Abstract
The fringing reefs in Opunohu Bay and Cook’s Bay in Moorea, French Polynesia are an important component of Moorea’s fringing reef system, as they comprise approximately 20% of the island’s perimeter. The two bays are assumed to have differing benthic communities due to differences in onshore land development and freshwater input, but observational studies of their benthic communities are rarely published. To address this information gap, we conducted a quadrat-based benthic survey to determine important drivers of coral spatial variation throughout the bays. Interestingly, we found that coral taxonomic richness and cover did not significantly vary between bays, and corals only declined at the sites nearest freshwater input. Instead, coral richness was significantly greater when the territorial herbivorous damselfish Stegastes nigricans was present. The majority of the corals we documented were relatively small (88% < 10 cm diameter), thus our results support a positive effect of S. nigricans on young coral diversity. Stegastes nigricans could potentially be creating a refuge inside their territories from scraper herbivores for many non-dominant corals, although other potential mechanisms and the context of these benefits warrant further investigation. We conclude that, on the fringing reefs of Moorea’s two major bays, spatial variation in coral richness is more strongly associated with patches of S. nigricans territories than larger-scale differences in onshore land development and distance from freshwater input.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatial variation in corals on tropical shallow reefs is influenced by the variation in biophysical drivers. Generally, abiotic variables such as sedimentation, high water turbidity, and nutrient loading can negatively affect corals, especially juveniles, by smothering them in sediments, reducing light available for photosynthesis, and supporting growth of competitor macroalgae (Erftemeijer et al. 2012; Mwachireya et al. 2017; Ceccarelli et al. 2019). Nearshore reefs can be a particularly challenging habitat for corals to survive, as all of these abiotic variables increase with terrestrial runoff (Mwachireya et al. 2017; Ceccarelli et al. 2019). An important biotic variable that affects coral spatial variation is the presence of herbivorous territorial damselfish (i.e., farmer damselfish). They are ubiquitous on many coral reefs (Ceccarelli et al. 2001) and create patches of epilithic algal matrix (hereafter turf) that is characteristically high in cover and height, by excluding other herbivores (Hata and Ceccarelli 2016). This territorial behavior can potentially have positive or negative effects on corals.
Corals have shown reduced growth, survival, and abundance in farmer damselfish territories (Potts 1977; Casey et al. 2015; Schopmeyer and Lirman 2015), likely due to competition with algae, and some damselfish species directly bite at corals, causing tissue mortality (Hata and Ceccarelli 2016; Hata et al. 2020). However, high coral recruitment (Sammarco and Carleton 1981; Gleason 1996), diversity (Sammarco and Williams 1982; Done et al. 1991; Gochfeld 2010), and density (Done et al. 1991; Gleason 1996) have also been correlated with farmer damselfish territories. A majority of these studies suggested, though it has rarely been experimentally tested, that the reason for the positive association between farmer damselfish territories and corals is that the territories provide refuge from corallivory (Gochfeld 2010) and intense herbivory that could incidentally damage corals (Sammarco and Carleton 1981; Sammarco and Williams 1982; Done et al. 1991; Gleason 1996; Gochfeld 2010). The ubiquity of farmer damselfish and their complex relationship with corals makes them a potentially important player in benthic spatial variation, especially for coral recruitment and recovery after disturbances.
In the south-central Pacific, the coral reefs surrounding the island of Moorea have been regarded as resilient due to their history of rapid recovery from frequent acute disturbances such as storms, Acanthaster planci outbreaks, and bleaching events (Adjeroud et al. 2018). This record of fast recovery is likely due to fast-growing coral species (Kayal et al. 2015; Adjeroud et al. 2018) and strong herbivore suppression of algae (Adam et al. 2011; Han et al. 2016). However, most of what we know about coral reef recovery in Moorea comes from research conducted on Moorea’s outer reef slope (Adjeroud et al. 2009; Holbrook et al. 2016; Lamy et al. 2016), which is in front of the reef crest and furthest from land. These studies may not be comparable to the habitats that are closer to land such as the back reef (directly behind the reef crest) and fringing reef (immediately adjacent to shore).
The back and fringing reefs are shallower than the outer reef and all have different benthic communities and fish communities (Adjeroud 1997; Han et al. 2016). Particularly, the outer reef has greater biomass of parrotfishes and surgeonfishes, while the back and fringing reef have greater biomass of the farmer damselfish Stegastes nigricans (Han et al. 2016), the main species of farmer damselfish in Moorea. This spatial variation in fish communities could have important implications for the benthic community. Moorea also has fringing reef habitat within two long narrow bays (Cook’s Bay and Opunohu Bay) that make up approximately 20% of the island’s perimeter, although the benthic community of this particular habitat is understudied. Local human impacts in both bays’ watersheds include onshore development and agriculture, and extensive dredging occurred on the bays’ reefs in the 1960s and 1970s (Fauchille 2003). All these activities could increase sedimentation and nutrient loading in the bays (Boutillier and Duane 2006; Lin and Fong 2008; Fong et al. 2020), which in turn are likely to affect benthic cover.
Cook’s Bay, also known as Paopao Bay, is generally regarded as more anthropogenically impacted compared to Opunohu Bay because it has more development surrounding it, including the island’s largest city Paopao in its watershed (Recensement de la population 2017). The most inland ends of both bays have freshwater stream input and the benthos generally has higher sediment levels, from runoff, than the bays’ mouths adjacent to the ocean. Two decades before the present study, Adjeroud and Salvat (1996) and Adjeroud (1997) documented a gradient of decreasing coral diversity and cover, decreasing water clarity, and increasing sediment cover from the mouths of both bays, going inland toward the freshwater input. Nutrient enrichment from an onshore shrimp farm has also been documented at the inland end of Opunohu Bay (Lin and Fong 2008). The lower coral diversity and cover near the ends of the bays with stream input were likely due to the negative effects of water turbidity, nutrients, and sediment on corals (Fabricius 2005). However, more recent work on the coral community within and between the bays has been limited.
