Abstract
In this study we examined abiotic and biotic factors that could potentially influence the presence of a non-indigenous seaweed, Eucheuma denticulatum, in two locations, one outside (Kane’ohe Bay, Hawai’i, USA) and one within (Mafia Island, Tanzania) its natural geographical range. We hypothesized that the availability of hard substrate and the amount of wave exposure would explain distribution patterns, and that higher abundance of herbivorous fishes in Tanzania would exert stronger top–down control than in Hawai’i. To address these hypotheses, we surveyed E. denticulatum in sites subjected to different environmental conditions and used generalized linear mixed models (GLMM) to identify predictors of E. denticulatum presence. We also estimated grazing intensity on E. denticulatum by surveying the type and the amount of grazing scars. Finally, we used molecular tools to distinguish between indigenous and non-indigenous strains of E. denticulatum on Mafia Island. In Kane’ohe Bay, the likelihood of finding E. denticulatum increased with wave exposure, whereas on Mafia Island, the likelihood increased with cover of coral rubble, and decreased with distance from areas of introduction (AOI), but this decrease was less pronounced in the presence of coral rubble. Grazing intensity was higher in Kane’ohe Bay than on Mafia Island. However, we still suggest that efforts to reduce non-indigenous E. denticulatum should include protection of important herbivores in both sites because of the high levels of grazing close to AOI. Moreover, we recommend that areas with hard substrate and high structural complexity should be avoided when farming non-indigenous strains of E. denticulatum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species are considered to be a major threat to global marine biodiversity and ecosystem services (Schaffelke and Hewitt 2007; Katsanevakis et al. 2014). Seaweeds (i.e., macroalgae) comprise a large part of non-indigenous species on a global scale (Bax et al. 2003; Schaffelke and Hewitt 2007). Non-indigenous seaweeds can become invasive and influence ecosystem characteristics and functions by altering habitat complexity (Veiga et al. 2014), community composition (Davidson et al. 2015), biodiversity (Casas et al. 2004; Schaffelke and Hewitt 2007) and ecosystem productivity (Sagerman et al. 2014). For example, invasive seaweeds have been reported to induce and/or amplify coral-to-algal phase shifts in tropical reef systems (Schaffelke et al. 2006; Williams and Smith 2007). Once a non-indigenous species has become established in a new area, it can be extremely difficult to eradicate (Critchley et al. 1986; Nyberg and Wallentinus 2005; Bax et al. 2008). Therefore, to better understand potential risks and environmental consequences of introductions of non-indigenous seaweeds, there is not only a need to document occurrence and spread, but also to identify environmental factors predicting their presence and abundance, especially if they become invasive.
The risk that non-indigenous seaweeds becomes invasive depends on a combination of species-specific traits, as well as biotic and abiotic conditions in the new environment (Nyberg and Wallentinus 2005). Environmental conditions known to influence invasions include wave exposure (Levin et al. 2002; D’Amours and Scheibling 2007), habitat complexity (Tamburello et al. 2013), diversity of primary producers (Kimbro et al. 2013), turf and crustose coralline algae cover (Britton-Simmons 2006; Vermeij et al. 2011), seaweed cover (Arenas et al. 2006), herbivory (Vermeij et al. 2009) and substrate availability (e.g., dead or living coral). Substrate availability has a prominent role in massive phase-shifts from coral to macroalgal dominance, a pattern observed in the aftermath of large-scale coral die-offs when substantial areas of hard substrate is made available to settling of algal propagules (McCook et al. 2001). Furthermore, less functionally diverse or reduced algal communities in the recipient system might facilitate establishment of a non-indigenous seaweed through decreased competition (Ceccherelli et al. 2002). Success by an invader might also be attributed to enemy release, i.e. the lack of consumers, or reduced biotic resistance within the recipient ecosystem (Parker et al. 2006; Kimbro et al. 2013).
One example of a deliberately introduced and (in certain locations) invasive species is the red seaweed Eucheuma denticulatum (Solieriaceae, Gigartinales, Rhodophyta). This tropical macroalgae grows naturally on hard substrates in Southeast Asia and East Africa and has been introduced to multiple countries for aquaculture purposes, with different environmental consequences (Conklin and Smith 2005; Chandrasekaran et al. 2008; Davidson et al. 2015; Castelar et al. 2015). In the early 1970s, E. denticulatum was introduced to Kane’ohe Bay, Hawai’i (USA) for growth studies (Glenn and Doty 1990; Rodgers and Cox 1999; Smith et al. 2002). Although it was believed that E. denticulatum would not be able to disperse over deeper waters between reefs (Russell 1983), surveys conducted > 25 years after the introduction have estimated a rate of spread of 250 m year−1 (Glenn and Doty 1990; Conklin and Smith 2005). Since then, this seaweed has been patchily distributed throughout the bay and has colonized a number of reefs, potentially overgrowing and shading reef-building corals (Conklin and Smith 2005). Important herbivores such as rabbitfishes (Siganidae) are absent on Hawai’i, which in combination with low preference for E. denticulatum by indigenous herbivores (Stimson et al. 2001; Stamoulis et al. 2017), could explain the high seaweed cover in locations within the bay (Conklin and Smith 2005; Fox et al. 2009; Hehre and Meeuwig 2015). Nevertheless, it remains unclear how this seaweed is influenced by biotic (e.g., herbivore abundance) and abiotic factors (e.g., wave exposure and substrate availability) in this geographic location. Herbivorous fishes and sea urchins can reduce biomass of E. denticulatum (Russell 1983; Neilson et al. 2018), but abundances and hence consumption rates might vary between locations (Stamoulis et al. 2017). Herbivory can be further influenced by seaweed cover (Stamoulis et al. 2017) and wave exposure, which can exclude herbivores with weaker swimming abilities (Bejarano et al. 2017). Furthermore, water motion (e.g., wave exposure or wind driven swell) might reduce biomass of E. denticulatum by the breaking of branches (but potentially facilitating spread), but also increase growth rates by higher rates of water exchange (Russell 1983; Glenn and Doty 1990; Rodgers and Cox 1999). Finally, distance to the source population of the original area of introduction (AOI) could potentially determine distribution patterns of E. denticulatum in early stages of an invasion.
Strains of Southeast Asian E. denticulatum were introduced for seaweed farming on the island of Zanzibar (Tanzania) in in the late 1980s (Lirasan and Twide 1993), although indigenous strains of E. denticulatum were already present along the East African coastline (Mshigeni 1984). Southeast Asian strains were selected for farming due to their faster growth rates (Lirasan and Twide 1993; Tano et al. 2015). With the epicenter on Zanzibar, farming practices using Southeast Asian seeding material have led to a spread of the non-indigenous strains over the East African region (Bryceson 2002; Rönnbäck et al. 2002). Currently, both indigenous and introduced strains are present in wild E. denticulatum populations around the island of Zanzibar, and the introduced strains dominate in some locations (Halling et al. 2013; Tano et al. 2015). However, the spread of introduced strains has not yet been confirmed in other areas in Tanzania, and no data exist on how biotic/abiotic factors may influence the presence of E. denticulatum in the East African seascape. In fact, there are only a few studies (Vermeij et al. 2009) comparing environmental factors that influence the distribution of non-indigenous seaweeds within and outside their natural biogeographical range.
Against this background, the objective of the present study was to identify biotic and abiotic factors influencing the distribution of E. denticulatum introduced in two contrasting geographical locations: Kane’ohe Bay (Hawai’i, USA) and Mafia Island (Tanzania). We hypothesized that (1) certain environmental variables would be more important in one geographical location than the other depending on site characteristics (e.g., habitat availability such as coral rubble) and history of introduction and (2) E. denticulatum is subjected to stronger top-down control by herbivorous communities in East Africa than in Hawai’i due to enemy release (i.e. the absence of certain consumers and low preference for E. denticulatum by native herbivores). Furthermore, we investigated if non-indigenous strains of E. denticulatum have spread from AOIs on Mafia Island.
