations among stochastic population growth rate and observed geographic rates of spread. Stochastic population growth rate explained a large amount of variation (79.3–92.1%) in annual rate of watershed spread of wild pigs. Our predicted probabilities of establishment and population growth can be used to inform surveillance and control efforts to reduce the potential for establishment and spread of wild pigs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasion of nonindigenous species is considered one of the most urgent problems affecting native ecosystems with 87% of imperiled species threatened by invasive species (Mack et al. 2000; McClure et al. 2018). Additionally, nonindigenous species threaten agricultural systems requiring considerable policy activity to mitigate invasive species concerns (Miller et al. 2018; Miller 2020). Existing theory indicates that the distribution and spread of invasive species is the result of complex ecological processes that include the frequency and size of introduction (propagule pressure), species-specific traits that provide a fitness advantage (high reproductive capacity and dispersal), and biotic and abiotic characteristics of the recipient ecosystem that limit or facilitate invasion (Simberloff 2009; Lustig et al. 2017). However risk assessments of establishment and spread typically lack explicit consideration of these interacting factors (Catford et al. 2011; Gallien et al. 2015) and broad scale predictions of invasive species distributions are typically based on static approaches linking species occurrence to biotic and abiotic factors (Guisan and Zimmermann 2000; Lustig et al. 2017). Mechanistic models that account for short-term population dynamics (i.e. transient dynamics) can improve prediction because they incorporate differing demographic processes that link the environmental conditions of a spatial location explicitly with the invasion process (Buhnerkempe et al. 2011; Miller 2017). Yet short-term population dynamics are rarely accounted for in spatial models of invasive species spread (Lustig et al. 2017).
When populations are not at equilibrium (e.g., the age structure is not the stable age structure and population growth deviates from the equilibrium population growth rate, λ), they are expected to exhibit transient dynamics (Caswell and Werner 1978; Hodgson and Townley 2004; Tremblay et al. 2015). Transient dynamics can cause populations either to grow or shrink at a much faster rate than would be expected under equilibrium conditions (Stott et al. 2011; McDonald et al. 2017). When a small group of conspecific individuals is introduced to a new location (a “propagule”), it is not likely to be a stable population, and transient dynamics can lead to either rapid growth and establishment of a population or local extinction (Iles et al. 2016). Further these transient dynamics can differ from dynamics simulated using approaches that assume asymptotic population growth (Bieber and Ruf 2005; Tabak et al. 2018).
Invasive wild pigs (Sus scrofa), also known as feral hogs, feral swine, or wild boar, are recognized as one of the most widespread and destructive invasive species in the world (Barrios-Garcia and Ballari 2012). Wild pigs are native to Eurasia and Northern Africa, but have been widely introduced for centuries, often deliberately, and now occupy every continent except Antarctica (Mayer and Brisbin 1991; Lewis et al. 2017). Once introduced spread rates have been estimated to range from 2.75 to 12.6 km per year (Singer 1981; Snow et al. 2017). In North America it is an extremely destructive invasive species that is of concern for human and animal health (Miller et al. 2017), causes significant economic damage (Engeman et al. 2004; Anderson et al. 2016), negatively impacts imperiled species (McClure et al. 2018), and is commonly introduced into new locations (Tabak et al. 2017; Hernandez et al. 2018). These concerns have generated significant Federal policy to mitigate these impacts with the US Department of Agriculture establishing the Animal Plant Health Inspection Service (APHIS) National Feral Swine Damage Management Program in 2013 aimed at reducing the spread of wild pigs in the United States (USDA, 2015; Miller et al., 2018).
Nevertheless, the distribution of wild pigs in Canada and the United States has increased dramatically in recent years (Michel et al. 2017; Snow et al. 2017), and research suggests that a major mechanism of their spread is translocation of individuals to augment populations for recreational hunting (Tabak et al. 2017). Wild pigs evolved as pulsed resource consumers of mast crops; they have larger litter sizes and reproduce more often under favorable forage conditions, and have reduced fecundity under poor forage conditions (Ostfeld and Keesing 2000). This variability in fecundity makes wild pigs especially susceptible to transient population dynamics (Bieber and Ruf 2005; Miller 2017; Tabak et al. 2018). Despite their ancestral dependence on mast crops, wild pigs have evolved to be dietary generalists, as agricultural crops can replace mast in their diets (Schley and Roper 2003; Rosell et al. 2012) and they currently thrive in ecosystems that lack mast producing species (Caley 1997; Choquenot and Ruscoe 2003). Nevertheless, their reproductive biology retains this variability of fecundity, which can cause populations to rapidly grow and establish following introduction in new environments, leading to the further expansion of this species. Despite significant efforts to forecast the spread of wild pigs (Snow et al. 2017), estimate the probability of occurrence (McClure et al. 2015), and predict the potential density of wild pig populations (Lewis et al. 2017) there are no available spatial predictions of population growth and establishment risk if wild pigs were released into a given spatial location.
Our objective was to predict the stochastic population growth rate and establishment probability of wild pigs (accounting for transient dynamics) following introduction into any location in North America. We then compared predicted stochastic population growth rate with observed geographic rates of spread to determine if increased population growth was associated with increased spread (e.g. invasion). According to foundational work of Fisher (1937), Skellam (1951) and later van den Bosch et al. (1992) the rate of spatial spread is expected to be proportional to the square root of the intrinsic population growth rate. However more recent empirical studies have compared rates of spatial spread in more than one type of habitat finding that environmentally dependent spread rates are common (Williamson and Harrison 2002; With 2002). Additionally, the spatial arrangement of habitat has been empirically demonstrated to drive spread rates in some systems (Bailey et al. 2000). To investigate the relationship between stochastic population growth rate and observed geographic rates of spread we used geospatial data on the distribution of mast producing tree species (a primary forage resource of wild pigs) and agricultural crops that can replace mast in their diets (Choquenot 1998; Choquenot and Ruscoe 2003) to predict population dynamics using transient population simulations. We simulated populations under different propagule sizes and for different amounts of time following introduction. By varying the initial population size and simulation time, we were able to identify areas in North America with high probability for establishment if wild pigs are introduced. Our findings can be used to inform proactive surveillance and removal efforts to reduce the potential establishment and spread of wild pigs.