Several years prior to the present study conducted in 2017, Moorea was impacted by an A. planci outbreak (2003–2010) and tropical storm Oli (2010) that drastically decreased coral cover on the north shore (Kayal et al. 2012; Adjeroud et al. 2018; Edmunds 2020). There are no published data on the bays’ fringing reefs from this time period to our knowledge, but we (Jacobs and Spies) observed higher coral cover in the bays prior to 2012, followed by low coral cover in 2014 (three years prior to this study). We also observed a potential positive relationship between juvenile coral abundance and S. nigricans territories in 2014. This potential relationship is consistent with previous work that has also found higher coral density and recruitment rates in S. nigricans territories on Moorea’s fringing reefs (Done et al. 1991; Gleason 1996; Gochfeld 2010).
In order to better understand the current status of the coral community on the fringing reefs of Moorea’s two major bays and determine what best predicts coral cover and diversity in the bays, we conducted an observational study. Particularly, we aimed to assess if coral cover and diversity varied based on S. nigricans territory presence, or if location within bays (i.e., distance from stream) or between bays (i.e., in Cook’s vs. Opunohu Bay) was more important. Our objectives were to (1) characterize the benthic cover and water quality in the two bays’ fringing reefs and (2) determine how coral diversity and cover varied based on presence of S. nigricans and location within and among the bays.
Methods
Study site
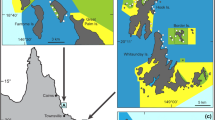
This study took place April 24 to May 25, 2017 on the fringing reefs of the eastern sides of the two major bays in Moorea, French Polynesia, an island in the tropical south-central Pacific. Opunohu Bay (17° 29′ 34″ S, 149° 51′ 20″ W) and Cook’s Bay (17° 29′ 38″ S, 149° 49′ 20″ W) are relatively similar in shape: narrow elongated bodies of water (approx. 3.0 km × 0.7 km), both with relatively low wave action.
Study design and data collection
Due to the lack of ecological information on the bays’ reefs and the differences in onshore development between the two bays, our objective was to characterize the benthic communities and water quality (clarity, temperature, salinity, conductivity) in the bays. To do this, we established five relatively equally spaced sites along a north–south gradient on the east side of each bay for a total of ten sites in the study (Fig. 1). Site location within bay served as a measure of distance from stream input (and its associated terrigenous matter), as both bays have streams emptying into their most-inland (i.e., most southern) ends. Each site was a 50 m × 5 m rectangular region along the border of the reef edge. Within each site’s rectangular region, we randomly placed fifteen quadrats by generating fifteen different xy coordinates within each site. The reef edge ran parallel to shore and was demarcated by a sharp drop off from < 2 m depth on the fringing reef platform to the deeper bay area (> 15 m depth). The average quadrat depth was 1.7 m (SE ± −0.1).
Site map of observational survey conducted in Opunohu Bay (17° 29′ 34″ S, 149° 51′ 20″ W) and Cook’s Bay (17° 29′ 38″ S, 149° 49′ 20″ W), on the north shore of Moorea, French Polynesia. Five sites were spaced roughly equidistant from each other on the eastern sides of each of the bays along the well-defined edge (i.e., crest) of the fringing reef (sites not drawn to scale). The most inland sites (5 and 10) were closest to stream input (a). Each site had 15 quadrats randomly placed within a site’s 50 m × 5 m potential sampling region (b). The rectangular sampling region was created by running a 50 m transect along the reef edge and a 5 m transect perpendicular to the reef edge (going shoreward away from the edge). We determined placement of each quadrat by using a random number generator to choose one number between 0 and 50 and one number between 0 and 5 to generate an xy coordinate for the quadrat within the rectangular region
To determine water quality, we took one set of measurements at each site at 3 m depth at the reef edge. We measured temperature, conductivity, and salinity with a YSI 556 water quality meter. To determine average water clarity, we took horizontal Secchi disk measurements via SCUBA at 3 m depth. We measured clarity twice with different divers and averaged the measurements in order to account for potential observer bias. All water quality measurements were taken on two consecutive days between the hours 0830 and 1530 when weather conditions were minimally overcast with low wind. There were no major rainfall events preceding the water quality measurements.
To characterize the benthic community, we conducted quadrat surveys via SCUBA and snorkel using a 1m2 point-count quadrat. The quadrat was strung with monofilament line in nine horizontal and vertical lines, creating 81 intersecting points, under which we identified benthic substrate or organism. We characterized the benthos beneath each point as one of the following: rock, sediment, macroalgae, turf, crustose coralline algae (hereafter CCA), hard coral, all other invertebrates, or debris (i.e., trash). We identified macroalgae to genus, measured average turf height (n = 10 haphazardly placed measurements per quadrat), quadrat depth and noted if the quadrat overlapped with a S. nigricans territory or not. Because the exact boundaries of a S. nigricans territory are not always clearly delineated, we determined territory presence in the quadrat based on (1) presence of S. nigricans before the quadrat was placed down, and (2) territorial activity of the S. nigricans once the quadrat was placed. The territorial activities that we monitored for included: returning to the quadrat area once the quadrat was placed, chasing other fish away from the quadrat, and biting at the algae in the quadrat. Stegastes nigricans is the main farmer damselfish species in Moorea, and we did not observe any other farmer damselfish species in this study.
