Abstract
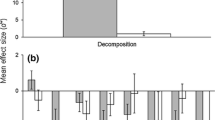
We quantified the effects of invasive Phragmites australis on estuarine faunal communities using meta-analysis to compare invaded to uninvaded marshes and then evaluated whether ecological restoration could reverse those effects. Relative to uninvaded marshes, the quantity and condition of fauna in invaded marshes was significantly poorer. We detected negative impacts to fauna residing in the mid-Atlantic but not in New England and to fauna utilizing the marsh surface but not to those inhabiting tidal creeks. By taxonomic group, we found that the invasion negatively affected nekton but not invertebrates. Both adult and sub-adult nekton were adversely affected, although the magnitude of the effect on the sub-adults was four times greater than that for adults. Our results indicate that negative effects on fauna within the mid-Atlantic region largely drove the overall results. When restored marshes were compared to uninvaded marshes there were no significant differences across all metrics assessed, suggesting that the negative impacts of the invasion were reversed. A separate qualitative review of trophic data indicated that benthic microalgae and dominant vascular plants are important primary producers at the base of the food web in uninvaded, invaded, and restored salt marshes but the overall quantity and importance of microalgae to diet decreased in highly invaded systems due to decreased light, potentially reducing energy availability. Our analyses revealed that while estuarine communities are adversely affected by P. australis, impacts vary by region, habitat, taxonomic group, and life history stage and that restoration can reverse long-term effects over relatively short time scales.



Similar content being viewed by others
References
Able KW, Hagan SM (2000) Effects of common reed (Phragmites australis) invasion on marsh surface macrofauna: response of fishes and decapod crustaceans. Estuaries 23(5):633–646
Able KW, Hagan SM (2003) Impact of common reed, Phragmites australis, on essential fish habitat: influence on reproduction, embryological development, and larval abundance of mummichog (Fundulus heteroclitus). Estuaries 26(1):40–50
Able KW, Hagan SM, Brown SA (2003) Mechanisms of marsh habitat alteration due to Phragmites: response of young-of-the-year mummichog (Fundulus heteroclitus) to treatment for Phragmites removal. Estuaries 26(2B):484–494
Adams DC, Gurevitch J, Rosenberg MS (1997) Resampling tests for meta-analysis of ecological data. Ecology 78(5):1277–1283
Allen EA, Fell PE, Peck MA, Gieg JA, Gutiike CR, Newkirk MD (1994) Gut contents of common mummichogs, Fundulus heteroclitus L., in a restored impounded marsh and in natural reference marshes. Estuaries 17(2):462–471
Angradi TR, Hagan SM, Able KW (2001) Vegetation type and the intertidal macroinvertebrate fauna of a brackish marsh: Phragmites vs. Spartina. Wetlands 21(1):75–92
Arnqvist G, Wooster D (1995) Meta-analysis: synthesizing research findings in ecology and evolution. Trends Ecol Evol 10(6):236–240
Benoit LK, Askins RA (1999) Impact of the spread of Phragmites on the distribution of birds in Connecticut tidal marshes. Wetlands 19(1):194–208
Brawley AH, Warren RS, Askins RA (1998) Bird use of restoration and reference marshes within the Barn Island wildlife management area, Stonington, Connecticut, USA. Environ Manage 22(4):625–633
Brittain RA, Schimmelmann A, Parkhurst DF, Craft CB (2012) Habitat use by coastal birds inferred from stable carbon and nitrogen isotopes. Estuaries Coast 35:633–645
Buchsbaum RN, Catena J, Hutchins E, James-Pirri MJ (2006) Changes in salt marsh vegetation, Phragmites australis, and nekton in response to increased tidal flushing in a New England salt marsh. Wetlands 26(2):544–557
Burdick DM, Dionne M, Boumans RM, Short FT (1997) Ecological responses to tidal restorations of two northern New England salt marshes. Wetl Ecol Manag 4(2):129–144
Burk I (1877) List of plants recently collected on ship’s ballast in the neighborhood of Philadelphia. P Acad Nat Sci Phila 29:105–109
Bushaw-Newton KL, Kreeger DA, Doaty S, Velinsky DJ (2008) Utilization of Spartina- and Phragmites-derived dissolved organic matter by bacteria and ribbed mussels (Geukensia demissa) from Delaware Bay salt marshes. Estuaries Coast 31:694–703