Methods
Predicting stochastic population growth and establishment
We used transient population dynamics models to simulate population trajectories for invasive wild pig populations under different types of environments that they could potentially experience in North America. Using the approach of Tabak et al. (2018) the demographic distribution in each subsequent year (\({n}_{t+1}\)) was estimated as,
where \({A}_{t}\) is the population projection matrix for year \(t\), that is specific to the environmental conditions (i.e. forage) experienced in that year. The projection matrix is standardized using \(\hat{A}_{t} = A_{t} /\lambda _{{\max }}\) where \({\lambda }_{max}\) is the dominant eigenvector of \({A}_{t}\). The standardized demographic distribution, \({\hat n}_{t}\), is the proportion of individuals in each age class at the current time step. Thus \(\left\| {\hat{A}_{t} \hat{n}_{t} } \right\|_{1}\)is the 1-norm of the product of \(\hat{A}_{t} \hat{n}_{t}\) and is equal to the reactivity of the population (Neubert and Caswell 1997; Townley et al. 2007; Stott et al. 2011).
Following Tabak et al. (2018) for each simulated environment initial population sizes of 5, 10, and 20 were used as the number of females introduced in the first year of simulations. These initial population sizes were selected for two reasons. First these sizes represent a range of initial population sizes that could be introduced accidentally (i.e. escape from a farm) or intentionally to establish a population (i.e. transported via a truck or livestock trailer). Second, Tabak et al. (2018) found that wild pig populations could be established with as few as five individuals after accounting for transient dynamics. The individuals in each initial population were randomly assigned to age classes (juvenile, sub-adult, and adult) using Latin hypercube sampling (Xu et al. 2005) and in subsequent generations a sex ratio of 1:1 was assumed in litters.
We estimated stochastic population growth rate (\({\lambda }_{s}\)) and probability of establishment for wild pig populations in these environments and in environments with agricultural subsidy using the methods and the R scripts of Tabak et al. (2018). Using the methods of Tabak et al. (2018), Bierzychudek (1982), and Zúñiga-Vega et al. (2007) \({\lambda }_{s}\) was calculated as \({\lambda }_{s}={e}^{r}\). Where \(r\) is the intrinsic rate of population growth calculated as the slope of the regression between the logarithm of simulated population size and time. A simulated population was considered established if \({\lambda }_{s}\) > 1 and the population size at the end of simulation was ≥ 60. Tabak et al. (2018) found that \({\lambda }_{s}\) stabilized at 60 individuals across a range of environments. Simulations were run for 1, 5, or 10 years representing a range of times post-introduction that a population might be identified and management to control the population might start.
Survival and fecundity rates for different mast qualities (poor, intermediate, and good) provided in Tabak et al. (2018) and Bieber and Ruf (2005) were used (see Supplemental Table S1). These vital rates are currently the only available estimates that demonstrate variation by age and forage condition for wild pigs. They have been used to estimate the influence of forage quality on asymptotic population growth (Bieber and Ruf 2005) as well as the influence of forage quality on transient population growth and population establishment (Tabak et al. 2018).
Environmental conditions and making geographic predictions of stochastic population growth and establishment
Following Tabak et al. (2018) we simulate three broad types of environmental conditions to which wild pigs might be introduced in North America: (1) an environment with one mast tree species (poor forage quality), (2) an environment with a mast community (intermediate quality), and (3) an environment with agricultural subsidy (good quality). We conducted simulations in these three environments under nine different introduction scenarios using initial population sizes of 5, 10, or 20 as the number of females introduced at the beginning of the simulations. Data are scarcely available for the quality of mast from trees over time. However available empirical data and theory indicates that increasing the number of masting species in a community will increase both the frequency of years with mast as well as the intensity (i.e. number of trees producing mast) of the mast in good years (Koenig et al. 1994; Touzot et al. 2020) (see supplemental Figure S4). In the literature we found historic data from one common mast species, Fagus sylvatica, over 114 years (Hilton and Packham 2003) and from a community of five mast tree species, Quercus spp., over 12 years (Koenig et al. 1994). Unfortunately, data are unavailable for mast records from areas with 2–4 mast species and from areas with > 5 mast species. Therefore, we assumed that five mast species represented the maximum population growth potential resulting from their consumption of mast crops. For areas with 2–4 mast species, we estimated \({\lambda }_{s}\) and establishment probability using loess regression, where the predictor variable was the number of mast species (1 or 5) and the response variable was \({\lambda }_{s}\) or establishment probability. To calculate the \({\lambda }_{s}\) in areas with both mast and agriculture, we estimated \({\lambda }_{s}\) as the mean between the growth rate estimated as a result of mast and the growth rate estimated as a result of agricultural subsidy. To calculate establishment probability in such situations, we took the sum of probability of establishment that was estimated as a result of mast species and that resulting from agricultural subsidy.
There are clearly limitations in our estimation of growth rate and of establishment probability as we extrapolated to areas with numbers of mast trees for which we do not know the frequency of mast and we are unaware of how wild pig growth potential changes in the presence of both agriculture and mast trees. We also only considered the effects of forage on population growth, but other factors might affect growth rate (e.g., climate could have strong effects on survival) or dispersal from adjacent populations might serve as demographic rescue. However, we are interested in the general trends of population growth and establishment across the continent more than precisely estimating these rates.
To apply these growth rates and establishment probabilities to geographic locations, we used a geospatial layer depicting the number of mast species in each 1 km2 and the presence of crops that can replace mast in wild pigs’ diets using the USDA’s CropScape data (NASS, 2019). A detailed description of how the mast species data was generated is provided in the supplemental information. CropScape data are only available for USA, and we wanted to extend our model results to all of North America, so we also used the consensus land-cover layer for North America (Tuanmu and Jetz 2014) and compared this with the results in USA that were produced using the detailed CropScape data (Fig S3). Since the maps created using these two datasets were similar for the USA, we assume that it is reasonable to use the consensus land-cover and apply our results to all of North America. We have provided these mast layers as well as all other data layers used in the supplemental.
