Abstract
For more than a century, bacterial infections caused by Vibrio spp. have affected various species of mussels around the world, with limited insights into the responses implemented by mussels against these infections. A combination of chemical analyses and carefully selected biological endpoints from haemolymph and tissues can be used to identify the welfare status of mussels and potentially protect aquatic ecosystems from catastrophic health threats. Recent developments in biomarker identification tools, such as omics and bioinformatics, have been successfully applied to evaluate the effect of environmental pollutants and other chemicals on mussels. However, the application of biomarkers to assess mussel health is limited. This review describes the available scientific literature on biomarker research for Vibrio-mussel interactions, and those aspects related to mussel health and disease assessment, grouped as biomarkers of exposure, effects, and susceptibility. From the review, it is clear that when integrated biomarkers are used, they can provide a deeper understanding of the relative health and potential susceptibility of mussels for better management practices. Furthermore, health biomarker data can be used to build resilience in mussels against climate change conditions, strengthen biosecurity management programs, improve farming and processing efficiency, and add value in terms of market-desirable traits. These data hold promise for advancing sustainability efforts within the aquaculture industry.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological contamination of aquatic environments, by means of bacteria, viruses, parasites, and harmful algal blooms, can have a significant impact on marine organisms, the environment, and human health. These biological contaminants can disrupt living organisms at various scales (from cell to ecosystem) (Zaghloul et al. 2020). To mitigate the potential adverse effects of biological contamination, it is essential to develop tools for monitoring impacts as diagnostic and early-warning systems.
Biological monitoring, often referred to as biomonitoring, involves the ongoing and systematic observation of living organisms or their natural responses within the environment to assess environmental quality (Prabhakaran et al. 2017). This approach typically centres around the examination of specific biomarkers in selected sentinel species, known as “biomonitors”, which serve as indicators of interest (Dallarés et al. 2018).
Organisms must possess specific attributes to be considered a potential biomonitor species for assessing aquatic diseases (Holt and Miller 2010). These characteristics encompass being: (1) abundant in the aquatic environment, (2) easy to handle, assemble, and identify; (3) relatively long-lived; (4) of an appropriate size for tissue sampling; (5) adaptable to laboratory conditions; (6) suitable for assessing aquatic ecosystem health; (7) capable of accumulating disease markers at levels present in their surroundings without experiencing detrimental effects; and (8) able to demonstrate a swift response to early exposure to various aquatic pathogens. Bivalve molluscs, especially mussel species, adequately fulfil these criteria (Fig. 1). Therefore, they are recognised as reliable models for monitoring diseases in aquatic ecosystems (Newton and Cope 2006; Lucy et al. 2008; Geba et al. 2020; Bigot-Clivot et al. 2022; Chahouri et al. 2022).
Research in the field of environmental science extensively documents the use of biomarkers to assess marine water contamination, which has been widely used and validated in both laboratory and field studies (Langston et al. 2007; Amiard et al. 2000; Lomartire et al. 2021). Biomarkers can be used during pollution monitoring to help detect problems caused by hazardous substances at the sub-cellular level, before they become evident at higher biological levels (Langston et al. 2007). Consequently, there has been a growing need to develop reliable biomarkers to identify and monitor the effects of contaminants in mussels, especially at low concentrations (Leprêtre et al. 2022). Research in the field of marine environmental monitoring aims to establish specific limits for the application of biomarkers. These limits are determined by the regular and systematic use of natural responses of living organisms as indicators. The International Council for Exploration of the Sea (ICES) and the Oslo–Paris Commission (OSPAR) have jointly established a framework for a comprehensive evaluation of the impact of contaminants in coastal and offshore regions (Hagger et al. 2006). Baseline assessment criteria represent the normal biological state in healthy organisms, while ecotoxicological assessment criteria signify the point at which significant immediate and long-term detrimental biological consequences are likely to manifest (Leprêtre et al. 2022). Most of our knowledge about applying threshold values in biomonitoring studies comes from detecting non-biological contaminants, for example, xenobiotics and radiation. These studies have mainly focused on incorporating environmental factors that amplify their effects in natural settings (Pain et al. 2007; Kamel et al. 2014), but they have not concentrated on examining the connection between biomarker responses and microbiological contamination. Since microbiological contamination often goes hand in hand with chemical pollution and climate change (e.g. marine heatwaves, ocean acidification), it makes sense to investigate how organisms respond to different levels of microbiological contamination (Goh et al. 2017).