Materials and methods
Description of study sites
Kane’ohe Bay, Hawai’i, USA
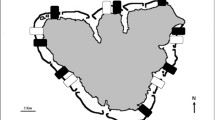
Kane’ohe Bay (21°28′N; 157°48′W) is a semi-enclosed, 46-km2 bay located on the east coast of the island of Oahu, Hawai’i (Bahr et al. 2015a, b; Stimson et al. 2001; Fig. 1a). Fringing reefs border the coastline and a 5-km barrier reef/sand bar protects the bay against the open ocean in the eastward direction (Stimson et al. 2001). Approximately 70 patch reefs composed of coral rubble and live coral (mainly Montipora capitata and Porites compressa) are scattered throughout the bay, of which most rise to less than 1 m below the surface (Bahr et al. 2015a; Stimson et al. 2001). Between the patch reefs, water depth ranges between 10 and 15 m and substrate consists of rubble, coral, mud, and sand (Bahr et al. 2015a). Corals and seaweeds are restricted to the shallower areas, most likely due to the high turbidity within the bay (Stimson et al. 2001). Tides are semi-diurnal with a mean amplitude of 0.7 m (Ringuet and Mackenzie 2005).
During the twentieth century, Kane’ohe Bay was subjected to several major disturbance events such as dredging/removal of reefs, freshwater inflows, coral bleaching, substantial sewage discharges, and introduction of non-indigenous seaweed species (Jokiel et al. 1993; Smith et al. 2002; Bahr et al. 2015a, b). Currently, four introduced seaweeds are abundant throughout the bay: Acanthophora spicifera, E. denticulatum, Gracilaria salicornia and Kappaphycus alvarezii (Stamoulis et al. 2017). Populations of E. denticulatum in Kane’ohe Bay consist of haplotype E32, which is of Southeast Asian origin (Zuccarello et al. 2006; Conklin et al. 2009). No commercial farming of E. denticulatum has been conducted on Oahu, and growth trials were abandoned in 1977 (Glenn and Doty 1990; Conklin and Smith 2005). Since then, no new introductions have been made and all E. denticulatum in Kane’ohe Bay therefore originate from the experiments conducted in the 1970s. Substantial efforts have been made to decrease the abundance of E. denticulatum and K. alvarezii in Kane’ohe Bay, including manual removal and the use of a biocontrol agent, the sea urchin Tripneustes gratilla (Neilson et al. 2018).
Mafia Island, Tanzania
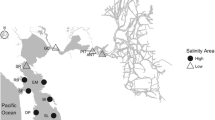
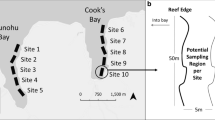
Mafia Island (7°40′S; 39°41′E) is the main island in a small archipelago situated 20 km from the Tanzanian mainland, south of the island of Zanzibar (Fig. 1b; McClanahan et al. 2008). The southeastern part of the island is included in a large (822 km2) marine protected area, the Mafia Island Marine Park (MIMP). MIMP was established in 1995 (Garpe and Öhman 2003; Gaspare et al. 2015) and prohibits the use of destructive fishing methods, but artisanal fisheries by local communities are allowed in certain zones in the outer part of the protected area (McClanahan et al. 2008).
Mafia Island is subjected to the East African Coastal Current (EACC) and semidiurnal tides with a mean amplitude of 3.3 m (Garpe and Öhman 2003). The eastern side, where this study was conducted, is protected by fringing reefs, but tides and monsoonal patterns create strong and complex currents that can reach up to 6 knots (Garpe and Öhman 2003; Berkström et al. 2013; Gaspare et al. 2015). The study area consists of a diverse and pristine patchwork of seagrass meadows, patch reefs, sandy areas, and seaweed beds, and is characterized by high biodiversity of scleractinian corals and fish (Horrill et al. 1996; Garpe and Öhman 2003; Berkström et al. 2013). Small-scale farming of Southeast Asian strains of E. denticulatum in the archipelago was initiated in the beginning of the twenty-first century (Torre-Castro et al. 2012; Msuya et al. 2014) and now occurs on Mafia Island, Chole Island, Juani Island and Jibondo Island (I. Bryceson, pers. comm.).
Field survey
The field survey was conducted in 2016 during July (Kane’ohe Bay, Hawai’i) and September to November (Mafia Island, Tanzania). The survey in Kane’ohe Bay coincided with the warmer season (May–September) with mean sea surface temperature ~ 27° C (Jokiel 1991) and the survey on Mafia Island with the cooler season (June–October), mean sea surface temperatures of ~ 26° C (McClanahan et al. 2007). While the fieldwork occurred during different seasons, the sea surface temperature was similar in the two locations. The field surveys consisted of belt transects (25 m × 2 m) in which we estimated benthic habitat characteristics, algal abundance, herbivore abundance and grazing on E. denticulatum fronds. A total of 100 transects were conducted: 52 in Kane’ohe Bay and 48 on Mafia Island (Table 1).
Habitat and Eucheuma denticulatum surveys
Within the belt transects habitat characteristics and seaweed cover were estimated by a snorkeler (M. Eggertsen). All transects were conducted in depths 0.5–3.5 m, as E. denticulatum is rarely found deeper (Russell 1983). Transects were placed at least 10 m apart, and locations were selected to encompass variation in environmental conditions, including benthic substrate composition, rugosity, wave exposure, depth and distance to areas of introductions (AOI) or seaweed farms. The location of the AOI of E. denticulatum in Kane’ohe Bay (i.e., the area where growth experiments were conducted) was derived from literature (Russell 1983). Reefs in Kane’ohe Bay that had been subjected to sea urchin transplantations or manual removal of E. denticulatum were also identified from the literature (Neilson et al. 2014, 2018). Two of these reefs (# 14 and 15) were surveyed in the present study. On Mafia Island, AOIs were defined as locations where seaweed farming was active, or where farming had ceased during the previous year, but farms and pieces of loose seaweeds still remained (visual observations by M. Eggertsen and D.H. Chacin). Information of locations where farming activities had previously been conducted was not available.
Benthic variables were visually estimated in 2 × 2 m sections along the transect line. Substrate composition (percent cover), E. denticulatum (percent cover), and the type of substrate where E. denticulatum was attached was identified. Bottom rugosity was visually estimated on a 1–5 scale following Gratwicke and Speight (2005) where 1 is completely flat and 5 denoting very high structural complexity. The percent cover of other fleshy macroalgae (as potential competitors), crustose coralline algae (CCA), algal turf and live corals was also documented. At both ends of each transect, GPS coordinates were recorded allowing for geographical positioning (± 5 m). All distances were measured in ArcMap 10.5 and measured as linear distance. Depth was measured at both ends of the transect with a dive computer (Suunto Vyper), and a mean value was calculated.
Grazing estimations
Grazing intensity on E. denticulatum found in transects was estimated using a 7-grade percent scale (1, 5, 10, 25, 50, 75, 100). Grazing was defined as 100% when all tips of branches within a patch of E. denticulatum had been removed, 50% when half of all tips had been removed and so on. It was also noted whether the grazing scars were caused by fish, urchins, or smaller invertebrates. Fish inflict straight bite marks, urchins cause irregular bites on the thallus with jagged edges, and invertebrates leave small cavities on the thallus (Hay 1981).
Estimations of relative wave exposure
Wave exposure is a major structuring force in marine communities (Harrold et al. 1988; Friedlander et al. 2003; Chollett and Mumby 2012). Here, relative wind fetch was calculated and used as a proxy for wave exposure (Burrows 2012). First, a shapefile was created in ArcGIS (ArcGIS 10.5), in which the locations of transects were projected and all land areas defined with polygons. Shallow reef areas exposed during low tide were also identified as land (objects reducing waves). Wave fetch was then calculated in R version 3.3.1 using the “fetchR” package (Seers 2017). To be able to detect both large- and fine-scale variation in relative wave/wind exposure, the fetch was set to a maximum of 10 km. The number of wind directions per measuring point was set to 36. Finally, a weighted mean fetch (depending on the frequency of different wind directions) was calculated in Excel, using wind data from Iowa Environmental Mesonet (Iowa State University; https://mesonet.agron.iastate.edu/request/download.phtml) for each respective month (July for Kane’ohe Bay and September–November for Mafia Island) and location (station Kaneohe_MCAS/OAHU and Dar es Salaam AR, respectively). A mean value for the start and end point of each transect was used in the calculations.