Determining rates of spread
We estimated the geographic rate of spread of wild pigs in the contiguous United States using data from the National Feral Swine Mapping System (Corn and Jordan 2017). These data describing the known distribution of wild pigs were compiled at irregular intervals from 1982 to 2008 and annually since 2008. They are the best available data describing the known distribution of wild pigs over the past 36 years and have been used to forecast the spread of wild pigs (Snow et al. 2017), estimate the probability of occurrence (McClure et al. 2015), determine wild pig agricultural damage risk (Miller et al. 2017), predict federal policy to control wild pigs (Miller et al. 2018), and determine the risk wild pigs pose to imperiled species (McClure et al. 2018). Polygons representing the known geographic extent of established wild pig populations (defined as populations present for two or more years with evidence of reproduction) are reported to the National Feral Swine Mapping System nationally by wildlife professionals in state wildlife resource agencies and the United States Department of Agriculture. The resulting distribution data is curated for accuracy with national data available annually.
Polygons representing the observed distribution for 1982, 1988, 2004, 2008, 2013, 2017, and 2021 were aggregated to watershed boundary data as described in McClure et al. (2015) to discretize consistent, comparable, and ecologically relevant sampling units. We selected the sub-watershed unit (hydrologic unit code 12; HUC12) as our primary sampling unit for several reasons. First theory indicates that rates of spread are primarily driven by population growth rate and dispersal (van den Bosch et al. 1992). Our interest here is to investigate associations among population growth rate and spread rate. To account for potential dispersal, HUC12 watersheds were selected because they encompass potential dispersal processes. Natural wild pig dispersal distance in North America has been reported to range from 0.5 to 2.4 km (Casas-Díaz et al. 2013) and empirical data suggests low dispersal rates (Stubbe et al. 1989; Truvé and Lemel 2003; Truvé 2004). The median distance from the centroid to the furthest edge of HUC12 watersheds with wild pigs occurring at any time point is 9.6 km and shortest distance was 3.2 km. Because HUC12 watersheds are larger than the expected dispersal distance we assume that they account for potential dispersal occurring. Second watersheds are frequently used as an ecologically relevant sampling unit for large scale studies (Odum and Barrett 1971; Montgomery et al. 1995; McClure et al. 2018) have been used to model the occurrence of wild pigs and other species (Collins and Glenn 1990; Peterson et al. 2003; McClure et al. 2015, 2018), and are expected to represent a discrete set of biotic and abiotic factors (McClure et al. 2015).
We chose to use watershed rates of spread rather than a linear measure of spread distance for several reasons. First, there is uncertainty in the polygons describing the known range of wild pigs in each year and converting these data to watershed level presence / absence smooths over potential uncertainties but also obscures the linear spread distance. Second, watershed rate of spread allows for the measure of range contraction (negative rates) while a linear measure of spread does not provide a useful measure of contraction. Lastly, wild pigs are frequently moved long distances and released by humans (Tabak et al. 2017). This means that an initial introduction into a new region will have a very large linear measure while subsequent spread will have shorter linear distances and is expected to be driven by local environmental conditions that drive population growth rates.
The annual watershed rate of spread among HUC12 watersheds was then calculated for three coarser watershed scales – sub-region (HUC4), basin (HUC6), sub-basin (HUC8). This was done because the spatial scale used for analysis can influence inference (Farnsworth et al. 2006). We calculated the annual rate (watershed/year) of spread (\(\theta\)) for each watershed\(j\) in each period \(t\) as,
where \({\eta }_{i,t}\) is the count of HUC12 watersheds occupied by wild pigs in the coarser watershed \(j\) in year \(t\), and \(T\) is the number of years between \(t\) and \(t+1\). We also calculated the mean annual rate of spread from 1982 to 2013, the final year in this range was the beginning of a National Feral Swine Damage Management Program to control wild pigs, and from 2013 to 2017 which represents the period after the National Program began.
Associations among stochastic population growth rate and annual rate of spread
To determine if the predicted stochastic population growth rate (\({\lambda }_{s}\)) was associated with the annual rate of spread (\(\theta\)) in watersheds we regressed the annual rate of spread, \(\theta\), on the mean watershed stochastic population growth rate, \({\lambda }_{s}\). To investigated potential non-linear relationship we included a quadratic term (\({\lambda }_{s}^{2}\)) for watershed stochastic population growth rate. Estimates of \({\lambda }_{s}\) included three different initial populations sizes over three establishment time periods resulting in nine estimates of \({\lambda }_{s}\) per watershed. To account for these differences in initial population size and establishment period lengths we included establishment time (1, 5, or 10 years) and the propagule size (5, 10, or 20 individuals) as fix effect terms in the regression models. We used ordinary least-squares assuming a Gaussian error structure. We used adjusted R2 as a measure of goodness of fit of each model (Kutner et al. 2005). We used the percent of model deviance explained by each predictor as a measure of strength of association.
Results
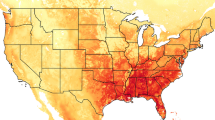
Our spatial estimates of \({\lambda }_{s}\) that used the detailed cropscape data for the US were similar to estimates using the cultivated layer that is available for all of North America (Fig. 1). Since these estimates were similar and using the cultivated data allows our results to have broader interpretation, we focus on the continental scale. With a small initial population size (propagule size = 5), mean \({\lambda }_{s}\) was < 1, regardless of the number of years that populations persisted following introduction (Fig. 1 and Table S7). As we increased propagule size and the number of years of simulation, \({\lambda }_{s}\) increased so that with a propagule size of 20 and ten years of simulation, \({\lambda }_{s}\) was > 1 for the majority of North America (mean = 1.16). We found the highest growth rates in the upper Midwestern United States, regions along the Mississippi river, and the southern prairie region of Canada.