Biomarkers can be grouped as exposure, effect, and susceptibility biomarkers, as well as general stress and specific biomarkers, which will be explained later in this review. Various diagnostic biomarker techniques have been developed to monitor the health of aquatic ecosystems and are known for their accuracy and cost-effectiveness in early-warning microbial contamination. Biomarkers utilised when dealing with microbiological contamination can identify molecular, biochemical, physiological, genetic, and cellular changes in organisms exposed to biological contaminants (Qureshi and Niazi 2020). These changes occur due to the interaction of three factors: the host, the microbes, and the environment (Lane et al. 2022), known as the epidemiological triangle. The scientific literature on microbiological contamination has enhanced our understanding of how organisms react to microbes by triggering identifiable biomarker responses. In this review, we will demonstrate this concept by focusing on mussels affected by marine bacteria in the genus Vibrio, and their associated biomarker responses. This bacterial group makes an ideal model / biological entity for testing ecological theories, because the bacteria species within are ubiquitous in the aquatic environment, use different hosts across trophic levels, and are relatively easy to manipulate in the laboratory (Chimalapati et al. 2020; Destoumieux-Garzón et al. 2020). Additionally, Vibrio spp. have been implicated in mussel mortality events (Li et al. 2020; Nguyen and Alfaro 2020), manifesting in diseases and infections which can impact wild mussel populations and the commercial mussel farming sector (Azizan et al. 2023d). It is important, to note that variations in biomarker responses within aquatic environments are also often influenced by a range of environmental factors, such as temperature, salinity, diet, and seasonal changes (Hagger et al. 2006). Besides the impact of abiotic factors, it is essential to consider the potential influence of inherent differences in morphology and biochemical/physiological conditions among exposed organisms when measuring biomarker responses. Biological variability may encompass variations in biomarker reactions between genders, age groups, sizes, genotypes, and/or at different stages of reproduction or growth (Hook et al. 2014).
In this review, we present mussel biomarkers previously identified and their relevance to mussel health monitoring programs, particularly in the context of exposure to Vibrio spp. pathogens. This work includes 91 peer-reviewed articles which fulfil five required criteria. The papers had to (1) report on a Vibrio spp. that had been found to infect and/or inhabit a mussel species, (2) report Vibrio spp. infection concentrations (CFU/mL) which the mussel was exposed to, (3) include a robust experimental design with replication of control and bacterial treatments, (4) utilise analytical methods to measure the mussel’s response to infection, and (5) report biomarker responses following infection. These biomarker responses to various environmental pollutants are discussed as well as their potential as monitoring tools in biomonitoring programs.
Pathogenesis of vibrios
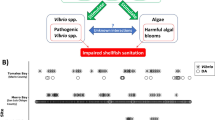
In the late nineteenth and early twentieth centuries, knowledge of shellfish-transmitted bacterial diseases emerged, with Vibrio spp. identified to cause about 20% of the outbreaks (Potasman et al. 2002). These Vibrio species in the Family Vibrionaceae, are versatile gamma-proteobacteria found in various aquatic environments (Thompson et al. 2004; Takemura et al. 2014). These bacteria encompass eight genera, including Aliivibrio, Echinimonas, Enterovibrio, Grimontia, Photobacterium, Salinivibrio, Vibrio, and the newly described Thaumasiovibrio, with more species yet to be found (Sawabe et al. 2013; Amin et al. 2017). Their metabolic flexibility and genetic variability contribute to their high colonisation potential, with Vibrios prevalent in diverse organisms, including plants, algae, zooplankton, and molluscs (Kesarcodi-Watson et al. 2009a; McFall-Ngai et al. 2013; Takemura et al. 2014; Le Roux et al. 2016; Le Roux and Blokesch 2018; Destoumieux-Garzón et al. 2020). Vibrio spp. are often described as pathogens of mussels, as they damage the host upon infection and can threaten shellfish farming (Travers et al. 2015; Destoumieux-Garzón et al. 2020). Investigating factors that contribute to Vibrio abundance is important to support public health programs and for economic trading.