Abundance and biomass of herbivores
Herbivores were surveyed by counting fish and sea urchins along each transect. Herbivorous fish were identified to the lowest taxonomical level (usually species or genus), and body size was estimated to the closest 1 cm, following Tano et al. (2017). To minimize potential disturbance to fishes, surveys were conducted 5 min after the transect line was placed. A snorkeler swam twice along the transect at ~ 0.1 m s−1, first documenting all easily visible fish species, and second, all cryptic species. To facilitate species identification, each snorkeler was equipped with a camera (Canon Powershot G7x Mark II and Canon WP-DC54 underwater housing) used to record unfamiliar species. To avoid potential bias regarding length estimations, size trial estimations were done prior to the study so that all snorkelers were calibrated with each other and any possible biases were consistent. All transect surveys were performed during high tides between 09:30–16:00. Literature was used to define fishes as herbivores (e.g., Froese and Pauly 2017), and in cases where it was not possible to identify a fish to species level, the trophic group for the family in question was used (e.g., juvenile scarine labrids). Although not all herbivorous fishes remove fleshy seaweeds intentionally (e.g., fishes targeting epiphytes can also remove parts of seaweeds), the overall effect of herbivory suppresses algal biomass and promotes coral cover (Bellwood et al. 2004; Mumby 2006, 2016), and thus analyses were performed on the total herbivorous fish assemblage. Herbivorous fish biomass was calculated using species-specific length–weight relationships for total length (TL) of each individual. If the species could not be determined or if species-specific values were lacking, relationships for the same subfamily were used instead. To describe the herbivorous fish assemblage, fishes were classified into juveniles, subadults and adults based on length estimations using the 1/3 and 2/3 cutoff method (Nagelkerken and Van der Velde 2002; Tano et al. 2017). If information on length at maturity (Lm) was available for a particular species, these values were used instead of the 1/3 method (see Tano et al. 2017). Lm values were extracted from FishBase (www.fishbase.org) and literature (DeMartini et al. 2005; Mangi and Roberts 2006; Taylor and Choat 2014).
Genetic sampling and analysis
Molecular analysis was used to determine which E. denticulatum strains that were dominant in Kane’ohe Bay and to be able to identify introduced (Southeast Asian origin) versus indigenous individuals (East African origin) on Mafia Island. An algal patch of E. denticulatum was considered as one individual and one frond was collected from each such algal patch along transects (Ntotal = 167). This sampling method was used because E. denticulatum (especially when grazed intensely) is rarely identifiable as individuals, but rather grows as dense patches which can cover a large area. The fronds were then dried and stored individually in sampling containers with silica gel. In the lab, total genomic DNA was isolated using a modified CTAB extraction, based on the protocol by Zuccarello and Lokhorst (2005). In short, a small piece of tissue was soaked in 500 μl CTAB buffer (Karolinska University Laboratory) for 2 h and homogenized using glass, metal, and ceramic beads in FastPrep MP24 (Nordic Biolabs) at a speed of 6.0, time for 40 s, which was repeated 5 times. The homogenized tissue was incubated overnight at 56 °C in 5 μl RNAse A (1000 mg ml−1, Thermo Scientific) and 10 μl proteinase K (20 mg ml−1, Thermo Scientific), and then extracted with chloroform:isoamyl alcohol (24:1, 500 μl) and centrifuged for 10 min (14,000 rpm). DNA suspended in the aqueous phase was carefully separated from the interphase (300–450 μl) and re-extracted in chloroform:isoamyl alcohol (24:1). DNA was precipitated using ice-cold isopropanol (100%) and incubated in –20° C for 30 min and centrifuged for 20 min in 14,000 rpm to retain a DNA pellet. The pellet was washed with 70% ethanol with subsequent centrifugation of (10 min, 14,000 rpm), air-dried and dissolved in 100 μl 0.1 × TE buffer. DNA yield and quality were estimated on agarose gel (stained with SYBRSafe; 5 μl 100 ml−1, for 30 min, Life technologies) and Nanodrop 2000 spectrophotometer. DNA was then stored in −80 °C freezer.
For identification of different haplotypes, the mitochondrial cox2-3 spacer was used, as described by Zuccarello et al. (1999). PCR purification and Sanger sequencing (forward and reverse) were carried out by Macrogen Europe Inc., using an ABI3730XL sequencer. Quality evaluation and alignment of sequences was conducted using MEGA 6.0. Haplotypes were aligned manually and identified using reference sequences (Zuccarello et al. 2006; Halling et al. 2013; Tano et al. 2015). A haplotype was considered new if there were ≥ 1 single nucleotide polymorphism (SNP) difference between the haplotype in question and reference haplotypes. All new haplotypes were carefully checked using chromatograms, reassuring that differences in SNPs were not due to insufficient quality of sequences. Ambiguous sequences/haplotypes were corrected using the chromatograms.
Statistical analyses
To explore which abiotic and biotic factors that influence the presence of E. denticulatum, a mean value for each variable was calculated for each transect. Data from Kane’ohe Bay and Mafia Island were analyzed separately, as the two sites have different environmental conditions and the same factors might not have the same impact at the different geographical locations.
There was high incidence of zero-values in the cover of of E. denticulatum (> 60% of transects) but preliminary analyses using zero-inflated poisson models (ZIPs) showed a poor model fit to assumptions. Moreover, % cover in transects where E. denticulatum was present was generally low (≤ 24%), average cover of E. denticulatum per transect in Kane’ohe Bay was 3.3% and on Mafia Island 1%, and in transects where E. denticulatum was found 7.8% and 3.5%, respectively. Consequently, we converted all E. denticulatum cover data into presence (1) or absence (0), and then tested the influence of environmental variables using mixed logistic regression. Variables influencing presence/absence of E. denticulatum in Kane’ohe Bay were tested with binomial generalized mixed effects models (GLMMs), using the R packages “lme4” (Bates et al. 2015), “glmmADMB” (Fournier et al. 2012; Skaug et al. 2016). Because at least two transects were sampled in each same patch reef or area in both Kane’ohe Bay and Mafia Island (Fig. 1), “reef” was initially included in all models as a random factor. Furthermore, because the variable “reef treatment” (“manual removal/sea urchin transplantations” or “none”) was non-normality distributed and not possible to transform satisfactorily, this variable was excluded. Hence, the random factor “reef” includes both potential variation caused by reef treatment and spatial grouping of reefs. Because no variation was added to the Mafia Island data set depending on “reef”, generalized linear models (GLMs) from package “stats” (R Core Team 2017) were used. Predictor variables [biomass of herbivorous fishes, total herbivore abundance (ind. transect−1), abundance of sea urchins (ind. transect−1), number of other seaweed species, cover of other seaweed species, turf cover, CCA cover, live coral cover, rugosity, relative wave exposure, depth, amount of soft substrate, amount of dead coral rubble and distance to AOI] were checked for multicollinearity by pairwise comparison using the Spearman rank test and by evaluating variation inflation factor (VIF) values (Zuur et al. 2010). Predictor variables with VIF-values ≥ 2 were removed from the same model. Model selection was performed by starting with the full model (including all predictors). Non-significant variables were then removed one by one until the most parsimonious model remained, based on Akaike’s Information Criterion corrected for small sample sizes (AICc) (Johnson and Omland 2004). If ΔAICc ≥ 2, the model with the lowest AICc value was considered the most parsimonious one. Each model was then tested for interaction effects against all environmental variables (allowed in the same model based upon Spearman rank and VIF tests). Prior to model fitting, normal distributions of predictor variables were visually examined by basic diagnostic plots, and if needed, transformation log(x + 1) and rescaling to size range were performed. All final models were tested with influence measures, Cessie van Houwelingen test and Pearsons x2 test for assumptions for binomial GLM and GLMM.