We photographed every coral in each quadrat using a Nikon Coolpix S33 camera. Each coral was photographed next to a ruler for scale (Fig. 2). We later reviewed the photographs to determine coral colony abundance per square meter, to identify corals to the lowest taxonomic level possible (species or genus) for taxonomic richness, and to estimate each coral’s diameter by visual comparison with the ruler. Coral colony abundance was the total number of individual colonies per m2 quadrat, while coral cover was measured at the number of intersection points that overlapped with hard coral in the 81-point-count m2 quadrat. Corals were small on average and the corals present in a quadrat often were not captured by the point-counts, so diversity metrics were calculated based on all corals in the quadrat as opposed to the point-count data. All data analyzed during the current study are available in the Mendeley Data repository [https://data.mendeley.com/datasets/whxw97nw86/1]; code is available at https://github.com/allieblanchette/Bays-damselfish-paper.
Data analysis
We used R Studio (R version 3.6.1) statistical software to meet our objectives of investigating correlations between corals and S. nigricans or other potential drivers (e.g., water clarity) in the two bays. First, we determined whether water clarity and corals (cover, richness, size) varied between Opunohu and Cook’s Bays, as well as with increasing distance from stream input (i.e., site location within bay). To do this, we fit a full Gaussian generalized linear model for each water clarity and coral response variable, with site (i.e., distance from stream input; continuous variable) and Bay as the predictor variables, plus an interaction term. To test whether site or Bay significantly affected water clarity and corals, and if these predictors had a significant interaction, we ran a type III ANOVA on the full model. Gaussian models were appropriate for these data because the level of replication for the coral response variables was the averaged value per site (averaged across 15 quadrats per site), and both the coral and water clarity response variables met the assumptions of normality and homogeneity of variance. Coral average size was log-transformed in order to meet these assumptions. We used an alpha of 0.05 and Bonferroni error correction.
Next, we assessed the coral community composition of the two bays’ fringing reefs, excluding site 5 in Opunohu Bay and site 10 in Cook’s Bay, both of which were majority sediment and turf and largely lacking corals. To determine if water clarity predicts coral taxonomic richness, cover, or size on the hard substrate reef, we fit Gaussian generalized linear models for each coral response variable with water clarity as the predictor variable. Gaussian models were appropriate for these data because the level of replication for the coral response variables was the averaged value per site (averaged across 15 quadrats per site) and the response variables met the assumptions of normality and homogeneity of variance. To test whether water clarity significantly affected corals, we used chi-squared log-likelihood ratio tests. We used an alpha of 0.05 and Bonferroni error correction.
To explore how corals related to the presence of S. nigricans territories and other benthic variables, we first ran a principal component analysis (PCA) on coral taxonomic richness, algal turf percent cover, average turf height, macroalgae percent cover, and CCA percent cover per m2 quadrat. Each point in the PCA represents a quadrat, and each quadrat was color-coded to represent presence or absence of a S. nigricans territory. All of the benthic data were z-scored to be on the same scale. To explore how coral taxa related to each other and the presence of S. nigricans territories, we ran another PCA on abundance of each coral taxa as measured by the total number of individual colonies per m2 quadrat. Each point in the PCA represents a quadrat, and each quadrat was color-coded to represent presence or absence of a S. nigricans territory.
To estimate how corals (taxon abundances, taxonomic richness, cover, and size) and turf (cover and height) depend on S. nigricans territory presence, we fit generalized linear mixed effects models. Stegastes nigricans territory was modeled as a fixed effect and site was modeled as a random effect (random intercepts). We fit the response variables of coral taxon abundances, taxonomic richness, coral cover, and turf cover with negative binomial distributions using the glmer.nb function in the R package lme4 (Bates et al. 2015). Coral and turf cover were measured as the number of points in the 81-point-count m2 quadrats that overlapped with hard coral or turf. Coral taxon abundances, taxonomic richness, coral cover, and turf cover followed a Poisson distribution, as they were right-skewed count data with a high frequency of low values. Negative binomial was appropriate for these metrics because it accounts for possible over-dispersion in Poisson-distributed data. Many of the coral taxa were at low abundances and did not have sufficient data for the models. Thus, the models of abundances per taxon were only run for taxa that were present in > 15% of quadrats and were present in all sites being assessed (i.e., Sites 1–4 and 6–9). We fit coral average size and turf average height per quadrat with Gaussian distributions using the lmer function in the R package lme4 (Bates et al. 2015). To meet the assumptions of normality and homogeneity of variance, we log-transformed the coral size and turf height data. To test whether S. nigricans territory significantly affected corals and turf, we used chi-squared log-likelihood ratio tests. We used an alpha of 0.05 and Bonferroni error correction.
Results
Characterizing benthic cover
The average benthic composition across all 150 quadrats was 39.7% (SE ± 2.0) turf, 19.4% (SE ± 1.3) macroalgae, 17.3% (SE ± 1.4) CCA, 14.7% (SE ± 2.3) sandy sediment, and 5.3% (SE ± 0.5) live hard coral. The remaining 4% of benthic cover for our sites was composed of bare carbonate rock, other invertebrates, and debris (i.e., trash). The most common macroalgae was Dictyota sp. (41% of the macroalgae documented), and the average turf height was 3.3 mm (SE ± 0.2). This heterogeneous benthic composition was evident across all sites, except for those closest to stream input (site 5 in Opunohu Bay and site 10 in Cook’s Bay), which had relatively high levels of sandy sediment (Fig. 3).
Benthic percent cover of different substrates, averaged across 15 1m2 quadrats per site. Sites indicated by bay name (O—Opunohu Bay; C—Cook’s Bay) and site number. The gray dashed line indicates average coral cover (5.3%) across the two bays. The black dashed line indicates average coral cover (4.2%) measured across LTER sites 1 and 2 on the north shore fringing reef outside of the bays in 2017. CCA stands for Crustose Coralline Algae
S. nigricans territories overlapped with 47 of the 150 total quadrats (31%), but were absent from all quadrats in the sites closer to stream, including site 5 in Opunohu Bay and sites 9 and 10 in Cook’s Bay (Fig. 4a & 4b). When S. nigricans territories were present, the average turf height was higher (5.5 mm ± 0.4 SE) than when territories were absent, as was the average turf cover (62.5% ± 2.6 SE). When S. nigricans territories were absent, average turf height and cover were significantly lower (Table 1): height 2.3 mm (± 0.1 SE), cover 29.3% (± 1.9 SE).