Vibriosis in mussels is less recognised compared to in other bivalves (such as oysters and clams), since mussels are often seen as resilient to Vibrio infections with few mass mortality events linked to Vibrio spp. (Stabili et al. 2005). Mussel mass mortalities have been attributed to environmental factors, chemical contaminants, intraspecific competition, and predation (Domeneghetti et al. 2014; Charles et al. 2020a; Romero et al. 2014). Vibrio pathogens are frequently detected in impacted mussel populations. For example, V. splendidus has been reported in diseased adult Mytilus edulis (Ben Cheikh et al. 2016, 2017), haemolytic Vibrio parahaemolyticus has been found in farmed green mussel samples (Ong et al. 2023), and V. splendidus and V. coralliilyticus/neptunius–like strains have been shown to be associated with high mortality rates in larval Perna canaliculus (Kesarcodi-Watson et al. 2009b). Despite the various mussel mortality and Vibrio spp. associated studies, it is still uncertain whether Vibrio pathogens cause mussel mortality under normal conditions and/or in the presence of additional stressors (Eggermont et al. 2017; Destoumieux-Garzón et al. 2020; Lupo et al. 2021; Richard et al. 2021; Harrison et al. 2022).
Infections of Vibrio spp. in mussels also rely on various factors that enable these bacteria to colonise the host, evade immune responses, invade host tissues, and persist within the host (Fig. 2). Vibrios utilise a range of molecular strategies to infect mussels, although our insights largely stem from a limited number of model strains, including V. splendidus (Zhang and Li 2021), V. parahaemolyticus (Ashrafudoulla et al. 2019), V. alginolyticus (Najwaa et al. 2015), V. tasmaniensis LGP32, V. aestuarianus 01/032 (Destoumieux-Garzón et al. 2020), and V. crassostreae (Islam et al. 2022). However, previous studies have identified several virulence factors contributing to the virulence of this bacterial group. Outer membrane proteins (OMP), thermolabile haemolysin (TLH) encoded by tlh genes, collagenases, gene toxR, toxRS, and cholera toxins (Najwaa et al. 2015), as well as biofilm associated genes (thermostable direct haemolysin encoded by tdh gene; VP950 encoding a lipoprotein-related protein), adhesion factors (Ashrafudoulla et al. 2019), and the quorum sensing system as regulatory factor (Islam et al. 2022), have been described as contributing factors in mussel pathogenesis.
Despite advances in our understanding of how pathogenic Vibrio spp. interact with mussels, further research is crucial for effectively using biomarkers to monitor mussel health (Newton and Cope 2006; Charles et al. 2020b; Destoumieux-Garzón et al. 2020). Future studies targeting Vibrio spp. infections in mussels should (1) acknowledge that Vibrio spp. infections are among crucial factors influencing mussel physiology across all life stages; (2) recognise the ubiquitous nature of Vibrio spp. in the marine environment, including within sediments, biofilms, and aquaculture farms with various marine life coexisting; (3) consider how environmental conditions, such as temperature, pH, salinity, nutrient levels, and seasonal variations impact Vibrio spp. presence and their concentrations (Vezzulli et al. 2013; de Souza Valente and Wan 2021); (4) understand that Vibrio spp. transmission occurs primarily through waterborne routes and direct contact; (5) be conscious that pathogen responses in nature are more complex than in the laboratory; (6) establish comprehensive baseline biomarker data gathering to differentiate between normal variability and Vibrio pathogen–induced stress; (7) comprehend that if the assessment method oversimplifies pathogenicity, it can lead to misidentification of Vibrio spp. at low concentrations; and (8) target disease progression studies from early to pathological stages, to provide a direct measurement of the Vibrio spp. exposure over time. Ultimately, comprehensive knowledge of the pathophysiology and pathogen identification are essential for the initial identification of candidate biomarkers, which is, gaining insight into both the effects of the Vibrio pathogen on the mussel (host) and the subsequent responses of the mussel. For biomarkers to serve as reliable and useful tools for detecting and monitoring aquatic environmental risks, it is advised to stablish these criteria for each candidate biomarker.