The mean establishment probability was very low (< 0.03) when the initial population size was 5 female wild pigs (Fig. 2 and Table S8). When propagule size was increased to 20 and simulations ran for 5 to 10 years mean establishment probability increased to 0.497. Establishment probabilities were high in the same locations we found the highest growth rates (Figs. 1 and 2). Establishment probabilities approached 1 for many locations in the upper Midwestern United States and regions along the Mississippi river.
Annual spread rate
The annual spread rate (\(\theta\)) was proportionally consistent across the three watershed scales, however it demonstrated strong spatial heterogeneity (Fig. 3, see supplemental Figure S1 and Tables S2-S4 for all watershed scales). Mean annual rates of spread changed among time periods (Fig. 4) with the greatest rates of spread (watershed/year) occurring from 2008 to 2013 across all watershed scales (\({\theta }_{HUC4}\)=5.80, \({\theta }_{HUC6}\)=3.67, \({\theta }_{HUC8}\)=0.85; see supplemental for all spread rates including linear rates of spread). Annual rates of spread demonstrated spatial heterogeneity and were not constantly greatest along the northern extent of wild pig distribution. During the earliest periods from 1982 to 2004 spread rates were positive across the majority of the wild pig distribution. From 2004 to 2013 watersheds along the Red River, Rio Grande River, and Ohio River consistently had the highest spread rates. Annual spread rates declined 82% after the establishment of the National Feral Swine control program in 2013 with 65.2–69.0% of watersheds having spread rates ≤ 0 (Table S2). The highest rates of spread after 2013 were confined to watersheds in the southeastern portion of the United States.
Annual watershed level spread rate (watersheds/year) for wild pigs from 1982 to 2021 for HUC6 level watershed (see supplemental for other watershed scales). Red indicates positive rates of spread and blue indicates negative rates of spread (i.e. contractions in the number of occupied watersheds). Light gray indicates rates of spread that were zero and dark gray indicates areas with no wild pigs present
Mean annual rates (watersheds/year) with confidence intervals for spread (Panel a), gain (Panel b) and loss (Panel c) rates for wild pigs from 1982 to 2021. Spread rates (Panel a) were positive from 1982 until 2013 after which they slowed and demonstrated higher loss rates. A national wild pig control program was initiated in 2013 with the objective of reducing the range of wild pigs in the United States
Association of spread rate and stochastic population growth rate
We found significant relationships between the annual rate of spread and stochastic population growth rates. Stochastic population growth rate explained a large amount of variation (79.3–92.1%) in annual rate of watershed spread of wild pigs across all time periods (Table 1). The linear term for the mean stochastic population growth rate explained the largest amount of deviation (Table 1 and S6) while the non-linear term for mean stochastic population growth rate was a significant term in most models but explained on average 4.01% (0.05 − 9.19%) of total deviance (Table 1). Propagule size and length of time for establishment were not significant in any models (Figure S3) and accounted for little to no deviance (Table S6). For all periods prior to the National Feral Swine control program, except for 1982 to 1988, population growth rates were a significant positive predictor of the annual rate of spread for all watershed scales (Fig. 5). Models including all periods explained a large amount of variation in rate of spread with adjusted R2 values ranging from 0.784 to 0.917 (Table 1 and Table S7). There was a difference among models that included all periods prior to (1982–2013) and after (2013–2021) the establishment of the National Feral Swine control program. Models including all periods from 1982 to 2013 had adjusted R2 values ranging from 0.887 to 0.913 while models including all periods from 2013 to 2021 had adjusted R2 values ranging from 0.46 to 3.26. For the period after the establishment of the Feral Swine control program (2013 to 2021), population growth rates were either not a significant predictor or had a negative association with annual watershed rate of spread.
Regression coefficients with 95% confidence intervals for linear models regressing wild pig spread rate on stochastic population growth rate. Spread rate was increasingly positively associated with stochastic population growth rate in all time periods except the period from 2013 to 2021. A national wild pig control program was initiated beginning in 2013 with the goal of reducing the range of wild pigs in the United States
Discussion
Explicitly accounting for initial population size and age structure allowed us to account for transient dynamics and demographic stochasticity that influence predictions of population growth and probability of establishment. Our geospatial projections of stochastic population growth rate and establishment probability (Figs. 1 and 2) highlight areas at increased risk of successful establishment of wild pig populations if introduced. The strong correlation between our estimates of stochastic population growth (\({\lambda }_{s}\)) and the annual watershed rate of spread (\(\theta\)) supports the validity of our population growth estimates. In other systems, transient population growth rate has been identified as important in predicting establishment success and long term viability of invading species (Iles et al. 2016). The relationship we found between population growth and watershed rate of spread corresponds to existing theory suggesting the rate of spatial spread is expected to be proportional to the intrinsic population growth rate (van den Bosch et al. 1992). However positive associations between population growth rate and spatial spread varied across time and watershed indicating that spread rates are potentially influenced by environmental or other more local factors such as the spatial arrangement of habitat (Bailey et al. 2000; Williamson and Harrison 2002; With 2002). Beyond aligning with established ecological theory our results can be used to target areas for surveillance that have higher potential for wild pig establishment and rapid population growth allowing for early identification of wild pigs and facilitating efforts to minimize population expansion once new wild pig populations are identified.
Applying mechanistic models to geospatial projections
While mechanistic models are often used for distribution and niche modeling for invasive species (Kearney and Porter 2009; Peterson et al. 2015), population dynamic processes across spatial scales are more commonly evaluated using statistical models to correlate population processes with spatial covariates (but see Chandler et al., 2018; Quintana-Ascencio et al., 2018). Correlative spatial models are convenient, especially in systems with less biological data, because they do not require an understanding of the mechanistic links between an organism and its environment. However, mechanistic processes tend to limit the expansion of species distributions, so it can be useful to build models that evaluate mechanistic processes across large spatial extents when the goal is to predict the growth and establishment potential of a species. Furthermore, for populations that experience transient dynamics, mechanistic models may be more applicable than correlative statistical models. When a population is introduced into a new environment, it will usually exhibit transient dynamics (Iles et al. 2016) and wild pigs are especially prone to transient dynamics due to their evolution as pulsed resource consumers (Bieber and Ruf 2005; Tabak et al. 2018).