Differences in grazing intensity between Kane’ohe Bay and Mafia Island were analyzed by Kruskal-Wallis rank sum test. The same type of test was used to compare the level of grazing scars on E. denticulatum among different patch reefs in Kane’ohe Bay. A two-way ANOVA was used to test for differences in grazing among sampling sites on Mafia Island and if this was dependent on distance to AOIs. Transects were classified as “close” if they were located at distances < 1 km from an AOI or “far” if > 1 km. To test for an effect of distance from AIOs on the proportion of non-indigenous and indigenous strains of E. denticulatum on Mafia Island, all E. denticulatum individuals found in transects were used. Proportion values were calculated for each sampling area (Chole Channel, Jibondo, Juani, Kitutia, Kulawe, Mwamba mkuu, Mwamba mkuu mdogo), and these were classified as “close” if they were located at distances < 1 km from an AOI or “far” if > 1 km. Origin of seaweeds were obtained from the DNA analyses and the proportion of non-indigenous and indigenous strains of E. denticulatum was analyzed with a Pearson chi square test. All statistical analyses were performed in R version 3.3.1 (R Core Team 2017).
Results
Factors influencing the presence of Eucheuma denticulatum
Several environmental characteristics differed between Kane’ohe Bay and Mafia Island (Table 1). E. denticulatum and live coral cover were higher in Kane’ohe Bay whereas cover and number of other seaweed species, biomass of herbivorous fish and amount of soft substrate were higher on Mafia Island (Table 1). However, the abundance of herbivorous fish and distances to AOIs were similar between the two locations.
Kane’ohe Bay
The likelihood of finding E. denticulatum increased with wave exposure (p < 0.05, Table 2; Fig. 2a) and was dependent on site (“reef”). E. denticulatum was only found in 23 of the 52 transects sampled, mainly in the northern and central part of Kane’ohe Bay (Fig. 1). At the AOI at Coconut Island, E. denticulatum was not found, and it was also absent from the southern inshore areas. Cover of E. denticulatum was generally low, ranging from 1.2—24.2% among transects where it was present. The distribution was patchy, with cover reaching up to 50% at heavily colonized subsections within transects. The substrate to which E. denticulatum was attached consisted almost exclusively of coral (living coral or coral rubble) with a high degree of structural complexity, mainly P. compressa.
Scarine labrids were the most abundant herbivores, and the majority consisted of juveniles or subadults. Few fish exceeded 15 cm, although larger individuals were observed on the barrier reef flat outside the transects. Adult Zebrasoma flavescens and Z. veliferum were frequently observed grazing on E. denticulatum, but these species were not abundant within the transects.
Mafia Island
The likelihood of finding E. denticulatum decreased with distance from AOIs (p = 0.009, Table 3) and increased with cover of dead coral rubble (p = 0.006, Table 3). There was also a significant interaction between the two variables (p = 0.02, Fig. 3), where occurrence of E. denticulatum decreased less with distance from AOIs in the presence of dead coral rubble. There was also a (weak) interactive effect of distance from AOIs and live coral (p = 0.042, Table 3), but not from live coral cover only (p = 0.527, Table 3).
Interaction plot of the generalized linear model (GLM) displaying fit of model with the interaction between distance to area of introduction (AOI) and cover of dead coral rubble (black and dashed lines) for presence of Eucheuma denticulatum (log odds) on Mafia Island. Shaded areas denote partial residuals. “High” (black line) denotes cover of dead coral rubble in an interval of 92.5–61.7%, “intermediate” (dashed line) cover of 61.7–30.8% and “low” (dotted line) cover of 30.8–0%
On Mafia Island, E. denticulatum cover was considerably lower (0.1–11% in transects where it was encountered) than in Kane’ohe Bay. Average cover per transect was 1% (all transects), and 3.4% in transects where E. denticulatum was encountered. The distribution of E. denticulatum was very patchy. Larger patches (up to 4 m2) with high cover of E. denticulatum (> 40%) were only found within 50 m of AOIs. Further away only solitary individuals were found. Coral rubble (usually remnants from branching corals such as Acropora spp.) was the most common substrate for attachment, followed by sponges and small rocks. No E. denticulatum was found in transects with high live coral cover.
Herbivorous fish assemblages were dominated by scarine labrids, consisting mainly of juveniles and subadults. Larger (adult) acanthurids, kyphosids and siganids were observed within the study area, but rarely in the transects.
Grazing intensity
In Kane’ohe Bay, E. denticulatum was estimated to be grazed 100% by fish (all fronds cropped). There were no significant differences in abundance of herbivorous fishes among sites (ANOVA, f = 3. 9, df = 6, p > 0.05). Almost all E. denticulatum observed in the study were cropped below the level of coral branches (Fig. 6a).
Compared to Kane’ohe Bay, a lower grazing pressure of E. denticulatum was found on Mafia Island (average 50%) (Kruskal-Wallis rank sum test, chi-square = 20.031, df = 1, p < 0.001; Fig. 4). Even though sea urchins (Diadema savignyi, D. setosum, Echinometra mathaei and Echinothrix diadema) were more abundant on Mafia Island than in Kane’ohe Bay, fish were still the most common grazers on E. denticulatum based on the type of grazing scars observed. The amount of grazing scars decreased with distance from AOI (ANOVA, f = 5.33, df = 1, p < 0.05).
Amount of grazing measured as % of grazing scars on individual Eucheuma denticulatum fronds found in transects on Mafia Island (N = 12) and in Kane’ohe Bay, Oahu (N = 22). Fronds on Mafia Island were on average grazed 60.3% and in Kane’ohe Bay 98.9%. Horizontal lines denote median values, boxes 25th and 75th percentiles, and error bars 95% confidential intervals. Filled circles denote outliers
Genetic composition
All identified E. denticulatum from Hawai’i (N = 21) belonged to a single haplotype (E32), the Southeast Asian haplotype previously described by Zuccarello et al. (2006). Seaweeds from Mafia Island consisted of a mix of seven haplotypes: one Southeast Asian and commonly farmed haplotype (E13, N = 30), three East African haplotypes [E60, KOM3, PAC5 (Zuccarello et al. 2006; Halling et al. 2013; Tano et al. 2015); N = 15, 1, and 13, respectively], and three newly identified haplotypes [MAF2 (MH115464), MAF4 (MH115465) and MAF6 (MH115466); N = 1, 3, and 4, respectively]. All new identified strains were single nucleotide polymorphisms (SNPs) of haplotype E60 (Fig. 5). Seaweeds sampled close to farms (≤ 50 m) consisted solely of introduced haplotypes (Fig. 5), while transects further away from AOIs had higher proportions of indigenous strains (chi-squared = 151.7, df = 1, p < 0.001). At the most remote location (Kitutia) only one individual of E. denticulatum was found, which belonged to the indigenous haplotype KOM3.
Overview of identified haplotypes of Eucheuma denticulatum and geographical location within the study area. Pie charts display composition of E. denticulatum haplotypes at sampling locations. Haplotype E13 is of Southeast Asian origin, and all the others are native to East Africa. Due to patchy seaweed distribution, sample size differs between locations. Filled (black) circles denote sampling points where E. denticulatum was found
Discussion
This study shows that different factors predicted the presence of E. denticulatum in the two geographic locations. Relative wave exposure increased the presence of E. denticulatum in Hawai’i, but had no effect on Mafia Island. Furthermore, E. denticulatum was more grazed in Kane’ohe Bay than on Mafia Island, which refutes our hypothesis that presence of E. denticulatum is regulated by herbivory to a higher degree on Mafia Island than in Kane’ohe Bay. On Mafia Island, however, the amount of hard substrate (dead coral rubble and live coral cover) close to areas of introductions (AOIs) was the main factor predicting the presence of E. denticulatum but not in Kane’ohe Bay.