Biomarker characteristics and potential roles
Biomarkers can be characterised in numerous ways. To ensure consistency, the following definitions for sets of biomarkers are used in this review, as derived by Kroon et al. (2017). Firstly, biomarkers of exposure are markers that indicate a response following exposure, generally an early response signal to stressors and contaminants. For example, these are markers which tell us what happens as soon as mussels are exposed to Vibrio spp. Secondly, biomarkers of effect are markers representative of changes due to exposure, typically associated with a specific condition. For example, these markers showcase the physiological response of mussels due to infection with Vibrio spp. Thirdly, biomarkers of susceptibility are markers indicative of an organism’s likeliness to develop a disease, based on the organism’s inherent ability to respond to a challenge. For example, these markers may be used to determine if mussels from selective breeding programs will be able to survive Vibrio spp. infections (Fig. 3). Considering that biomarkers lie on a continuum, it is important to keep in mind that it may be difficult to distinguish between biomarkers of exposure and effect (Hook et al. 2014).
For a biomarker to be used in risk assessments linked to ecosystem and organismal health, they need to meet certain criteria (Oliver and Fisher 1999; Newton and Cope 2006; Moore et al. 2007; Ryan et al. 2007; Hook et al. 2014; Kroon et al. 2017; Brosset et al. 2021). Even though the criteria are fluid, with large variations for discovery to validated biomarkers, the following qualities are desired when looking for biomarkers (Fig. 3). An ideal biomarker would: (1) be easy and safe to collect, transport, and store; (2) be easy to measure and analyse; (3) possess a long half-life, with clarity on the time-relationship from exposure to response and persisting effects; (4) be relatively cheap to monitor; (5) be robust and rapid to analyse; (6) be representative of a large sample size; (7) show sensitivity to the variations in populations (e.g. from different geographical regions, seasonal trends, gender differences); (8) be distributed over wide spatial and temporal ranges of health outcomes; (9) allow quantifiable results; and (10) result in reproducible results.
In mussel health assessment, biomarkers can be detected in haemolymph, extrapallial fluid, and soft tissues, such as gills, digestive glands, gonads, and adductor muscle. The samples may then be used to form a biosignature profile describing the biochemical and physiological state of the host as a whole (Newton and Cope 2006; Brooks et al. 2009; Waller and Cope 2019). Linking biomarkers with mussel health can be of great value for diagnosis of disease (e.g. vibriosis), as they can be useful in the following areas: (1) early suspicion of disease which could trigger further study of a site; (2) confirmation and classification of disease severity; (3) identification of high risks cohort; (4) rationalising treatments; (5) assessing responses to treatment; (6) predicting outcomes; and (7) monitoring tool to follow the health of a site over time (World Health Organization 1993, Paillard et al. 2004; Gestal et al. 2008; Brosset et al. 2021).
Biomarkers detected in mussels in response to Vibrio spp. infection
Mussels are constantly being challenged by changing environments, making them more susceptible to diseases (Delorme et al. 2021). Consequently, numerous approaches have been developed to evaluate mussel health, with a growing focus on the investigation of physiological biomarker responses to stress. A critical review of the current literature on biomarker research provides an opportunity to synthesise the accumulated knowledge and assess their potential applications.
Biomarkers of exposure
Biomarkers of exposure can indicate an early response to infection (exposure) and are generally indicative of the extent to which an organism is exposed to a given stressor. While focusing on Vibrio spp. infections in mussels, biomarkers of exposure are classified as biotransformation intermediates, oxidative stress markers, metabolites and proteins, haematological parameters, and immunological parameters.