Comparison to other studies
Our estimates of stochastic population growth and establishment probability correspond well to the reported establishment of wild pigs throughout the United States and the prairie provinces of central Canada (Lewis et al. 2017, 2019; Michel et al. 2017). However, our estimates of establishment probability differ in several ways from the predicted spread of wild pigs in the United States (Snow et al. 2017). We found high establishment probability throughout the upper Mississippi and Ohio River drainages while previous studies predicted lower probability of spread in these areas. The historical spread of wild pigs in the United States demonstrated by Snow et al. (2017) implicitly includes factors such as human translocation of wild pigs which previous studies have found to be important in the spread of wild pigs (Tabak et al. 2017). The differences between Snow et al. (2017) and our results may stem from differences in the frequency of introduction, that is likely driven by anthropogenic factors. This indicates that while the probability of establishment is generally high in some regions of the United States, the historical frequency of introduction has likely been low. The distribution of our predicted probability of establishment and stochastic population growth rates generally correspond well with the predicted equilibrium population density of wild pigs by Lewis et al. (2017).
Additionally Tabak et al. (2018), which is the basis for the approach used in this study, found that fewer individuals are required to establish a population of wild pigs when compared with other studies (Howells and Edwards-Jones 1997; Leaper et al. 1999). These previous studies use more traditional population modeling approaches that have an underlaying assumption of asymptotic growth and population at the stable-stage distribution. While these studies accounted for stochasticity either in demographics or environment, they do not explicitly account for the interaction of age structure and vital rates that can result in transient effects that either amplify or attenuate population growth (Caswell and Werner 1978; Townley et al. 2007). Explicitly modeling transient dynamics provides important insights into the effects of altering age structure and the corresponding changes in population size (Stott et al. 2011). Studies have demonstrated that greater than 50% of population dynamics can result from transient effects (Ellis and Crone 2013; McDonald et al. 2016) and empirical studies have documented the interaction of demographics and initial population size on growth rates (Komers and Curman 2000). Further predictions of population trajectories that account for transient dynamics can be very different than methods using asymptotic growth approaches for wild pigs (Bieber and Ruf 2005; Tabak et al. 2018). Interestingly a retrospective analysis of exotic ungulate introductions found that introductions with as few as six individuals were successful which is similar to the propagule size of ten that Tabak et al. (2018) found to be a threshold for wild pigs and is similar to our results (Forsyth and Duncan 2001).
The differences among the predictions of wild pig spread (Snow et al. 2017), population density (Lewis et al. 2017), and probability of establishment (McClure et al. 2015) are not mutually exclusive. The differences among these results is largely due to each study focusing on a different component of the invasion process - introduction, establishment, population growth, and spread (Reise et al. 2006). These differences highlight the need to develop integrated mechanistic approaches that explicitly include factors that drive introduction pressure (e.g. Snow et al. (2017) and Tabak et al. (2017)) probability of establishment and population growth (e.g. our study), and factors regulating long-term population growth and equilibrium population size (e.g. Lewis et al., 2017). Accounting for the invasion process in a spatially explicit context would allow for greatly improved predictions of establishment risk and consequences once established.
Management implications
Establishment probability was very low when initial population size was small and when populations had fewer years to become established (Fig. 3), because populations in these simulations were unlikely to meet one of the criteria for establishment: a population size of 60 individuals in the final simulation year. This indicates that there may be the opportunity to prevent population establishment if actions are undertaken quickly upon discovery of new introductions. With larger initial populations and more time following introduction, the potential for population establishment increased dramatically, indicating that it is best to prevent introductions of large numbers of individuals and to begin interventions early. Reducing the frequency and size (number of animals) of releases of wild pigs can reduce establishment probability, but these goals may be difficult to implement, as movement and release of wild pigs is common and has been documented on multiple continents (Hampton et al. 2004; Tabak et al. 2017; Hernandez et al. 2018). This is often complicated by limitations of current tools available for detecting newly released pigs. Tools such as pig detection dogs (Keiter et al. 2016) and passive monitoring using camera traps (Tabak et al. 2019) are difficult to deploy over large geographic extents for long periods of time. Our results can be used to refine where monitoring using these tools is conducted, improving the chances of early detection of pigs. When considering management to prevent population establishment, it is important to note that our methods only account for a single introduction event so likely represent a conservative estimate of establishment probability. Wild pigs are often repeatedly introduced to the same location (Mayer and Brisbin 1991), which can increase establishment probability through demographic rescue of the founder population (Hufbauer et al. 2015).
Extensions
Our method of estimating stochastic population growth and establishment success has some important limitations that offer opportunities to extend our approach. Complete data on the frequency and intensity of mast and how these change with the size and community structure of mast species is limited. To address this data limitation, we extrapolated using available data, however there is uncertainty in our approach for making this extrapolation. The collection of data describing mast frequency and intensity over large geographies in North America would have significant value for population dynamic modeling of many species that consume seasonal mast and fruit. Additionally, we used data linking how mast frequency and intensity affect vital rates of wild pigs from Europe (Briedermann 1967; Bieber and Ruf 2005); data describing this relationship in other continents are needed to better extrapolate globally. In our predictions the primary driver of wild pig vital rates was assumed to be access to mast. However, previous studies have indicated that precipitation and temperature can influence survival of younger age classes impacting transient population dynamics of wild pigs (Miller 2017). Currently most studies reporting survival, litter size, and frequency of litters and how these factors correlate with environmental conditions are from wild pig populations in Europe. Studies collecting these vital rates for wild pig populations in North American would be of great value. We also do not account for inter and intra species interactions that may also be limiting for founder populations. For example Lewis et al. (2017) found that predator species richness was negatively associated with wild pig population density. Accounting for these interactions within a dynamical modeling framework that accounts for transient dynamics would be an exciting extension of our work. Lastly, our matrix model approach does not account for non-linearity in population growth (e.g., density dependence) that we assume maybe influencing the vital rates from Bieber and Ruf (2005), although, Lewis et al. (2019) found that many wild pig populations in the United States may be at or near equilibrium. Despite these limitations, predicted population growth and the spread rate of wild pigs in areas where they have been introduced were highly correlated. The positive correlation indicates that despite the data uncertainties, our projections are reasonable estimates of population growth and establishment potential for wild pigs.