Studies have identified water movement and water exchange as important factors influencing productivity among seaweeds, because a high degree of water movement (to a certain extent) enhances CO2 concentration in the water and thus increases nutrient and gas exchange between the macroalgae and the surrounding water (Hurd 2000). In Kane’ohe Bay, water movement is mainly generated through wave and wind exposure, whereas within the Mafia seascape, water movement is also driven by large tidal differences, potentially explaining why wave exposure is not as important on Mafia Island. However, water movement might have various and ambiguous impacts on algal assemblages, e.g., reducing biomass by dislodging thalli or branches due to mechanical stress (and thus possibly facilitating spread; Jackelman and Bolton 1990; Rodgers and Cox 1999), or a positive impact by decreasing herbivory because of exclusion of fishes with weaker swimming abilities (Bejarano et al. 2017). Water movement probably has an important role in the dispersal of E. denticulatum (Russell 1983), which is similar to other non-indigenous seaweeds with vegetative reproduction, such as Hypnea musciformis (Vermeij et al. 2009).
The survey of grazing scars and the restriction of E. denticulatum to protected microhabitats (i.e., in high complexity habitats) suggests that populations in Kane’ohe Bay are intensely grazed by herbivorous fish, although this was not supported by fish biomass and abundance data obtained from the field study. Biomass of herbivorous fishes was considerably lower than in other studies on the Hawai’ian islands (e.g., Kauai ~ 12 g m−2, Maui ~ 20 g m−2, this study ~ 1 g m−2) and Oahu as a whole (~ 10 g m−2; Helyer and Samhouri 2017; Gorospe et al. 2018). Avoidance behavior of fish towards observers is a potential risk when conducting underwater visual census (Kulbicki 1998; Edgar et al. 2004) and might explain why fish biomass was not a predictor of E. denticulatum in our study. Adult herbivores displayed a stronger avoidance behavior than juvenile and subadult individuals, of which the latter two were dominant in our surveys and thus resulting in low biomass estimates. Future studies should explore whether the use of sampling methods that reduce the observer effect, e.g., remote underwater video (RUV), can better resolve the potential relationship between non-indigenous seaweed densities and herbivorous fishes. According to our grazing scar inventory, native herbivores in Kane’ohe Bay might have the ability to control E. denticulatum biomass, given the low seaweed cover observed during the survey year (2016). Additionally, the substrate on which this seaweed is usually found (dead coral rubble) hampers predation as many herbivorous fish species forage on less structurally complex surfaces (Brandl and Bellwood 2014). Such microhabitat topography has been shown to be an important factor in structuring tropical seaweed assemblages by creating grazing refuges in high-complexity reefscapes (Poray and Carpenter 2014).
On Mafia Island, E. denticulatum was also grazed but not to the same extent as in Kane’ohe Bay. According to the degree of grazing scars, seaweeds growing close to AOIs were grazed much more than seaweeds growing further away. Similarly, the likelihood of finding E. denticulatum was also higher close to the AOIs. These observations may be explained by several mechanisms. Seaweeds in farms were generally also heavily grazed (D.H. Chacin, pers. obs.), and seaweed farms are known to attract siganids (Eklöf et al. 2006), which are efficient browsers and croppers of seaweeds (Bennett and Bellwood 2011; Hoey et al. 2013). This observation is in line with findings from Kenya and Southeast Asia, where there is a positive relationship between farming of eucheumoid seaweeds and siganid fisheries (Hehre and Meeuwig 2016; Anyango et al. 2017). Few siganids were encountered in the transects, but many were spotted in the surveyed areas. It is possible that the presence of active seaweed farms on Mafia Island, by attracting and concentrating herbivorous fishes, reduces grazing intensity on seaweeds outside farms. In support of this idea, a previous study from Kane’ohe Bay showed that fish preference for non-indigenous seaweeds reduced grazing on other algae (Stimson et al. 2001). Moreover, E. denticulatum located further away from farms grew mainly as solitary plants instead of in large patches, and fronds also grew more cryptically (under ledges, in cracks) at the more remote sites than closer to farms (Fig. 6b, c). These growth patterns might have made fronds at remote sites more difficult to detect by herbivorous fishes.
Photos of Eucheuma denticulatum at different sampling sites; a E. denticulatum in Kane’ohe Bay, Hawai’i. Seaweeds are heavily grazed and cropped below branches of scleractinian corals. b Southeast Asian (E13) E. denticulatum growing on a patch of coral rubble on Mafia Island. c An East African E. denticulatum (haplotype KOM3) growing under a rock at Kitutia, Mafia Island
On Mafia Island, distance to AOI was a significant factor for predicting the presence of E. denticulatum, because the likelihood of finding E. denticulatum was much higher closer to AOIs. This pattern was not observed in Kane’ohe Bay, but because the original AOI were the growth trial took place 40 years ago (and no new introductions have been made since) did not contain any E. denticulatum at the time of our study, reefs with higher cover might act as new “seeding points/AOIs” and mask this effect by constituting “stepping stones” for spread of the species throughout the bay. Although not investigated in the present study, the number of years a non-indigenous species has been present in the recipient ecosystem might be an important factor influencing which environmental variables that predict presence and spread of an invader.
The other main factor predicting presence of E. denticulatum on Mafia was the amount of dead coral rubble and live coral cover close to seaweed farms. Coral rubble originating from branching corals (e.g., acroporids) provides a three-dimensional structure, which may favor colonization by E. denticulatum if pieces of thallus can get intercepted and entangled in branches long enough for holdfasts to develop. Sexual reproduction is not common for this species, so dispersal is limited to thallus fragmentation and water movement (Rodgers and Cox 1999; Conklin and Smith 2005). The ability of E. denticulatum to regrow and form attachments from small thallus fragments (Conklin and Smith 2005) in combination with the high percentage of introduced seaweed haplotypes found on rubble close to farms (100% of Southeast Asian origin), suggests that algal fragments from farms have dispersed and reattached to hard substrate in adjacent areas. If no or little suitable habitat was present, cover of introduced haplotypes was very low, which in theory should also reduce the risk of spread. Considering that seaweed farms were first introduced to Mafia Island around the year 2000, these results imply a slightly slower spread than documented for Kane’ohe Bay (Conklin and Smith 2005), which might depend on the availability of suitable substrate.
Different patterns of genetic structure of E. denticulatum populations were found between the two geographical locations, with only one haplotype (E32 from Southeast Asia) present in Kane’ohe Bay, but seven haplotypes on Mafia Island, of which only one was from Southeast Asia (E13). On Mafia Island, six native haplotypes of E. denticulatum were present in low quantities and exhibited a sparse coverage, and a higher proportion of Southeast Asian haplotypes were found closer to AOIs (Fig. 5). Furthermore, the Southeast Asian haplotype generally had a high cover (Fig. 6b), likely due to new propagules from farms (see above), in combination with higher growth rates (Tano 2016). These results confirm that non-indigenous strains of E. denticulatum have spread to Tanzanian reefs, similar to patterns observed on Zanzibar (Halling et al. 2013; Tano et al. 2015). Earlier studies have shown that native haplotypes of E. denticulatum exhibit lower growth rates than introduced haplotypes (Lirasan and Twide 1993; Mtolera et al. 1995), indicating that East African haplotypes are less competitive and may therefore not dominate reef communities in a detrimental way.
Establishment of Southeast Asian haplotypes outside farms may result in a shift from indigenous to introduced E. denticulatum within the Tanzanian seascape, with considerably lower genetic diversity as a consequence (Tano et al. 2015). However, baseline data on densities, cover, and settling substrate of wild eucheumoid populations prior to seaweed farming is lacking. Also, further research is needed to examine if the introduced haplotypes have a negative effect on corals in Tanzania similar to that documented in Kane’ohe Bay (Conklin and Smith 2005; Neilson et al. 2018). To minimize further spread of non-indigenous haplotypes in Tanzania, we suggest avoiding placing seaweed farms close to areas with hard substrate with a high degree of three-dimensional complexity. Threshold values may vary with geographical location and need to be adjusted depending on currents, tides, and herbivorous communities.