Biotransformation intermediates
The glutathione antioxidant system is vital for cellular defence against free radicals and oxidants (Meister 1988). Glutathione (GSH) neutralises free radicals and reactive oxygen species (ROS), enzymatically converting GSH to glutathione disulfide (GSSG), which is then reduced back to GSH by nicotinamide adenine dinucleotide phosphate (NADPH)–dependent glutathione reductase. GSH also forms glutathione-S-transferase (GST) during detoxification processes. Furthermore, GSH is essential for activating immune cells during immune challenges (Wu et al. 2004). GSH levels are widely used as a biomarker of metal, organic hydrophilic contaminants, and some types of pesticides exposure (Laitano and Fernández-Gimenez 2016). Laboratory-based studies infecting mussels with Vibrio splendidus, V. anguillarum, and V. harveyi have shown increased GST enzyme activity (Canesi et al. 2010; Liu et al. 2015). Metabolites linked to GSH:GSSH and total GSH have also been found to be increased in mussels (P. canaliculus) infected with Vibrio spp. DO1 (V. coralliilyticus/neptunius–like isolate) (Nguyen et al. 2019a). In addition, GST has been detected in M. galloprovincialis, and Elliptio complanata following V. anguillarum infection (Canesi et al. 2010; Wu et al. 2013; Ji et al. 2013; François et al. 2015), and are closely tied to antioxidant defence systems, resulting in ROS production (Table S1). However, caution should be exercised when measuring biotransformation intermediates, as these compounds are typically unstable or can be converted into other forms. This complicates the pre-analytical handling of samples before measuring the response (Langston et al. 2007; Ho et al. 2013).
Oxidative stress markers
Biomarkers related to oxidative stress are frequently used in mussel assessment after exposure to pathogenic bacteria, as summarised in Table S2. Oxidative stress results from an imbalance in the production of oxidising species (e.g. ROS and oxygen free radicals) (Kroon et al. 2017; Delorme et al. 2021). Prolonged oxidative stress can cause enzyme inactivation, lipid peroxidation (LPOx), deoxyribonucleic acid (DNA) damage, and lead to apoptosis or cell necrosis. It has been well established that mussels have an effective antioxidant system, enabling a balance of basal level free radical production (Kroon et al. 2017). Oxidative stress markers, such as enzymatic superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPOx), LPOx, ROS, defensin, lysozyme, and metallothionein, have been explored as indicators of oxidative stress in mussels exposed to Vibrio spp. In all assays that rely on individual antioxidant enzymes, it is essential to establish well-defined baseline levels and precisely measure the natural variations.
Superoxide dismutase (SOD)
This enzyme converts superoxide anions to hydrogen peroxide, of which catalase (CAT) detoxifies, maintaining a balance in hydrogen peroxide (H2O2) levels (Kroon et al. 2017), and prevents the generation of highly toxic hydroxide ions (OH−) (Fridovich 1973). Serving as a first line of defence against reactive oxygen species (ROS), superoxide dismutase (SOD) is classified into three main types: copper/zinc (Cu/Zn)–SOD found in eukaryotic cytosols, manganese (Mn)–SOD located in mitochondria, and iron (Fe)–SOD present in bacteria (Geret et al. 2004). SOD activity significantly increased in Mytilus edulis exposed to various Vibrio strains (V. tubiashii, V. splendidus, V. parahaemolyticus, V. tubiashii, V. splendidus, and V. alginolyticus), suggesting a protective mussel response against free radical damage caused by bacterial infection (Parry and Pipe 2004; Tanguy et al. 2013a; Hernroth et al. 2016). This protective response, of elevated SOD activity, is similarly observed in M. galloprovincialis and Pinna nobilis when challenged with V. harveyi and other Vibrio spp. (Künili et al. 2021). Both Cu/Zn-SOD and Mn-SOD play pivotal roles in mitigating oxidative damage and may be a sensitive marker for bacterial infection. Cu/Zn-SOD, in particular, is essential for mussel metabolism, acting as an efficient scavenger of free radicals, while Mn-SOD, originating as a mitochondrial precursor protein, becomes crucial in the cytoplasm after the cleaving of signal peptides (Wang et al. 2010; Wu et al. 2017; Sendra et al. 2020). To date, the extensive research into the application and validation of SOD measures has resulted in a simple low-cost spectrophotometric assay and also molecular techniques are being routinely employed to measure SOD in invertebrate species (Wang et al. 2010; Wu et al. 2017; Sendra et al. 2020).