Conclusion
Risk assessments of establishment and spread of invasive species can be improved by using mechanistic models that consider the frequency and size of introduction along with the biotic and abiotic characteristics of the recipient ecosystem, while accounting for short-term population dynamics can improve prediction. Accounting for short-term population dynamics in spatial models of invasive species spread will become increasingly important as variation in climatic conditions increases. For many invasive species, including wild pigs, increasing temperature is expected to increase invasion success and expand regions with the potential for invasion (Vetter et al. 2015). Spatial models that account for short-term population dynamics can allow for uncertainties resulting from increased climate variation to be explicitly considered, improving predictions. An important extension of our work is to integrate, using mechanistic approaches that account for short-term population dynamics, ecological and anthropocentric processes that determine introduction, establishment, and spread of invasive species.
Data Availability
The datasets generated during and/or analyzed as part of this study are provided in the electronic supplementary material.
References
Anderson A, Slootmaker C, Harper E, Holderieath J, Shwiff SA (2016) Economic estimates of feral swine damage and control in 11 US states. Crop Prot 89:89–94. https://doi.org/10.1016/j.cropro.2016.06.023
Bailey DJ, Otten W, Gilligan CA (2000) Saprotrophic invasion by the soil-borne fungal plant pathogen Rhizoctonia solani and percolation thresholds. New Phytol 146(3):535–544
Barrios-Garcia MN, Ballari SA (2012) Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol Invasions 14(11):2283–2300
Bieber C, Ruf T (2005) Population dynamics in wild boar Sus scrofa: ecology, elasticity of growth rate and implications for the management of pulsed resource consumers. J Appl Ecol 42(6):1203–1213
Bierzychudek P (1982) The demography of jack-in‐the‐pulpit, a forest perennial that changes sex. Ecol Monogr 52(4):335–351
Briedermann L (1967) Studies on the diet of wild boar in lowland of the German Democratic Republic. German Academy of Agricultural Sciences, Berlin
Buhnerkempe MG, Burch N, Hamilton S, Byrne KM, Childers E, Holfelder KA, McManus LN, Pyne MI, Schroeder G, Doherty PF Jr (2011) The utility of transient sensitivity for wildlife management and conservation: bison as a case study. Biol Conserv 144(6):1808–1815
Caley P (1997) Movements, activity patterns and habitat use of feral pigs (Sus scrofa) in a tropical habitat. Wildl Res 24(1):77–87
Casas-Díaz E, Closa-Sebastià F, Peris A, Torrentó J, Casanovas R, Marco I, Lavín S, Fernández-Llario P, Serrano E (2013) Dispersal record of wild boar (Sus scrofa) in northeast Spain: implications for implementing disease-monitoring programs. Wildl Biology Pract 9(3):19–26
Caswell H, Werner PA (1978) Transient behavior and life history analysis of teasel (Dipsacus sylvestris Huds). Ecology 59(1):53–66
Catford JA, Downes BJ, Gippel CJ, Vesk PA (2011) Flow regulation reduces native plant cover and facilitates exotic invasion in riparian wetlands. J Appl Ecol 48(2):432–442
Chandler RB, Hepinstall-Cymerman J, Merker S, Abernathy-Conners H, Cooper RJ (2018) Characterizing spatio-temporal variation in survival and recruitment with integrated population models. The Auk: Ornithological Advances 135(3):409–426
Choquenot D (1998) Testing the relative influence of instrinsic and extrinsic variation in food availability on feral pig populations in Australia’s rangelands. J Anim Ecol 67(6):887–907
Choquenot D, Ruscoe WA (2003) Landscape complementation and food limitation of large herbivores: habitat-related constraints on the foraging efficiency of wild pigs. J Anim Ecol 72(1):14–26
Collins SL, Glenn SM (1990) A hierarchical analysis of species’ abundance patterns in grassland vegetation. Am Nat 135(5):633–648
Corn JL, Jordan TR (2017) Development of the national feral swine map, 1982–2016. Wildl Soc Bull 41(4):758–763
Ellis MM, Crone EE (2013) The role of transient dynamics in stochastic population growth for nine perennial plants. Ecology 94(8):1681–1686
Engeman RM, Smith HT, Severson R, Severson MA, Woolard J, Shwiff SA, Constantin B, Griffin D (2004) Damage reduction estimates and benefit-cost ratios for feral swine control from the last remnant of a basin marsh system in Florida. Environ Conserv 31(3):207–211
Fisher RA (1937) The wave of advance of advantageous genes. Annals of eugenics 7(4):355–369
Forsyth DM, Duncan RP (2001) Propagule size and the relative success of exotic ungulate and bird introductions to New Zealand. Am Nat 157(6):583–595
Gallien L, Mazel F, Lavergne S, Renaud J, Douzet R, Thuiller W (2015) Contrasting the effects of environment, dispersal and biotic interactions to explain the distribution of invasive plants in alpine communities. Biol Invasions 17(5):1407–1423
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Model 135(2–3):147–186
Hampton JO, Spencer PB, Alpers DL, Twigg LE, Woolnough AP, Doust J, Higgs T, Pluske J (2004) Molecular techniques, wildlife management and the importance of genetic population structure and dispersal: a case study with feral pigs. J Appl Ecol 41(4):735–743
Hernandez FA, Parker BM, Pylant CL, Smyser TJ, Piaggio AJ, Lance SL, Milleson MP, Austin JD, Wisely SM (2018) Invasion ecology of wild pigs (Sus scrofa) in Florida, USA: the role of humans in the expansion and colonization of an invasive wild ungulate. Biol Invasions 20:1865–1880. https://doi.org/10.1007/s10530-018-1667-6