Compared to previous studies of E. denticulatum in Kane’ohe Bay, we found considerably lower cover which also consisted of smaller and heavily grazed seaweeds (Conklin and Smith 2005; Neilson et al. 2018). We can only speculate about the scarcity found in the present study. First, in the attempt to capture a wide range of environmental conditions (including different substrates) our study did not specifically target areas which were already colonized by E. denticulatum. Likely, this choice increased the number of transects where E. denticulatum was not observed (i.e., “absent”). Second, there might be seasonal or annual fluctuations in cover that were beyond the scope of this study.
Different factors predict the presence of E. denticulatum in the two studied locations, and this result supports previous suggestions that intrinsic characteristics of the recipient ecosystem are crucial for influencing species introductions that result in invasions (Bulleri et al. 2008). Furthermore, we cannot rule out the possibility that since our surveys occurred during different seasons, E. denticulatum phenology could have played a role in the patterns observed. We therefore recommend studies that investigate seasonality of macroalgal abundance across geographic locations. To understand and predict consequences of introductions of non-indigenous species or haplotypes, such factors need to be identified. Risk assessments that combine data on species traits with local environmental conditions (e.g., wave exposure, herbivore abundances or high cover of suitable substrate) might be a useful approach. However, as illustrated here, depending on the characteristics of the study sites direct comparisons may be difficult. There are large differences in both temporal and spatial scale, as Kane’ohe Bay is considerably smaller than the study area on Mafia Island, and the introductions occurred much earlier (1970s vs. 2000s). Also, Kane’ohe Bay has been subjected to multiple disturbances resulting in loss of live hard coral cover, while the Mafia Island seascape is relatively pristine. However, overfishing of herbivores, combined with disturbances causing coral die-offs that increase the amount of advantageous substrate for E. denticulatum, might result in increases in seaweed biomass also on Mafia Island.
Conclusions
Here we show that the presence of the introduced E. denticulatum in Kane’ohe Bay and on Mafia Island are predicted by different factors. Moreover, the introduction of E. denticulatum might not have inferred similar detrimental effects on Mafia Island that have been observed in Kane’ohe Bay. On Mafia Island, suitable substrate (dead coral rubble) and distance to AOI constrains the establishment of introduced haplotypes. We therefore recommend that E. denticulatum biomass should be continuously monitored in Mafia Island and farm locations carefully planned to avoid placement near areas with abundant hard substrate, i.e. habitat patches with a high degree of complexity that may act as stepping stones for spread of introduced haplotypes. In Kane’ohe Bay the abundance of herbivores (sea urchins and fish) likely have the ability to reduce biomass of E. denticulatum, making it desirable to maintain high densities of these consumers to reduce the risk for further spread and invasions.
References
Anyango JO, Mlewa CM, Mwaluma J (2017) Abundance, diversity and trophic status of wild fish around seaweed farms in Kibuyuni, South Coast Kenya. Int J Fish Aquat Stud 5:440–446
Arenas F, Sánchez I, Hawkins SJ, Jenkins SR (2006) The invasibility of marine algal assemblages: role of functional diversity and identity. Ecology 87:2851–2861. https://doi.org/10.1890/0012-9658(2006)87[2851:TIOMAA]2.0.CO;2
Bahr KD, Jokiel PL, Toonen RJ (2015) The unnatural history of Kāne‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ 3:e950. https://doi.org/10.7717/peerj.950
Bahr KD, Jokiel PL, Rodgers KS (2015) The 2014 coral bleaching and freshwater flood events in Kāne’ohe Bay, Hawai’i. PeerJ 3:e1136. https://doi.org/10.7717/peerj.113
Bates B, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Bax N, Williamson A, Aguero M, Gonzalez E, Geeves W (2003) Marine invasive alien species: a threat to global biodiversity. Mar Policy 27(4):313–323
Bax N, Carlton JT, Mathews-Amos A et al (2008) The control of biological invasions in the world’s oceans. Conserv Biol 15:1234–1246. https://doi.org/10.1111/j.1523-1739.2001.99487.x
Bejarano S, Jouffray J-B, Chollett I, Allen R, Roff G, Marshell A, Steneck R, Ferse S, Mumby P (2017) The shape of success in a turbulent world: wave exposure filtering of coral reef herbivory. Funct Ecol 31:1312–1324. https://doi.org/10.1111/1365-2435.12828
Bellwood DR, Hughes TP, Folke C, Nystrom M (2004) Confronting the coral reef crisis. Nature 429:827–833
Bennett S, Bellwood D (2011) Latitudinal variation in macroalgal consumption by fishes on the Great Barrier Reef. Mar Ecol Prog Ser 426:241–252. https://doi.org/10.3354/meps09016
Berkström C, Jörgensen T, Hellström M (2013) Ecological connectivity and niche differentiation between two closely related fish species in the mangrove–seagrass–coral reef continuum. Mar Ecol Prog Ser 477:201–215. https://doi.org/10.3354/meps10171
Brandl SJ, Bellwood DR (2014) Individual-based analyses reveal limited functional overlap in a coral reef fish community. J Anim Ecol 83:661–670. https://doi.org/10.1111/1365-2656.12171
Britton-Simmons KH (2006) Functional group diversity, resource preemption and the genesis of invasion resistance in a community of marine algae. Oikos 113:395–401
Bryceson I (2002) Coastal aquaculture developments in Tanzania: sustainable and non-sustainable experiences. West Indian Ocean J Mar Sci 1(1):1–10
Bulleri F, Bruno JF, Benedetti-Cecchi L (2008) Beyond competition: incorporating positive interactions between species to predict ecosystem invasibility. PLoS Biol 6:e162. https://doi.org/10.1371/journal.pbio.0060162
Burrows M (2012) Influences of wave fetch, tidal flow and ocean colour on subtidal rocky communities. Mar Ecol Prog Ser 445:193–207. https://doi.org/10.3354/meps09422
Casas G, Scrosati R, Piriz ML (2004) The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina). Biol Invasions 6:411–416
Castelar B, de Siqueira MF, Sánchez-Tapia A, Reis RP (2015) Risk analysis using species distribution modeling to support public policies for the alien alga Kappaphycus alvarezii aquaculture in Brazil. Aquaculture 446:217–226. https://doi.org/10.1016/j.aquaculture.2015.05.012
Ceccherelli G, Piazzi L, Balata D (2002) Spread of introduced Caulerpa species in macroalgal habitats. J Exp Mar Biol Ecol 280:1–11. https://doi.org/10.1016/S0022-0981(02)00336-2
Chandrasekaran S, Nagendran NA, Pandiaraja D, Krishnankutty N, Kamalakannan B (2008) Bioinvasion of Kappaphycus alvareziion corals in the Gulf of Mannar. India. Cur Sci 94(9):1167–1172
Chollett I, Mumby PJ (2012) Predicting the distribution of Montastraea reefs using wave exposure. Coral Reefs 31:493–503. https://doi.org/10.1007/s00338-011-0867-7
Conklin EJ, Smith JE (2005) Abundance and spread of the invasive red algae, Kappaphycus spp., in Kane’ohe Bay, Hawai’i and an experimental assessment of management options. Biol Invasions 7:1029–1039. https://doi.org/10.1007/s10530-004-3125-x
Conklin KY, Kurihara A, Sherwood AR (2009) A molecular method for identification of the morphologically plastic invasive algal genera Eucheuma and Kappaphycus (Rhodophyta, Gigartinales) in Hawaii. J Appl Phycol 21:691–699. https://doi.org/10.1007/s10811-009-9404-2
Critchley AT, Farnham WF, Morrell SL (1986) An account of the attempted control of an introduced marine alga, Sargassum muticum, in southern England. Biol Conserv 35:313–332