Canty A, Ripley B (2011) Boot: bootstrap R (S-Plus) functions. R package version 1.3-3
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot 64:261–273
Chambers RM, Havens KJ, Killeen S, Berman M (2008) Common reed Phragmitesaustralis occurrence and adjacent land use along estuarine shoreline in Chesapeake Bay. Wetlands 28(4):1097–1103
Chambers RM, Meyerson LA, Dibble KL (2012) Ecology of Phragmites australis and responses to tidal restoration. In: Roman C, Burdick D (eds) Tidal marsh restoration: a synthesis of science and management. Island Press, Washington, DC
Currin CA, Wainright SC, Able KW, Weinstein MP, Fuller CM (2003) Determination of food web support and trophic position of the mummichog, Fundulus heteroclitus, in New Jersey smooth cordgrass (Spartina alterniflora), common reed (Phragmites australis), and restored salt marshes. Estuaries 26:495–510
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431
Davison AC, Hinkley DV (1997) Bootstrap methods and their applications. Cambridge Univ Press, Cambridge
Deegan LA, Garritt RH (1997) Evidence for spatial variability in estuarine food webs. Mar Ecol Prog Ser 147:31–47
Deegan LA, Bowen JL, Drake D, Fleeger JW, Friedrichs CT, Galván KA, Hobbie JE, Hopkinson C, Johnson DS, Johnson JM, LeMay LE, Miller E, Peterson BJ, Picard C, Sheldon S, Sutherland M, Vallino J, Warren RS (2007) Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecol Appl 17(5):S42–S63
Dibble KL, Meyerson LA (2012) Tidal flushing restores the physiological condition of fish residing in degraded salt marshes. PLoS ONE 7(9):e46161
Dionne M, Short FT, Burdick DM (1999) Fish utilization of restored, created, and reference salt-marsh habitat in the Gulf of Maine. Am Fish Soc Symp S22:384–404
Eberhardt AL, Burdick DM, Dionne M (2010) The effects of road culverts on nekton in New England salt marshes: implications for tidal restoration. Restor Ecol 19. doi:10.1111/j.1526-100X.2010.00721.x
Efron B (1987) Better bootstrap confidence intervals (with discussion). J Am Stat Assoc 82:171–200
Fell PE, Weissbach SP, Jones DA, Fallon MA, Zeppieri JA, Faison EK, Lennon KA, Newberry KJ, Reddington LK (1998) Does invasion of oligohaline tidal marshes by reed grass, Phragmites australis (Cav.) Trin. ex Steud., affect the availability of prey resources for the mummichog, Fundulus heteroclitus L.? J Exp Mar Biol Ecol 222:59–77
Fry B (2006) Stable isotope ecology. Springer, USA
Gaertner M, Den Breeyen A, Hui C, Richardson DM (2009) Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: a meta-analysis. Prog Phys Geogr 33(3):319–338
Galván K, Fleeger JW, Fry B (2008) Stable isotope addition reveals dietary importance of phytoplankton and microphytobenthos to saltmarsh infauna. Mar Ecol Prog Ser 359:37–49
Galván K, Fleeger JW, Peterson B, Drake D, Deegan LA, Johnson DS (2011) Natural abundance stable isotopes and dual isotope tracer additions help to resolve resources supporting a salt marsh food web. J Exp Mar Biol Ecol 410:1–11
Gratton C, Denno RF (2005) Restoration of arthropod assemblages in a Spartina salt marsh following removal of the invasive plant Phragmites australis. Restor Ecol 13(2):358–372
Gratton C, Denno RF (2006) Arthropod food web restoration following removal of an invasive wetland plant. Ecol Appl 16:622–631
Grothues TM, Able KW (2003a) Discerning vegetation and environmental correlates with subtidal marsh fish assemblage dynamics during Phragmites eradication efforts: interannual Trend Measures. Estuaries 26(2B):574–586
Grothues TM, Able KW (2003b) Response of juvenile fish assemblages in tidal salt marsh creeks treated for Phragmites removal. Estuaries 26(2B):563–573
Gurevitch J, Hedges LV (1999) Statistical issues in ecological meta-analyses. Ecology 80(4):1142–1149
Hagan SM, Brown SA, Able KW (2007) Production of mummichog (Fundulus heteroclitus): response in marshes treated for common reed (Phragmites australis) removal. Wetlands 27(1):54–67
Hauber DP, Saltonstall K, White DA, Hood CS (2011) Genetic variation in the common reed, Phragmites australis, in the Mississippi River Delta marshes: evidence for multiple introductions. Estuaries Coast 34:851–862
Hedges LV, Olkin I (1985) Statistical methods for meta-analysis. Academic Press, Florida