Hilton G, Packham J (2003) Variation in the masting of common beech (Fagus sylvatica L.) in northern Europe over two centuries (1800–2001). Forestry 76(3):319–328
Hodgson DJ, Townley S (2004) Methodological insight: linking management changes to population dynamic responses: the transfer function of a projection matrix perturbation. J Appl Ecol 41(6):1155–1161
Howells O, Edwards-Jones G (1997) A feasibility study of reintroducing wild boar Sus scrofa to Scotland: are existing woodlands large enough to support minimum viable populations. Biol Conserv 81(1–2):77–89
Hufbauer RA, Szűcs M, Kasyon E, Youngberg C, Koontz MJ, Richards C, Tuff T, Melbourne BA (2015) Three types of rescue can avert extinction in a changing environment. Proc Natl Acad Sci 112(33):10557–10562
Iles DT, Salguero-Gómez R, Adler PB, Koons DN (2016) Linking transient dynamics and life history to biological invasion success. J Ecol 104(2):399–408
Kearney M, Porter W (2009) Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett 12(4):334–350
Keiter DA, Cunningham FL, Rhodes Jr OE, Irwin BJ, Beasley JC (2016) Optimization of scat detection methods for a social ungulate, the wild pig, and experimental evaluation of factors affecting detection of scat. PLoS ONE 11(5):e0155615
Koenig WD, Mumme RL, Carmen WJ, Stanback MT (1994) Acorn production by oaks in central coastal California: variation within and among years. Ecology 75(1):99–109
Komers PE, Curman GP (2000) The effect of demographic characteristics on the success of ungulate re-introductions. Biol Conserv 93(2):187–193
Kutner MH, Nachtsheim CJ, Neter J, Li W (2005) Applied linear statistical models. Irwin, McGraw-Hill
Leaper R, Massei G, Gorman M, Aspinall R (1999) The feasibility of reintroducing wild boar (Sus scrofa) to Scotland. Mammal Rev 29(4):239–258
Lewis JS, Corn JL, Mayer JJ, Jordan TR, Farnsworth ML, Burdett CL, VerCauteren KC, Sweeney SJ, Miller RS (2019) Historical, current, and potential population size estimates of invasive wild pigs (Sus scrofa) in the United States. Biol Invasions 21(7):2373–2384
Lewis JS, Farnsworth ML, Burdett CL, Theobald DM, Gray M, Miller RS (2017) Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci Rep 7:44152. https://doi.org/10.1038/srep44152
Lustig A, Worner SP, Pitt JP, Doscher C, Stouffer DB, Senay SD (2017) A modeling framework for the establishment and spread of invasive species in heterogeneous environments. Ecol Evol 7(20):8338–8348
Mack RN, Simberloff D, Mark Lonsdale W, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10(3):689–710
Mayer J, Brisbin I (1991) Wild pigs in the United States: their life history, morphology and current status. University of Georgia Press, Athens, Georgia, USA
McClure ML, Burdett CL, Farnsworth ML, Lutman MW, Theobald DM, Riggs PD, Grear DA, Miller RS (2015) Modeling and mapping the probability of occurrence of invasive wild pigs across the contiguous United States. PLoS ONE 10(8):e0133771. https://doi.org/10.1371/journal.pone.0133771
McClure ML, Burdett CL, Farnsworth ML, Sweeney SJ, Miller RS (2018) A globally-distributed alien invasive species poses risks to United States imperiled species. Sci Rep 8(1):5331. https://doi.org/10.1038/s41598-018-23657-z
McDonald JL, Franco M, Townley S, Ezard TH, Jelbert K, Hodgson DJ (2017) Divergent demographic strategies of plants in variable environments. Nat Ecol Evol 1(2):0029
McDonald JL, Stott I, Townley S, Hodgson DJ (2016) Transients drive the demographic dynamics of plant populations in variable environments. J Ecol 104(2):306–314
Michel NL, Laforge MP, Van Beest FM, Brook RK (2017) Spatiotemporal trends in canadian domestic wild boar production and habitat predict wild pig distribution. Landsc Urban Plann 165:30–38
Miller RS (2017) Interaction among societal and biological drivers of policy at the wildlife-agricultural interface. Colorado State University
Miller RS (2020) Wildlife in the United States. In: SM Opp and RJ Duffy (eds) Environmental Issues Today: Choices and Challenges [2 volumes]. ABC-CLIO, p 171
Miller RS, Opp SM, Webb CT (2018) Determinants of invasive species policy: print media and agriculture determine US invasive wild pig policy. Ecosphere 9(8):e02379. https://doi.org/10.1002/ecs2.2379
Miller RS, Sweeney SJ, Slootmaker C, Grear DA, Di Salvo PA, Kiser D, Shwiff SA (2017) Cross-species transmission potential between wild pigs, livestock, poultry, wildlife, and humans: implications for disease risk management in North America. Sci Rep 7(1):7821. https://doi.org/10.1038/s41598-017-07336-z
Montgomery DR, Grant GE, Sullivan K (1995) Watershed analysis as a framework for implementing ecosystem management 1. JAWRA J Am Water Resour Association 31(3):369–386
NASS (2019) CropScape-cropland data layer. N. A. S. S. United States Department of Agriculture. United States Department of Agriculture, National Agricultural Statistics Service, Washington DC
Neubert MG, Caswell H (1997) Alternatives to resilience for measuring the responses of ecological systems to perturbations. Ecology 78(3):653–665
Odum EP, Barrett GW (1971) Fundamentals of ecology. Saunders Philadelphia
Ostfeld RS, Keesing F (2000) Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol Evol 15(6):232–237
Peterson AT, Papes M, Kluza DA (2003) Predicting the potential invasive distributions of four alien plant species in North America. Weed Sci 51(6):863–868