D’Amours O, Scheibling RE (2007) Effect of wave exposure on morphology, attachment strength and survival of the invasive green alga Codium fragile ssp. tomentosoides. J Exp Mar Biol Ecol 351:129–142. https://doi.org/10.1016/j.jembe.2007.06.018
Davidson AD, Campbell ML, Hewitt CL, Schaffelke B (2015) Assessing the impacts of nonindigenous marine macroalgae: an update of current knowledge. Bot Mar 58:55–79
de la Torre-Castro M, Lyimo TJ, Association WIOMS (eds) (2012) People, nature, and research in Chwaka Bay, Zanzibar, Tanzania. Western Indian Ocean Marine Science Association, Zanzibar
De Martini EE, Friedlander AM, Holzwarth SR (2005) Size at sex change in protogynous labroids, prey body size distributions, and apex predator densities at NW Hawaiian atolls. Mar Ecol Prog Ser 297:259–271
Edgar GJ, Barrett NS, Morton AJ (2004) Biases associated with the use of underwater visual census techniques to quantify the density and size–structure of fish populations. J Exp Mar Bio 308(2):269–290
Eklöf JS, de la Torre-Castro M, Nilsson C, Rönnbäck P (2006) How do seaweed farms influence local fishery catches in a seagrass-dominated setting in Chwaka Bay, Zanzibar? Aquat Living Resour 19:137–147. https://doi.org/10.1051/alr:2006013
Fox R, Sunderland T, Hoey A, Bellwood D (2009) Estimating ecosystem function: contrasting roles of closely related herbivorous rabbitfishes (Siganidae) on coral reefs. Mar Ecol Prog Ser 385:261–269. https://doi.org/10.3354/meps08059
Friedlander AM, Brown EK, Jokiel PL, Smith WR, Rodgers KS (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22:291–305. https://doi.org/10.1007/s00338-003-0317-2
Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder M, Nielsen A, Sibert J (2012) AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw 27:233–249
Froese R, Pauly D (eds) (2017) FishBase. World Wide Web Electronical Publication
Garpe KC, Öhman MC (2003) Coral and fish distribution patterns in Mafia Island Marine Park, Tanzania: fish–habitat interactions. Hydrobiologia 498:191–211
Gaspare L, Bryceson I, Kulindwa K (2015) Complementarity of fishers’ traditional ecological knowledge and conventional science: contributions to the management of groupers (Epinephelinae) fisheries around Mafia Island, Tanzania. Ocean Coast Manag 114:88–101. https://doi.org/10.1016/j.ocecoaman.2015.06.011
Glenn EP, Doty MS (1990) Growth of the seaweeds Kappaphycus alvarezii, K. striatum and Eucheuma denticulatum as affected by environment in Hawaii. Aquaculture 84:245–255
Gorospe KD, Donahue MJ, Heenan A, Gove JM, Williams ID, Brainard RE (2018) Local biomass baselines and the recovery potential for Hawaiian coral reef fish communities. Front Mar Sci 5:162
Gratwicke B, Speight MR (2005) The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J Fish Biol 66:650–667
Halling C, Wikström SA, Lilliesköld-Sjöö G, Mörk E, Lundsör E, Zuccarello GC (2013) Introduction of Asian strains and low genetic variation in farmed seaweeds: indications for new management practices. J Appl Phycol 25:89–95. https://doi.org/10.1007/s10811-012-9842-0
Harrold C, Watanabe J, Lisin S (1988) Spatial variation in the structure of kelp forest communities along a wave exposure gradient. Mar Ecol 9:131–156
Hay ME (1981) Spatial patterns of grazing intensity on a caribbean barrier reef: Herbivory and algal distribution. Aquat Bot 11:97–109. https://doi.org/10.1016/0304-3770(81)90051-6
Hehre EJ, Meeuwig JJ (2015) Differential response of fish assemblages to coral reef-based seaweed farming. PLoS ONE 10:e0118838. https://doi.org/10.1371/journal.pone.0118838
Hehre EJ, Meeuwig JJ (2016) A global analysis of the relationship between farmed seaweed production and herbivorous fish catch. PLoS ONE 11:e0148250
Helyer J, Samhouri JF (2017) Fishing and environmental influences on estimates of unfished herbivorous fish biomass across the Hawaiian Archipelago. Mar Ecol Prog Ser 575:1–15. https://doi.org/10.3354/meps12235
Hoey AS, Brandl SJ, Bellwood DR (2013) Diet and cross-shelf distribution of rabbitfishes (f. Siganidae) on the northern Great Barrier Reef: implications for ecosystem function. Coral Reefs 32:973–984. https://doi.org/10.1007/s00338-013-1043-z
Horrill JC, Darwall WR, Ngoile M (1996) Development of a marine protected area: Mafia Island, Tanzania. Ambio 25(1):50–57
Hurd CL (2000) Water motion, marine macroalgal physiology, and production. J Phycol 36:453–472
Jackelman JJ, Bolton JJ (1990) Form variation and productivity of an intertidal foliose Gigartina species (Rhodophyta) in relation to wave exposure. Hydrobiologia 204(1):57–64
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108. https://doi.org/10.1016/j.tree.2003.10.013
Jokiel PL (1991) Jokiel's illustrated scientific guide to Kane'ohe Bay, O'ahu. Technical report, Hawai'i Institute of Marine Biology, University of Hawai'i, p 64, doi:https://doi.org/10.13140/2.1.3051.9360
Jokiel PL, Hunter CL, Taguchi S, Watarai L (1993) Ecological impact of a fresh-water “reef kill” in Kaneohe Bay, Oahu. Hawaii Coral Reefs 12:177–184
Katsanevakis S, Wallentinus I, Zenetos A, Leppäkoski E, Çinar ME, Oztürk B, Grabowski M, Golani D, Cardoso AC (2014) Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat Invasions 9:391–423. https://doi.org/10.3391/ai.2014.9.4.01
Kimbro DL, Cheng BS, Grosholz ED (2013) Biotic resistance in marine environments. Ecol Lett 16:821–833. https://doi.org/10.1111/ele.12106
Kulbicki M (1998) How the acquired behaviour of commercial reef fishes may influence the results obtained from visual censuses. J Exp Mar Bio 222(1–2):11–30
Levin PS, Coyer JA, Petrik R, Good TP (2002) Community-wide effects of nonindigenous species on temperate rocky reefs. Ecology 83:3182–3193. https://doi.org/10.1890/0012-9658(2002)083[3182:CWEONS]2.0.CO;2
Lirasan T, Twide P (1993) Farming Eucheuma in Zanzibar, Tanzania. Hydrobiol-HAGUE 260:353–353
Mangi SC, Roberts CM (2006) Quantifying the environmental impacts of artisanal fishing gear on Kenya’s coral reef ecosystems. Mar Pollut Bull 52:1646–1660. https://doi.org/10.1016/j.marpolbul.2006.06.006
McClanahan TR, Ateweberhan M, Muhando CA, Maina CA, Mohammed M (2007) Effects of climate and seawater temperature variation on coral bleaching and mortality. Ecol Monogr 77:503–525
McClanahan TR, Cinner J, Kamukuru AT, Abunge C, Ndagala J (2008) Management preferences, perceived benefits and conflicts among resource users and managers in the Mafia Island Marine Park. Tanzan Environ Conserv 35:340–350. https://doi.org/10.1017/S0376892908005250
McCook L, Jompa J, Diaz-Pulido G (2001) Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19:400–417. https://doi.org/10.1007/s003380000129
Mshigeni KE (1984) The red algal genus Eucheuma (Gigartinales, Solieriaceae) in East Africa: an underexploited resource. Eleventh International Seaweed Symposium. Springer, Dordrecht, pp 347–350
Msuya FE, Buriyo A, Omar I, Pascal B, Narrain K, Ravina J, Mrabu E, Waikibia J (2014) Cultivation and utilisation of red seaweeds in the Western Indian Ocean (WIO) Region. J Appl Phycol 26:699–705. https://doi.org/10.1007/s10811-013-0086-4
Mtolera MSP, Collén J, Pedersén M, Semesi AK (1995) Destructive hydrogen peroxide production in Eucheuma denticulatum (Rhodophyta) during stress caused by elevated pH, high light intensities and competition with other species. Eur J Phycol 30:289–297. https://doi.org/10.1080/09670269500651071
Mumby PJ (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101. https://doi.org/10.1126/science.1121129
Mumby PJ (2016) Stratifying herbivore fisheries by habitat to avoid ecosystem overfishing of coral reefs. Fish Fish 17:266–278. https://doi.org/10.1111/faf.12078