Catalase (CAT)
This is an enzyme which catalyses the decomposition of H2O2 into water and oxygen, thereby maintaining optimum H2O2, crucial for cell signalling processes (Nandi et al. 2019). The defensive role of CAT against H2O2 from pathogen infections is evident in various mussel tissues, including haemolymph (Table S2). In a study on M. galloprovincialis, catalase activity was stimulated in the digestive gland following infection with V. splendidus and V. anguillarum, respectively (Canesi et al. 2010). The use of CAT as a biomarker against V. splendidus LGP32, has been highlighted by Sendra et al. (2020), who reported up-regulation of catalase messenger RNA (mRNA) expression following infection in M. galloprovincialis. In a study by Bao et al. (2018), explicit expression of a McCAT gene was found in the hepatopancreas of M. coruscus following infection with V. parahemolyticus and A. hydrophila. In tissues of P. nobilis, co-infections between V. harveyi and other Vibrio spp. showed increased CAT activity revealing that the organism was facing oxidative stress due to the bacterial co-infection (Künili et al. 2021). These reports show that pathogenic bacteria could induce high expression of CAT in mussel tissues and CAT is a typical inducible protein, which plays an important immune defence role on the invasion of bacteria (Bao et al. 2018), and highlights CAT as a biomarker when concerned with prevention of oxidative stress damage.
Glutathione peroxidases (GPOx)
In the mitochondria (and sometimes in the cytosol), GPOx reduces H2O2 to water, and lipid peroxides to their corresponding alcohols. Specifically, this enzyme inhibits the lipid peroxidation process and thus protects cells from the harmful effects of oxidation (Vidal-Liñán et al. 2015). Currently, there are only two studies which report GPOX activity in mussels (P. nobilis and Bathymodiolus azoricus), mainly focused on detection of their presence or variation in response to Vibrio spp. infection. In the first study, GPox activity increased significantly in the gills and digestive glands of Pinna nobilis, measured by low-cost spectrometric assay following co-infection of Haplosporidium pinnae and multiple Vibrio infections (Künili et al. 2021). In the second study, glutathione peroxidase I (GPOx1) was up-regulated in digestive gland and gill tissues after mussels were challenged with V. alginolyticus, V. anguillarum, and V. splendidus suspensions (Martins et al. 2014). These studies reveal increased GPOX levels, which may be associated with a decrease in the harm caused by H2O2 generated after invasion by the pathogen (Ren et al. 2009). To date, discerning the causes of variability in GPOX levels has been challenging due to susceptibility to numerous biotic factors, such as seasonal variation (Martins et al. 2014). Future studies must consider measuring superoxide radicals, hydrogen peroxide concentration, and GPOX activity in mussels to comprehend the impact of Vibrio spp. infections.
Lipid peroxidation (LPOX)
LPOX occurs as a result of oxidation of polyunsaturated fatty acids due to oxidative stress, and can be quantified by measuring the LPOx degradation products, such as lysosomal lipofuscin, aldehydes, acetone, and malondialdehyde (MDA) (Kroon et al. 2017). Only a few studies have identified LPOX activity in mussels as indicator of oxidative stress caused by Vibrio spp. pathogens. In one example, it was found that both V. splendidus and V. anguillarum induced the accumulation of lysosomal lipofuscin, an end-product of lipid peroxidation, in the digestive gland of M. galloprovincialis (Canesi et al. 2010). Another study showed that freshwater mussels (Elliptio complanata) which were co-exposed to municipal effluents and V. anguillarum infection had increased level of MDA-TBA2 adducts [thiobarturic acid reactants (TBARS)] in the digestive glands (François et al. 2015). MDA levels were also observed to be higher in the gills of P. canaliculus under 24 and 120 h pathogenic bacterial (P. swingsii) challenge at different temperatures (16 °C and 24 °C) (Azizan et al. 2023b). These findings suggest dysfunction of the antioxidant system in mussels, as a measure of lipid peroxide performance as a consequence of bacterial infection. Lipids can be influenced by various abiotic- and biotic-driven differences in biomarker response. Therefore, elevated LPOx levels may not exclusively indicate Vibrio-related stress.