Peterson AT, Papeş M, Soberón J (2015) Mechanistic and correlative models of ecological niches. Eur J Ecol 1(2):28–38
Quintana-Ascencio PF, Koontz SM, Smith SA, Sclater VL, David AS, Menges ES (2018) Predicting landscape‐level distribution and abundance: integrating demography, fire, elevation and landscape habitat configuration. J Ecol 106(6):2395–2408
Reise K, Olenin S, Thieltges DW (2006) Are aliens threatening european aquatic coastal ecosystems? Helgol Mar Res 60(2):77
Rosell C, Navàs F, Romero S (2012) Reproduction of wild boar in a cropland and coastal wetland area: implications for management. Anim Biodivers Conserv 35(2):209–217
Schley L, Roper TJ (2003) Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops. Mammal Rev 33(1):43–56
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Singer FJ (1981) Wild pig populations in the national parks. Environ Manage 5(3):263–270
Skellam JG (1951) Random dispersal in theoretical populations. Biometrika 38(1/2):196–218
Snow NP, Jarzyna MA, VerCauteren KC (2017) Interpreting and predicting the spread of invasive wild pigs. J Appl Ecol 54(6):2022–2032
Stott I, Townley S, Hodgson DJ (2011) A framework for studying transient dynamics of population projection matrix models. Ecol Lett 14(9):959–970
Stubbe C, Mehlitz S, Peukert R, Goretzki J, Stubbe W, Meynhardt H (1989) Lebensraumnutzung und Populationsumsatz des Schwarzwildes in der DDR-Ergebnisse der Wildmarkierung. Beiträge zur Jagd-und Wildforschung 16:212–231
Tabak MA, Norouzzadeh MS, Wolfson DW, Sweeney SJ, VerCauteren KC, Snow NP, Halseth JM, Di Salvo PA, Lewis JS, White MD (2019) Machine learning to classify animal species in camera trap images: applications in ecology. Methods Ecol Evol 10(4):585–590. https://doi.org/10.1111/2041-210X.13120
Tabak MA, Piaggio AJ, Miller RS, Sweitzer RA, Ernest HB (2017) Anthropogenic factors predict movement of an invasive species. Ecosphere 8(6):e01844. https://doi.org/10.1002/ecs2.1844
Tabak MA, Webb CT, Miller RS (2018) Propagule size and structure, life history, and environmental conditions affect establishment success of an invasive species. Sci Rep 8(1):10313
Touzot L, Schermer É, Venner S, Delzon S, Rousset C, Baubet E, Gaillard JM, Gamelon M (2020) How does increasing mast seeding frequency affect population dynamics of seed consumers? Wild boar as a case study. Ecol Appl 30(6):e02134
Townley S, Carslake D, KELLIE-SMITH O, McCarthy D, Hodgson D (2007) Predicting transient amplification in perturbed ecological systems. J Appl Ecol 44(6):1243–1251
Tremblay RL, Raventos J, Ackerman JD (2015) When stable-stage equilibrium is unlikely: integrating transient population dynamics improves asymptotic methods. Ann Botany 116(3):381–390
Truvé J (2004) Pigs in space. Movement, dispersal and geographic expansion of wild boar (Sus scrofa) in Sweden. Goteborg University
Truvé J, Lemel J (2003) Timing and distance of natal dispersal for wild boar Sus scrofa in Sweden. Wildl Biology 9:51–57
Tuanmu MN, Jetz W (2014) A global 1-km consensus land‐cover product for biodiversity and ecosystem modelling. Glob Ecol Biogeogr 23(9):1031–1045
USDA (2015) Feral swine damage management: a National Approach. United States Department of Agriculture, Animal and Plant Health Inspection Service, Washington DC
van den Bosch F, Hengeveld R, Metz JAJ (1992) Analysing the velocity of animal range expansion. J Biogeog 5:135–150
Vetter SG, Ruf T, Bieber C, Arnold W (2015) What is a mild winter? Regional differences in within-species responses to climate change. PLoS ONE 10(7):e0132178
Williamson J, Harrison S (2002) Biotic and abiotic limits to the spread of exotic revegetation species. Ecol Appl 12(1):40–51
With KA (2002) The landscape ecology of invasive spread. Conserv Biol 16(5):1192–1203
Xu C, He HS, Hu Y, Chang Y, Li X, Bu R (2005) Latin hypercube sampling and geostatistical modeling of spatial uncertainty in a spatially explicit forest landscape model simulation. Ecol Model 185(2–4):255–269
Zúñiga-Vega JJ, Valverde T, Isaac Rojas-González R (2007) Lemos-Espinal JA (2007) Analysis of the population dynamics of an endangered lizard (Xenosaurus grandis) through the use of projection matrices. Copeia 2:324–335
Acknowledgements
We thank Colleen Webb for influencing our ideas related to population dynamics. We also thank research librarian Mary Foley for help with finding supporting literature.
Funding
This work was supported in part by the United States Department of Agriculture, Animal Plant Health Inspection Service National Feral Swine Damage Management Program.
Author information
Authors and Affiliations
Contributions
R.M., M.T., and D.W. contributed to the study conception and design. C.B. develop methods and data for mast tree species. M.T. and R.M. conducted transient population dynamics analysis. R.M. conducted statistical analysis of spread rates. D.W. aided in material preparation and data collection. The first draft of the manuscript was written by R.M. with contributions from M.T. and D.W. C.B, wrote supplemental information describing the mast tree species methods. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors they have no financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miller, R.S., Tabak, M.A., Wolfson, D.W. et al. Transient population dynamics drive the spread of invasive wild pigs and reveal impacts of management in North America. Biol Invasions 25, 2461–2476 (2023). https://doi.org/10.1007/s10530-023-03047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03047-x