Sum of Stegastes nigricans territories overlapping with quadrats, out of 15 total quadrats per site (a, b). Total abundances of each coral taxa per site, split between quadrats with S. nigricans territories present (c, d) or absent (d, f). Across all 10 sites, S. nigricans territories were present in 47 quadrats and absent from 103 quadrats
We photographed 1,424 individual coral colonies from the 150 quadrats. We identified 15 different taxonomic groups to species or genus, belonging to 9 families and 13 genera (Table 2). There was substantial coral biodiversity at each site, except for sites 5 and 10 (Fig. 4c, d). The top three most common taxa were Porites lobata (494 individuals), Leptastrea sp. (300 individuals), and Psammocora contigua (168 individuals). Coral diameter ranged from 0.3 to 51.0 cm, although 88% of all corals documented were under 10 cm and the average coral diameter was 5.0 cm (SE ± 0.1).
Variation within and between bays
Across all sites, water clarity was the only water parameter that varied; temperature, conductivity, and salinity did not (Table S1 in Online Resource 1). Water clarity significantly increased with increasing distance from stream input in each bay (Table 3; Fig. S1 in Online Resource 1). However, this correlation was largely driven by the sites closest to the stream end of the bay. When those sites (site 5 in Opunohu Bay and site 10 in Cook’s Bay) were excluded from the analysis, the relationship was no longer significant. As such, there was also no significant relationship between water clarity and corals (cover, taxonomic richness, or size) when those two end sites were excluded (Table S2 in Online Resource 1). Furthermore, corals did not significantly vary in cover, taxonomic richness, or size by bay or site, nor were there any significant interactions between bay and site for any of the water clarity and coral response variables (Table 3). Surprisingly, water clarity also did not vary significantly between bays, despite the difference in land use of the two watersheds.
Relationship between S. nigricans and corals
S. nigricans territories were associated with turf percent cover, turf height, and coral taxonomic richness, as is evident in principal component 1 in Fig. 5a (which explains 51.14% of the variation among benthic variables). There is relatively minimal variation in principal component 2 when S. nigricans were present, however, when they were absent, there is relatively greater variation, evidenced by an inverse relationship between CCA and macroalgae (Fig. 5a) on principal component 2 (which accounts for 21.19% of the variation among benthic variables). Together, principal components 1 and 2 explain 72.33% of the variation. Scores for the five principal components (Table S3) and scree plots (Fig. S2) are in Online Resource 1.
Principal Component Analysis (PCA) for benthic substrate types and coral taxonomic richness (a), and coral abundance by taxa (b). Variables in panel a were z-scored to be on a comparable scale. Sites 5 and 10 are excluded from both PCAs. Each point represents a quadrat (120 quadrats total): orange triangles are quadrats with a Stegastes nigricans territory present, blue circles are quadrats without a S. nigricans territory. CCA in panel a stands for Crustose Coralline Algae. Codes for coral taxa in panel b are: Ac—Astrea curta, Cs—Cyphastrea sp., Ff—Fungia fungites, Gp—Gardineroseris planulata, Hl—Herpolitha limax, La—Leptastrea sp., Lo—Leptoseris sp., Ms—Montipora sp., Pc—Pavona cactus, Pl—Porites lobata, Pm—Psammocora contigua, Pp—Psammocora profundacella, Pr—Porites rus, Ps—Pocillopora sp., Sa—Stylocoeniella armata
Many, but not all, coral taxa had a positive association in their abundances with S. nigricans territories, as is evident around PC1 in Fig. 5b (which explains 15.78% of variance in coral taxa abundances). Together, principal components 1 and 2 explain 26.45% of variation in the abundances of the 15 coral taxa. Scores for the 15 principal components (Table S4) and scree plots (Fig. S2) are in Online Resource 1. This positive association results in S. nigricans territory presence having a significant positive relationship with coral taxonomic richness (Table 1). Coral taxonomic richness was 4.4 taxa (SE ± 0.3) per m2 when a S. nigricans territory was present and 3.0 (SE ± 0.2) taxa per m2 when absent (Fig. 6a). The majority of coral taxa (9 out of 15 taxa) were more abundant on average when S. nigricans were present, while others (6 out of 15 taxa) were more abundant when S. nigricans were absent (Table 2, Fig. 6b). Some of the coral taxa significantly differed in their abundances in and out of S. nigricans territories, including Psammocora contigua (positive relationship), Cyphastrea sp. (positive relationship), and Porites lobata (negative relationship), but many of the taxa were not abundant enough to determine significant differences. Across taxa though, total coral abundance per m2 was higher when a S. nigricans territory was present: 13.7 (SE ± 1.2) individuals per m2 when a S. nigricans territory was present and 10.4 (SE ± 0.9) individuals per m2 when absent.
Coral cover did not significantly vary depending on whether S. nigricans were present (7.4% coral cover) or absent (5.3% coral cover). However, as noted above, it is evident that individual coral taxa showed general patterns of either a positive or negative relationship with S. nigricans. Coral average size was also not significantly correlated with S. nigricans. Corals were small whether S. nigricans were present or absent, although corals were slightly smaller on average when S. nigricans were present (4.8 cm) than absent (5.5 cm).