Hedges LV, Gurevitch J, Curtis P (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hendricks LG, Mossop HE, Kicklighter CE (2011) Palatability and chemical defense of Phragmites australis to the marsh periwinkle snail Littoraria irrorata. J Chem Ecol. doi:10.1007/s10886-011-9990-8
Hesterberg T, Moore DS, Monaghan S, Clipson A, Epstein R (2005) Bootstrap methods and permutation tests, 2nd edn. W. H. Freeman, New York
Holt ER, Buchsbaum R (2000) Bird use of Phragmites australis in coastal marshes of northern Massachusetts. In: Pederson J (ed) Marine bioinvasions: proceedings of a conference, January 24–27, 1999. MIT Sea Grant College Program vol 2, pp 232–240
Hunter KL, Fox DA, Brown LM, Able KW (2006) Responses of resident marsh fishes to stages of Phragmites australis invasion in three mid Atlantic estuaries. Estuaries Coast 29(3):487–498
James-Pirri MJ, Raposa KB, Catena JG (2001) Diet composition of mummichogs, Fundulus heteroclitus, from restoring and unrestricted regions of a New England (U.S.A.) salt marsh. Estuar Coast Shelf Sci 53:205–213
Jivoff PR, Able KW (2003) Blue crab, Callinectes sapidus, response to the invasive common reed, Phragmites australis: abundance, size, sex ratio, and molting frequency. Estuaries 26(2B):587–595
Kalies EL, Chambers CL, Covington WW (2010) Wildlife responses to thinning and burning treatments in southwestern conifer forests: a meta-analysis. For Ecol Manag 259:333–342
Kettenring KM, Reinhardt Adams C (2011) Lessons learned from invasive plant control experiments: a systematic review and meta-analysis. J Appl Ecol 48:970–979
Kimball ME, Able KW (2007) Nekton utilization of intertidal salt marsh creeks: tidal influences in natural Spartina, invasive Phragmites, and marshes treated for Phragmites removal. J Exp Mar Biol Ecol 346:87–101
Kimball ME, Able KW, Grothues TM (2010) Evaluation of long-term response of intertidal creek nekton to Phragmites australis (common reed) removal in oligohaline Delaware Bay salt marshes. Restor Ecol 18(5):772–779
Kneib RT (1986) The role of Fundulus heteroclitus in salt marsh trophodynamics. Am Zool 26:259–269
Kneib RT (1997) The role of tidal marshes in the ecology of estuarine nekton. Oceanogr Mar Biol Annu Rev 35:163–220
Kneib RT (2003) Bioenergetic and landscape considerations for scaling expectations of nekton production from intertidal marshes. Mar Ecol Prog Ser 264:279–296
Kulmatiski A, Beard KH, Meyerson LA, Gibson JC, Mock KE (2010) Nonnative Phragmites australis invasion into Utah wetlands. West N Am Nat 70(4):541–552
Lambertini C, Mendelssohn IA, Gustafsson MHG, Olesen B, Riis T, Sorrell BK, Brix H (2012) Tracing the origin of Gulf coast Phragmites (Poaceae): a story of long distance dispersal and hybridization. Am J Bot 99(3):538–551
Litvin SY, Weinstein MP (2003) Life history strategies of estuarine nekton: the role of marsh macrophytes, benthic microalgae, and phytoplankton in the trophic spectrum. Estuaries 26(2B):552–562
Litvin SY, Weinstein MP (2004) Multivariate analysis of stable-isotope ratios to infer movements and utilization of estuarine organic matter by juvenile weakfish (Cynoscion regalis). Can J Fish Aquat Sci 61:1851–1861
McClary M Jr (2004) Spartina alterniflora and Phragmites australis as habitat for the ribbed mussel, Geukensia demissa (Dillwyn), in Saw Mill Creek of New Jersey’s Hackensack Meadowlands. Urban Habitats 2(1):83–90
Meadows RE, Saltonstall K (2007) Distribution of native and introduced Phragmites australis in freshwater and oligohaline tidal marshes of the Delmarva Peninsula and southern New Jersey. J Torrey Bot Soc 134(1):99–107
Meyer DL, Johnson JM, Gill JW (2001) Comparison of nekton use of Phragmites australis and Spartina alterniflora marshes in the Chesapeake Bay, USA. Mar Ecol Prog Ser 209:71–84
Meyerson LA, Cronin JT (in revision) Evidence for multiple introductions of Phragmites australis to North America: detection of a new non-native haplotype. Biol Invasions
Meyerson LA, Chambers RM, Vogt KA (1999) The effects of Phragmites removal on nutrient pools in a freshwater tidal marsh ecosystem. Biol Invasions 1:129–136
Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay S (2000) A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetl Ecol Manage 8:89–103