Nagelkerken I, Van der Velde G (2002) Do non-estuarine mangroves harbour higher densities of juvenile fish than adjacent shallow-water and coral reef habitats in Curaçao (Netherlands Antilles)? Mar Ecol Prog Ser 245:191–204
Neilson BJ, Blodgett J, Gewecke C, Stubbs B, Tejchma K (2014) Kaneohe Bay, Oahu snap-assessment report. University of Hawaii, Social Science Research Institute, p 88
Neilson BJ, Wall CB, Mancini FT, Gewecke CA (2018) Herbivore biocontrol and manual removal successfully reduce invasive macroalgae on coral reefs. PeerJ 6:e5332. https://doi.org/10.7717/peerj.5332
Nyberg CD, Wallentinus I (2005) Can species traits be used to predict marine macroalgal introductions? Biol Invasions 7:265–279
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311:1459–1461. https://doi.org/10.1126/science.1121407
Poray AK, Carpenter RC (2014) Distributions of coral reef macroalgae in a back reef habitat in Moorea, French Polynesia. Coral Reefs 33:67–76. https://doi.org/10.1007/s00338-013-1104-3
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, https://www.R-project.org/
Ringuet S, Mackenzie FT (2005) Controls on nutrient and phytoplankton dynamics during normal flow and storm runoff conditions, southern Kaneohe Bay, Hawaii. Estuar Coasts 28:327–337
Rönnbäck P, Bryceson I, Kautsky N (2002) Coastal aquaculture development in Eastern Africa and the Western Indian Ocean: prospects and problems for food security and local economies. AMBIO J Hum Environ 31:537–542. https://doi.org/10.1579/0044-7447-31.7.537
Rodgers S, Cox EF (1999) Rate of spread of introduced Rhodophytes Kappaphycus alvarezii, Kappaphycus striatum, and Gracilaria salicornia and their current distribution in Kane’ohe Bay O’ahu Hawai’i. Pac Sci 53(3):232–241
Russell DJ (1983) Ecology of the imported red seaweed Eucheuma striatum Schmitz on Coconut Island, Oahu, Hawaii. Pac Sci 37(2):87–107
Sagerman J, Enge S, Pavia H, Wikström SA (2014) Divergent ecological strategies determine different impacts on community production by two successful non-native seaweeds. Oecologia 175:937–946. https://doi.org/10.1007/s00442-014-2938-2
Skaug H, Fournier D, Bolker B, Magnusson A, Nielsen A (2016) Generalized linear mixed models using 'AD Model Builder'. R package version 0.8.3.3.
Schaffelke B, Hewitt CL (2007) Impacts of introduced seaweeds. Bot Mar 50:397–417.https://doi.org/10.1515/BOT.2007.044
Schaffelke B, Smith JE, Hewitt CL (2006) Introduced macroalgae: a growing concern. J Appl Phycol 18:529–541. https://doi.org/10.1007/s10811-006-9074-2
Seers B (2017) fetchR: calculate wind fetch. R package version 2.1-0.https://CRAN.R-project.org/package=fetchR
Smith JE, Hunter CL, Smith CM (2002) Distribution and reproductive characteristics of nonindigenous and invasive marine algae in the Hawaiian Islands. Pac Sci 56:299–315. https://doi.org/10.1353/psc.2002.0030
Stamoulis KA, Friedlander AM, Meyer CG, Fernandez-Silva I, Toonen RJ (2017) Coral reef grazer-benthos dynamics complicated by invasive algae in a small marine reserve. Sci Rep 7:43819. https://doi.org/10.1038/srep43819
Stimson J, Larned S, Conklin E (2001) Effects of herbivory, nutrient levels, and introduced algae on the distribution and abundance of the invasive macroalga Dictyosphaeria cavernosa in Kaneohe Bay, Hawaii. Coral Reefs 19:343–357. https://doi.org/10.1007/s003380000123
Tamburello L, Benedetti-Cecchi L, Masini L, Bulleri F (2013) Habitat heterogeneity promotes the coexistence of exotic seaweeds. Oecologia 172:505–513. https://doi.org/10.1007/s00442-012-2510-x
Tano SA (2016) Seaweed in the tropical seascape: importance, problems and potential. Doctoral thesis, Department of Ecology, Environment and Plant Sciences, Stockholm University, Stockholm
Tano SA, Eggertsen M, Wikström SA, Berkström C, Buriyo A, Halling C (2017) Tropical seaweed beds as important habitats for juvenile fish. Mar Freshw Res. 68(10):1921–1934. https://doi.org/10.1071/MF16153
Tano SA, Halling C, Lind E, Buriyo A, Wikström SA (2015) Extensive spread of farmed seaweeds causes a shift from native to non-native haplotypes in natural seaweed beds. Mar Biol 162:1983–1992. https://doi.org/10.1007/s00227-015-2724-7
Taylor BM, Choat JH (2014) Comparative demography of commercially important parrotfish species from Micronesia. J Fish Biol 84:383–402. https://doi.org/10.1111/jfb.12294
Veiga P, Rubal M, Sousa-Pinto I (2014) Structural complexity of macroalgae influences epifaunal assemblages associated with native and invasive species. Mar Environ Res 101:115–123. https://doi.org/10.1016/j.marenvres.2014.09.007
Vermeij M, Dailer M, Smith C (2011) Crustose coralline algae can suppress macroalgal growth and recruitment on Hawaiian coral reefs. Mar Ecol Prog Ser 422:1–7. https://doi.org/10.3354/meps08964
Vermeij MJA, Smith TB, Dailer ML, Smith CM (2009) Release from native herbivores facilitates the persistence of invasive marine algae: a biogeographical comparison of the relative contribution of nutrients and herbivory to invasion success. Biol Invasions 11:1463–1474. https://doi.org/10.1007/s10530-008-9354-7
Williams SL, Smith JE (2007) A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu Rev Ecol Evol Syst 38:327–359. https://doi.org/10.1146/annurev.ecolsys.38.091206.095543
Zuccarello GC, Critchley AT, Smith J et al (2006) Systematics and genetic variation in commercial shape Kappaphycus and shape Eucheuma (Solieriaceae, Rhodophyta). J Appl Phycol 18:643–651. https://doi.org/10.1007/s10811-006-9066-2
Zuccarello GC, Lokhorst GM (2005) Molecular phylogeny of the genus Tribonema (Xanthophyceae) using rbcL gene sequencing data: monophyly of morphologically simple algal species. Phycologia 44(4):384–392
Zuccarello GC, West JA, Kamiya M, King RJ (1999) A rapid method to score plastid haplotypes in red seaweeds and its use in determining parental inheritance of plastids in the red alga Bostrychia (Ceramiales). Hydrobiologia 401:207–214
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems: data exploration. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
We would like to thank the Editor-in-Chief, the Associate Editor and the two anonymous reviewers who have contributed with valuable comments which have improved the manuscript. We also wish to thank Prof. John Stimson (University of Hawai’i) and Brian Nielson (DAR) for invaluable help at Oahu and the staff at the Hawai’i Institute of Marine Biology (HIMB) for their support during fieldwork in Kane’ohe Bay. Further, we want to thank Prof. Mats Grahn at Södertörn University for providing lab facilities for the molecular work. We also want to thank Carolina Åkerlund, Karlina See Kee, Yessenia Rojas, the staff at the Mafia Island Marine Park (MIMP), Big Blu Mafia Island Dive Centre and Mafia Island Diving for their support during field work on Mafia Island. Last but not least we want to thank our Tanzanian boat captain, Mr. Nahoda Salamala, for his invaluable help and local knowledge. The study on Mafia Island was supported by the Swedish Research Council (Grants No. 2015-05848 and 2014-03264).
Funding
Open access funding provided by Stockholm University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest.
The authors have no potential conflict of interest concerning the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eggertsen, M., Tano, S.A., Chacin, D.H. et al. Different environmental variables predict distribution and cover of the introduced red seaweed Eucheuma denticulatum in two geographical locations. Biol Invasions 23, 1049–1067 (2021). https://doi.org/10.1007/s10530-020-02417-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02417-z