Discussion
Opunohu Bay and Cook’s Bay are important components of Moorea’s fringing reef system, comprising approximately 20% of the island’s perimeter. Their benthic communities are assumed to vary between and within the bays, due to variation in onshore land development and freshwater input. Contrary to expectations, we found that coral cover and taxonomic richness did not significantly vary between or within bays. Instead, coral taxonomic richness was strongly associated with the territories of farmer damselfish S. nigricans. The results of this study provide nuance to a previous assumption about the condition of an understudied fringing reef habitat and highlight the importance of farmer damselfish in influencing coral spatial variation. Further investigation is needed on the potential mechanisms of famer damselfishes’ effects on corals and the spatiotemporal context of these effects.
Characterizing benthic cover and variation across sites
We found that water clarity significantly increased with increasing distance from stream input, though this was largely driven by the sites in each bay that had the lowest water clarity and were closest to the streams. Throughout the bays, most of the sites had a heterogeneous benthic composition, made up of multiple different substrate types, including turf, macroalgae, CCA, and hard corals, except for the two sites that were closest to stream input. The habitat of these two sites differed from the rest of the fringing reef in the bays because they were largely composed of soft sediment and had lower water clarity and coral cover. These patterns might evidence that the streams have short-reaching impacts (e.g., sedimentation from runoff) on the corals, which could be due to high sedimentation directly at the stream input or differential effects of sediments based on size and distance travelled. On average across all sites, corals had low percent cover and were relatively small with minimal variation in size. P. lobata was the most abundant coral species and 15 taxa were observed in total.
Our results are similar to previous characterizations from two decades earlier by Adjeroud and Salvat (1996) and Adjeroud (1997), who also found relatively low coral cover ranging from 0 to 12%; that water clarity increased with increasing distance from stream input; and that the sites closest to stream input were majority sediment. The reef community may have changed over the past two decades though, as we found fewer coral genera (13 genera) than Adjeroud and Salvat (1996) (17 genera) and Adjeroud (1997) (18 genera). However, those prior studies do not include raw data from which more formal tests for change could be performed. Additionally, differences in genus identification and sampling effort might exist between the previous studies and the current one. In the present study, we also documented greater turf cover and slightly lower CCA cover compared to Adjeroud and Salvat (1996) and Adjeroud (1997). Overall, while the reefs’ low coral cover appears to have stayed relatively constant over time, other aspects of the benthic community such as coral biodiversity, turf cover, and CCA cover have changed.
Cook’s Bay is generally regarded to have a less healthy reef than Opunohu Bay because of the development in Cook’s Bay’s watershed, leading to higher human waste and impact. For example, Cook’s Bay has greater nutrient loading (Holbrook et al., 2021). However, there has been minimal research to explore this assumption about the reef. Interestingly, we did not see any significant differences in water clarity or corals (cover, richness, size) between the two bays. Furthermore, while coral richness was previously correlated with increasing distance from stream input (Adjeroud 2000), we did not see any significant changes in coral richness with increasing distance from stream input. This difference in results might be due to higher coral richness at the bays’ mouths in the past (Adjeroud 2000).
When we conducted this study (2017), the average coral cover outside of the bays on the north shore of Moorea (LTER sites 1 and 2) was similar (4.2%, Edmunds 2020) to what we documented inside the bays (5.3%). However, the coral diversity was slightly higher in the bays (13 genera) than outside (9 genera) at LTER sites 1 and 2 (Edmunds 2020). There was relatively similar benthic cover of sand, turf, and macroalgae inside and outside of the bays (Edmunds 2020). The bays are typically assumed to have less healthy reefs than the rest of Moorea’s reef habitats due to their proximity to onshore development and low circulation. However, we found that the bays actually had comparable benthic cover and slightly higher coral genus richness than the north shore reefs outside of the bays in 2017, which might be a result of their common disturbance histories, such as an island-wide A. planci outbreak in 2003–2010 and tropical storm Oli in 2010 (Kayal et al. 2012; Adjeroud et al. 2018).
Lastly, we documented small coral average size (and no significant difference when S. nigricans territories were present or absent), relative to other fringing reef habitats. While 88% of the corals we documented were < 10 cm in diameter, other studies on fringing reefs outside of Moorea have documented a higher frequency of corals in the 10–20 cm range (Lirman and Fong 2007; Roth et al. 2010; Zhao et al. 2014). This difference in sizes across locations is likely due to differences in terrestrial runoff, human impacts, and disturbance histories among these reefs, as well as variation in coral composition and species growth rates. In the context of Moorea, small non-branching corals (0–10 cm diameter) have previously been documented as the most common size and shape on the north shore fringing reef near the bays (Done et al. 1991; Adjeroud et al. 2007). Thus, our result of small corals in the bays may be specific to the context of our study location, rather than a generalizable pattern across fringing reefs.
Relationship between S. nigricans and corals
We found that the territorial herbivorous damselfish (i.e., farmer damselfish) S. nigricans had a significant positive relationship with coral taxonomic richness, and that the majority of coral taxa were more abundant when a S. nigricans territory was present. Most of the coral taxa that were positively associated with territories had relatively low total abundance. Interestingly, this result suggests that S. nigricans might be supporting coral diversity by promoting the abundance of many non-dominant coral taxa.
Positive relationships between farmer damselfish and coral diversity have previously been documented (Sammarco and Williams 1982; Done et al. 1991; Gochfeld 2010), as well as coral recruitment (Sammarco and Carleton 1981; Gleason 1996) and density (Done et al. 1991; Gleason 1996). While we did not assess coral recruitment and we did not see a significant relationship between S. nigricans and coral size, the majority of corals in our study were relatively young, as 88% of them were smaller than 10 cm. Our results, combined with previous studies demonstrating a positive effect on coral recruitment (Sammarco and Carleton 1981; Gleason 1996), suggest that S. nigricans might have a positive effect on recruitment or juvenile corals. It has been suggested that the mechanism for the positive relationship between farmer damselfish and corals is that the damselfish indirectly create a refuge in their territories from corallivores and abrasion from herbivory (Gochfeld 2010; Kamath et al. 2019), which could be especially beneficial for young corals. Other potential mechanisms of farmer damselfish territoriality that might benefit corals include sediment removal (Tebbett et al. 2020) and nutrient provision (Blanchette et al. 2019), but these mechanisms are less understood.