Meyerson LA, Saltonstall K, Chambers RM (2009) Phragmites australis in Eastern North America: a historical and ecological perspective. In: Silliman BR, Grosholz E, Bertness MD (eds) Salt Marshes under global siege. University of California Press, Berkeley, pp 57–82
Molloy PP, Reynolds JD, Gage MJG, Mosqueira I, Cote IM (2008) Links between sex change and fish densities in marine protected areas. Biol Conserv 141:187–197
Moreno-Mateos D, Power ME, Comin FA, Yockteng R (2012) Structural and functional loss in restored wetland ecosystems. PLoS Biol 10(1). doi:10.1371/journal.pbio.1001247
Osgood DT, Yozzo DJ, Chambers RM, Jacobson D, Hoffman T, Wnek J (2003) Tidal hydrology and habitat utilization by resident nekton in Phragmites and non-Phragmites marshes. Estuaries 26(2B):522–533
Peterson BJ (1999) Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oecol 20(4):479–487
Posey MH, Alphin TD, Meyer DL, Johnson JM (2003) Benthic communities of common reed Phragmites australis and marsh cordgrass Spartina alterniflora marshes in Chesapeake Bay. Mar Ecol Prog Ser 261:51–61
Powell KI, Chase JM, Knight TM (2011) A synthesis of plant invasion effects on biodiversity across spatial scales. Am J Bot 98(3):539–548
Pyšek P, Jarosik V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Change Biol. doi:10.1111/j.1365-2486.2011.02636.x
Raichel DL, Able KW, Hartman JM (2003) The influence of Phragmites (common reed) on the distribution, abundance, and potential prey of a resident marsh fish in the Hackensack Meadowlands, New Jersey. Estuaries 26(2B):511–521
Raposa K (2002) Early responses of fishes and crustaceans to restoration of a tidally restricted New England salt marsh. Restor Ecol 10(4):665–676
Raposa K (2008) Early ecological responses to hydrologic restoration of a tidal pond and salt marsh complex in Narragansett Bay, Rhode Island. J Coast Res 55:180–192
Raposa KB, Roman CT (2001) Seasonal habitat-use patterns of nekton in a tide-restricted and unrestricted New England salt marsh. Wetlands 21(4):451–461
Raposa KB, Roman CT (2003) Using gradients in tidal restriction to evaluate nekton community responses to salt marsh restoration. Estuaries 26(1):98–105
Rey Benayas JM, Newton AC, Diaz A, Bullock JM (2009) Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science 325:1121–1124
Robertson TL, Weis JS (2005) A comparison of epifaunal communities associated with the stems of salt marsh grasses Phragmites australis and Spartina alterniflora. Wetlands 25(1):1–7
Robertson TL, Weis JS (2007) Interactions between the grass shrimp Palaemonetes pugio and the salt marsh grasses Phragmites australis and Spartina alterniflora. Biol Invasions 9:25–30
Roman CT, Niering WA, Warren RS (1984) Salt marsh vegetation change in response to tidal restriction. Environ Manage 8:141–150
Roman CT, Raposa KB, Adamowicz SC, James-Pirri MJ, Catena JG (2002) Quantifying vegetation and nekton response to tidal restoration of a New England salt marsh. Restor Ecol 10(3):450–460
Rooth JE, Court Stevenson J, Cornwell JC (2003) Increased sediment accretion rates following invasion by Phragmites australis: the role of litter. Estuaries 26(2B):475–483
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, URL: http://www.R-project.org/
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. P Natl Acad Sci 99:2445–2449
Silliman BR, Bertness MD (2004) Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conserv Biol 18(5):1424–1434
Stribling JM, Cornwell JC (1997) Identification of important primary producers in a Chesapeake Bay tidal creek system using stable isotopes of carbon and sulfur. Estuaries 20(1):77–85
Talley TS, Levin LA (2001) Modification of sediments and macrofauna by an invasive marsh plant. Biol Invasions 3:51–68
Vasquez EA, Glenn EP, Brown JJ, Guntenspergen GR, Nelson SG (2005) Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmites australis(Poaceae). Mar Ecol Prog Ser 298:1–8
Vilà M, Espinar J, Hejda M, Hulme PE, Jarosik V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities, and ecosystems. Ecol Lett 14:702–708
Wainright SC, Weinstein MP, Able KW, Currin CA (2000) Relative importance of benthic microalgae, phytoplankton, and the detritus of smooth cordgrass Spartina alterniflora and the common reed Phragmites australis to brackish-marsh food webs. Mar Ecol Prog Ser 200:77–91
Warren RS, Fell PE, Grimsby JL, Buck EL, Rilling GC, Fertik RA (2001) Rates, patterns, and impacts of Phragmites australis expansion and effects of experimental control on vegetation, macroinvertebrates, and fish within tidelands of the Lower Connecticut River. Estuaries 24(1):90–107
Warren RS, Fell PE, Rozsa R, Brawley AH, Orsted AC, Olson ET, Swamy V, Niering WA (2002) Salt marsh restoration in Connecticut: 20 years of science and management. Restor Ecol 10(3):497–513
Weinstein MP, Litvin SY, Bosley KL, Fuller CM, Wainright SC (2000) The role of tidal marsh as an energy source for marine transient and resident finishes: a stable isotope approach. T Am Fish Soc 129:797–810
Weinstein MP, Keough JR, Gutenspergen GR, Litvin SY (2003) Phragmites australis: a sheep in wolf’s clothing? Estuaries 26(2B):397
Weinstein MP, Litvin SY, Guida VG (2009) Essential Fish Habitat and wetland restoration success: a tier III approach to the biochemical condition of common mummichog Fundulus heteroclitus in common reed Phragmites australis and smooth cordgrass Spartina alterniflora-dominated salt marshes. Estuaries Coast 32:1011–1022
Weis JS (2005) Diet and food web support of the white perch, Morone americana, in the Hackensack Meadowlands of New Jersey. Environ Biol Fishes 74:109–113
Weis JS, Butler CA (2009) Salt marshes: a natural and unnatural history. Rutgers Univ Press, New Jersey
Weis JS, Weis P (2000) Behavioral responses and interactions of estuarine animals with an invasive marsh plant: a laboratory analysis. Biol Invasions 2:305–314
Weis JS, Windham L, Santiago-Bass C, Weis P (2002) Growth, survival, and metal content of marsh invertebrates fed diets of detritus from Spartina alterniflora Loisel. and Phragmites australis Cav. Trin. ex Steud. from metal-contaminated and clean sites. Wetl Ecol Manag 10:71–84
Weisberg SB, Lotrich VA (1982) The importance of an infrequently flooded intertidal marsh surface as an energy source for the mummichog Fundulus heteroclitus: an experimental approach. Mar Biol 66:307–310
Windham L, Lathrop RG Jr (1999) Effects of Phragmites australis (common reed) invasion on aboveground biomass and soil properties in brackish tidal marsh of the Mullica River, New Jersey. Estuaries 22(4):927–935
Windham L, Meyerson LA (2003) Effects of common reed (Phragmites australis) expansions on nitrogen dynamics of tidal marshes in the Northeastern U.S. Estuaries 26(2B):452–464
Woolcott CA (2005) Nekton use of Spartina alterniflora and Phragmites australis in the Hackensack Meadowlands. MS Thesis, Rutgers
Wozniak AS, Roman CT, Wainright SC, McKinney RA, James-Pirri MJ (2006) Monitoring food web changes in tide-restored salt marshes: a carbon stable isotope approach. Estuaries Coast 29(4):568–578
Yuhas CE, Hartman JM, Weis JS (2005) Benthic communities in Spartina alterniflora-and Phragmites australis-dominated salt marshes in the Hackensack Meadowlands, New Jersey. Urban Habitats 3(1):158–191
Acknowledgments
We thank Carla Lambertini, Daniel Simberloff, and an anonymous reviewer for their constructive comments that greatly improved this manuscript. Many thanks to Peter August for drafting the map of research sites for studies used in our analysis. We also thank the following agencies and organizations for their support: Environmental Protection Agency Science To Achieve Results Graduate Fellowship Program (FP-91710001-0), National Oceanic and Atmospheric Administration National Estuarine Research Reserve Graduate Fellowship Program (NA09NOS4200041), National Science Foundation Integrative Graduate Education and Research Traineeship Grant to the Coastal Institute at the University of Rhode Island (0504103), Philanthropic Educational Organization (Lellis-Dib3158688), Northeast Aquatic Plant Management Society, Rhode Island Natural History Survey and The Nature Conservancy of Rhode Island (Lellis-Dibble 05-30-09), University of Rhode Island Agricultural Experiment Station (RI00H-332, 311000-6044), University of Rhode Island Coastal Fellows Program, and the U.S. and Czech Fulbright Commissions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dibble, K.L., Pooler, P.S. & Meyerson, L.A. Impacts of plant invasions can be reversed through restoration: a regional meta-analysis of faunal communities. Biol Invasions 15, 1725–1737 (2013). https://doi.org/10.1007/s10530-012-0404-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0404-9