In contrast, farmer damselfish can also have negative effects on corals. Corals have exhibited reduced growth, survival, and abundance in farmer damselfish territories (Potts 1977; Casey et al. 2015; Schopmeyer and Lirman 2015) as well as tissue mortality, likely due to competition with the cultivated algal turf and damselfish directly biting at the coral tissue (Hata and Ceccarelli 2016; Hata et al. 2020). Therefore, the net effect of farmer damselfish on corals might be context-dependent, based on factors that vary within reef habitats as well as globally, such as coral life history, the intensity of damselfish farming, abundance of herbivores and corallivores, and coral community composition (Wellington 1982; White and O’Donnell 2010; Ladd et al. 2018).
While there was a significant relationship between coral diversity and S. nigricans, there was not one for coral cover. This may be due to the variation in relationship directions per coral taxa (negative, neutral, or positive) neutralizing any net effect of S. nigricans on coral cover. This lack of significant relationship aligns with other studies in Moorea (Feeney et al. 2021) and the Caribbean (Vermeij et al. 2015). Ultimately, the long-term relationship between farmer damselfish and corals remains unknown. While juvenile corals might benefit from being inside farmer damselfish territories (Sammarco and Carleton 1981; Gleason 1996; Gochfeld 2010), their growth could be slowed (Potts 1977; Schopmeyer and Lirman 2015) and the damselfish might eventually attack the corals (Hata and Ceccarelli 2016; Hata et al. 2020). However, it is also possible that the farmer damselfish will eventually leave the territory (Hata and Ceccarelli 2016; Hata et al. 2020) when the corals get larger. Further research is needed on the long-term stability and lifespan of farmer damselfish territories and on the survival and growth of the corals within them. Of particular importance is whether the corals are able to reach reproductive maturity and contribute to the local species pool, which could support longer-term coral reef resilience.
Two of the genera (Porites and Pocillopora) that did not appear to benefit from the presence of S. nigricans are ones that have been especially important for recovery after disturbance events on Moorea’s outer reef slope (Berumen and Pratchett 2006; Pratchett et al. 2011; Trapon et al. 2011; Bramanti and Edmunds 2016; Adjeroud et al. 2018). Porites lobata was more abundant when S. nigricans territories were absent, and Porites rus and Pocillopoa sp. had nearly equivalent abundances, whether S. nigricans were present or absent. This may be a common pattern, as other studies have documented either a negative or neutral relationship between farmer damselfish and Porites (Sammarco and Carleton 1981; Done et al. 1991; Gochfeld 2010; Casey et al. 2015). Stegastes nigricans are more abundant on the fringing reef than the outer reef slope (Han et al. 2016) and are likely to be more ecologically important there. This emphasizes the importance of spatial ecological variation among reef habitats (e.g., outer reef slope vs. fringing reef).
It is important to note several caveats in our observational study. First, our water quality observations do not account for temporal variation such as large rainfall events, which affect water turbidity and salinity within and possibly between bays. Additionally, we only assessed the eastern sides of each bay. On the western sides, potentially different water quality and anthropogenic impacts such as dredging (Fauchille 2003) could affect benthic communities differently than the eastern sides. Importantly, our work does not document a similar recovery of fringing reefs to their status prior to disturbance, as is generally seen on the outer reef slope of Moorea (e.g., Adjeroud et al. 2018). Porites rus is thought to have been the ecologically dominant coral on Moorea’s north shore fringing reefs prior to 2012, based on previous research on the north shore (Lenz and Edmunds 2017; Edmunds 2020) and anecdotal observation within the bays. However, there is limited historic data in the bays since Adjeroud (1997) and differences in sampling prevent exact comparison. Despite these caveats, our results provide valuable information on the bays’ fringing reefs, which are understudied relative to Moorea’s outer reef slope. Our results strongly suggest an influence of S. nigricans territories on coral diversity on the bays’ fringing reefs, a pattern that has been previously reported on the reef flats of Moorea’s north shore (Done et al. 1991; Gleason 1996). Lastly, because our study was observational, we cannot ultimately conclude if S. nigricans are causing high coral diversity or whether another confounding factor was affecting both the corals and S. nigricans. However, we identified a strong relationship between S. nigricans and coral diversity that could play an important role in coral reef resilience. To better understand the potential mechanisms and extent of this relationship, further research is needed to experimentally test the effects of S. nigricans, as well as explore the extent of this relationship over time and in other damselfish species, coral communities, and coral reef habitat types (i.e., beyond fringing reefs and beyond the south-central Pacific).
Spatial ecological variation in benthic and fish communities is common on coral reefs across scales: globally, across reef habitat types (e.g., fringing, outer, barrier) and within reef habitats (e.g., patches of farmer damselfish territories). Spatial variation in coral reefs is important as it can affect the ecological processes and patterns that support recovery and resilience. In this study, we characterized the benthic heterogeneity of an understudied habitat on two bays’ fringing reefs in the south-central Pacific that had recently experienced multiple acute disturbances. We found a strong positive relationship between farmer damselfish and coral diversity. We conclude that the unique territorial farming behavior of farmer damselfish could be important for coral reef resilience by maintaining coral diversity.
References
Adam TC, Schmitt RJ, Holbrook SJ, Brooks AJ, Edmunds PJ, Carpenter RC, Bernardi G (2011) Herbivory, connectivity, and ecosystem resilience: response of a coral reef to a large-scale perturbation. PLoS ONE 6:8
Adjeroud M (1997) Factors influencing spatial patterns on coral reefs around Moorea, French Polynesia. Mar Ecol Prog Ser 159:105–119
Adjeroud M (2000) Zonation of macrobenthic communities along two bays in an insular coral reef ecosystem (Moorea, French Polynesia). C R Acad Sci III 323:305–313
Adjeroud M, Salvat B (1996) Spatial patterns in biodiversity of a fringing reef community Along Opunohu Bay, Moorea, French Polynesia. Bull Mar Sci 59:175–187
Adjeroud M, Pratchett MS, Kospartov MC, Lejeusne C, Penin L (2007) Small-scale variability in the size structure of scleractinian corals around Moorea, French Polynesia: patterns across depths and locations. Hydrobiologia 589:117–126
Adjeroud M, Michonneau F, Edmunds PJ, Chancerelle Y, De Loma TL, Penin L, Thibaut L, Vidal-Dupiol J, Salvat B, Galzin R (2009) Recurrent disturbances, recovery trajectories, and resilience of coral assemblages on a South Central Pacific reef. Coral Reefs 28:775–780
Adjeroud M, Kayal M, Iborra-Cantonnet C, Vercelloni J, Bosserelle P, Liao V, Chancerelle Y, Claudet J, Penin L (2018) Recovery of coral assemblages despite acute and recurrent disturbances on a South Central Pacific reef. Sci Rep 8:1–8
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Berumen ML, Pratchett MS (2006) Recovery without resilience: Persistent disturbance and long-term shifts in the structure of fish and coral communities at Tiahura Reef, Moorea. Coral Reefs 25:647–653
Blanchette A, Ely T, Zeko A, Sura SA, Turba R, Fong P (2019) Damselfish Stegastes nigricans increase algal growth within their territories on shallow coral reefs via enhanced nutrient supplies. J Exp Mar Biol Ecol 513:21–26
Bramanti L, Edmunds PJ (2016) Density-associated recruitment mediates coral population dynamics on a coral reef. Coral Reefs 35:543–553
Casey JM, Choat JH, Connolly SR (2015) Coupled dynamics of territorial damselfishes and juvenile corals on the reef crest. Coral Reefs 34:1–11
Ceccarelli DM, Jones GP, McCook LJ (2001) Territorial damselfishes as determinants of the structure of benthic communities on coral reefs. Oceanogr Mar Biol Ann Rev 37:355–389
Ceccarelli DM, Evans RD, Logan M, Mantel P, Puotinen M, Petus C, Russ GR, Williamson DH (2019) Long-term dynamics and drivers of coral and macroalgal cover on inshore reefs of the Great Barrier Reef Marine Park. Ecol Appl 30:e02008
Done TJ, Dayton PK, Dayton AE, Steger R (1991) Regional and local variability in recovery of shallow coral communities: Moorea, French Polynesia and central Great Barrier Reef. Coral Reefs 9:183–192
Erftemeijer PLA, Riegl B, Hoeksema BW, Todd PA (2012) Environmental impacts of dredging and other sediment disturbances on corals: a review. Mar Pollut Bull 64:1737–1765
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146
Feeney WE, Bertucci F, Gairin E, Siu G, Waqalevu V, Antoine M, de Loma TL, Planes S, Galzin R, Lecchini D (2021) Long term relationship between farming damselfish, predators, competitors and benthic habitat on coral reefs of Moorea Island. Sci Rep 11:14548
Fong CR, Gaynus CJ, Carpenter RC (2020) Extreme rainfall events pulse substantial nutrients and sediments from terrestrial to nearshore coastal communities: a case study from French Polynesia. Sci Rep 10:1–8
Gleason MG (1996) Coral recruitment in Moorea, French Polynesia: the importance of patch type and temporal variation. J Exp Mar Biol Ecol 207:79–101
Gochfeld D (2010) Territorial damselfishes facilitate survival of corals by providing an associational defense against predators. Mar Ecol Prog Ser 398:137–148
Han X, Adam TC, Schmitt RJ, Brooks AJ, Holbrook SJ (2016) Response of herbivore functional groups to sequential perturbations in Moorea, French Polynesia. Coral Reefs 35:999–1009
Hata H, Takano S, Masuhara H (2020) Herbivorous damselfishes expand their territories after causing white scars on Porites corals. Sci Rep 10:16172
Holbrook SJ, Schmitt RJ, Adam TC, Brooks AJ (2016) Coral Reef Resilience, tipping points and the strength of herbivory. Sci Rep 6:35817
Holbrook SJ, Wencélius J, Dubel AK, Adam TC, Cook DC, Hunter CE, Lauer M, Lester SE, Miller SD, Rassweiler A, Schmitt RJ (2021) Spatial co-variation in nutrient enrichment and fishing of herbivores in an oceanic coral reef ecosystem. Ecol Appl 32:e2515
Kamath A, Pruitt JN, Brooks AJ, Ladd MC, Cook DT, Gallagher JP, Vickers ME, Holbrook SJ, Schmitt RJ (2019) Potential feedback between coral presence and farmerfish collective behavior promotes coral recovery. Oikos 128:482–492
Kayal M, Vercelloni J, De Loma TL, Bosserelle P, Chancerelle Y, Geoffroy S, Stievenart C, Michonneau F, Penin L, Planes S (2012) Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 7:e47363
Kayal M, Vercelloni J, Wand MP, Adjeroud M (2015) Searching for the best bet in life-strategy: A quantitative approach to individual performance and population dynamics in reef-building corals. Ecol Complex 23:73–84
Ladd MC, Miller MW, Hunt JH, Sharp WC, Burkepile DE (2018) Harnessing ecological processes to facilitate coral restoration. Front Ecol Environ 16(4):239–247
Lamy T, Galzin R, Kulbicki M, Lison de Loma T, Claudet J (2016) Three decades of recurrent declines and recoveries in corals belie ongoing change in fish assemblages. Coral Reefs 35:293–302
Lenz EA, Edmunds PJ (2017) Branches and plates of the morphologically plastic coral Porites rus are insensitive to ocean acidification and warming. J Exp Mar Biol Ecol 486:188–194
Lin DT, Fong P (2008) Macroalgal bioindicators (growth, tissue N, δ15N) detect nutrient enrichment from shrimp farm effluent entering Opunohu Bay, Moorea, French Polynesia. Mar Pollut Bull 56:245–249
Lirman D, Fong P (2007) Is proximity to land-based sources of coral stressors an appropriate measure of risk to coral reefs? An example from the Florida Reef Tract. Mar Pollut Bull 54:779–791
Mwachireya SA, Nzioka AM, Mutiso DN (2017) Coral Recruit-Algae Interactions in Coral Reef Lagoons are mediated by riverine influences. Int J Ecol 2017:e1351854
Potts DC (1977) Suppression of coral populations by filamentous algae within damselfish territories. J Exp Mar Biol Ecol 28:207–216
Pratchett MS, Trapon M, Berumen ML, Chong-Seng K (2011) Recent disturbances augment community shifts in coral assemblages in Moorea, French Polynesia. Coral Reefs 30:183–193
Roth L, Koksal S, van Woesik R (2010) Effects of thermal stress on key processes driving coral-population dynamics. Mar Ecol Prog Ser 411:73–87
Sammarco P, Williams A (1982) Damselfish Territoriality: influence on diadema distribution and implications for coral community structure. Mar Ecol Prog Ser 8:53–59
Schopmeyer SA, Lirman D (2015) Occupation dynamics and impacts of damselfish territoriality on recovering populations of the threatened staghorn coral, Acropora Cervicornis. Plos ONE 10:e0141302
Tebbett SB, Chase TJ, Bellwood DR (2020) Farming damselfishes shape algal turf sediment dynamics on coral reefs. Mar Environ Res 160:104988
Trapon ML, Pratchett MS, Penin L (2011) Comparative effects of different disturbances in coral reef habitats in Moorea, French Polynesia. J Mar Biol. https://doi.org/10.1155/2011/807625
Vermeij MJA, DeBey H, Grimsditch G, Brown J, Obura D, DeLeon R, Sandin SA (2015) Negative effects of gardening damselfish Stegastes planifrons on coral health depend on predator abundance. Mar Ecol Prog Ser 528:289–296
Wellington GM (1982) Depth zonation of corals in the Gulf of Panama: control and facilitation by resident reef fishes. Ecol Monogr 52:223–241
White J-SS, O’Donnell JL (2010) Indirect effects of a key ecosystem engineer alter survival and growth of foundation coral species. Ecology 91:3538–3548
Zhao MX, Yu KF, Zhang QM, Shi Q, Roff G (2014) Age structure of massive Porites lutea corals at Luhuitou fringing reef (northern South China Sea) indicates recovery following severe anthropogenic disturbance. Coral Reefs 33:39–44
Boutillier S, Duane T (2006) Land Use Planning to Promote Marine Conservation of Coral reef Ecosystems in Moorea, French Polynesia. Department of Landscape Architecture and Environmental Planning, University of California Berkeley. https://escholarship.org/uc/item/10f3q5p4
Décret du n 2017a-1681 du 13 décembre 2017a authentifiant les résultats du recensement de la population 2017 de Polynésie française. (2017). Journal Officiel de la Republique Francaise. https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000036196054
Edmunds, P. of Moorea Coral Reef LTER. 2020. MCR LTER: Coral Reef: Long-term Population and Community Dynamics: Corals, ongoing since 2005. knb-lter-mcr.4.38. 10.6073/pasta/10ee808a046cb63c0b8e3bc3c9799806
Fauchille A (2003) Les anciennes zones d’extraction de matériaux coralliens, île de Moorea, Polynésie française: Description, bilan écologique, réhabilitation. Documentation Ifrecor. http://ifrecor-doc.fr/items/show/1365
Hata H, Ceccarelli DM (2016) Farming behaviour of territorial damselfishes. In Biology of damselfishes. CRC Press, pp 122–152
Sammarco PW, Carleton JH (1981) Damselfish territoriality and coral community structure: Reduced grazing, coral recruitment, and effects on coral spat. In: Proceedings of the 4th International Coral Reef Symposium, vol 2, pp 525–535
Acknowledgements
We enthusiastically thank Dr. Andrew Rassweiler and anonymous reviewers for comments and discussion; and Dr. Daniel Okamoto for statistical support. We thank the government of French Polynesia for permitting this research and the UC Berkeley Richard B. Gump South Pacific Research Station for providing laboratory space and housing. We thank Jacob Chow for help collecting field data and identifying corals. This material is based upon work supported in part by UCLA’s Department of Ecology and Evolutionary Biology and Office of Instructional Development, the National Council for Scientific and Technological Development of Brazil (Turba) under Grant No. 209261/2014-5, the Holmes O. Miller Endowment Fund Scholarship for Field Study (Blanchette), the A. R. Wallace Scholarship for International Field and Marine Research (Franco), and the UCLA Academic Affairs Commission Travel Mini Grant (Franco).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Andrew Hoey
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blanchette, A., Spies, B., Eminhizer, S. et al. Effects of onshore development and damselfish (Stegastes nigricans) on coral richness in Opunohu Bay and Cook’s Bay in Moorea, French Polynesia. Coral Reefs 41, 987–999 (2022). https://doi.org/10.1007/s00338-022-02282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02282-3