Reactive oxygen species (ROS)
ROS production is necessary for the elimination of microorganisms, such as viruses, bacteria, fungi, and protozoa within the host, via the activation of respiratory burst (Deretic et al. 2013). ROS over-production tends to induce oxidative damage to various cellular components, such as lipids, proteins, and nucleic acids. In several studies, where mussels were infected with Vibrio spp., increased ROS was reported (Table S2). The bacterium V. splendidus enhanced significantly the up-regulation of ROS in haemolymph samples of M. edulis (Tanguy et al. 2013a). Increased ROS in M. galloprovincialis were reported following infected with V. anguillarum, V. alginolyticus, and V. splendidus LGP32 (García-García et al. 2008; Costa et al. 2009; Wang et al. 2013b; Sendra et al. 2020). P. canaliculus also showed increased ROS production in response to Vibrio spp. DO1 (V. coralliilyticus/neptunius–like isolate) infections (Nguyen et al. 2018b; Ericson et al. 2022). From these papers, it can be concluded that Vibrio spp. infections trigger oxidative burst, leading to the rapid production of ROS to combat and destroy invading bacteria. Along with higher ROS comes impaired cellular functions following oxidative damage, highlighting the need to measure oxidative stress markers in tandem to characterise the cellular redox status of mussels following Vibrio spp. infections.
Defensin and lysozyme
These are important defence molecules which play a vital role in anti-oxidative stress and immune defence systems (Zhao et al. 2010a, 2010b). Both are antibacterial components that have been characterised in marine molluscs (Oliver and Fisher 1999). After challenging M. galloprovincialis with V. harveyi, mRNA expression levels of these two stress-responsive genes were significantly up-regulated in the hepatopancreases of both male and female mussels (Liu et al. 2014b). Varying responses of defensin were further detected in different V. harveyi infected tissues, with the digestive gland defensin mRNA more up-regulated than gill samples (Liu et al. 2014b). Lysozyme-like activities were also reported in M. galloprovincialis following injection with V. alginolyticus, V. aestuarianus 01/032 (V.a.), V. splendidus LGP32, V. anguillarum, and in a second study injection with Micrococcus lysodeikticus, and exposure to temperature stress (Li et al. 2009; Balbi et al. 2013; Laith et al. 2021). Up-regulated defensin and low levels of lysozyme were also found in 2-day-old D-larvae of M. edulis following a 48-h Vibrio challenge, supporting involvement of these compounds in immune functions in the early development phase of mussels (Van Hung et al. 2019). Both defensin and lysozyme are antibacterial components that have been characterised in marine molluscs (Liu et al. 2013) and can serve as indicators of immune stress induced by Vibrio spp. infections. Additionally, these biomarkers have shown consistent changes due to a number of Vibrio species infections (Van Hung et al. 2019), making it a potential biomarker for assessing mussel health.
Metallothioneins (MTs)
MTs are highly conserved, low-molecular-weight, cysteine-rich non-inducible enzymatic proteins that participate in metabolism of essential metals, their detoxification, and scavenging of oxyradicals (Sigel et al. 2015). Aquatic pathogens or oxidative stress–producing chemicals trigger the expression of MT as a defence response. Canesi et al. (2010) reported that mussels (M. galloprovincialis) infected with both V. splendidus and V. anguillarum resulted in oxidative and immune stress with significant over-expression of MTs involved in antioxidant and immune functions, measured by molecular techniques. In a study by Ge et al. (2020), after infection by V. parahemolyticus, the expression of Mytilus coruscus metallothionein (McMT) in hepatopancreas tissue increased until 24 h later, and then showed a slow decline. Bacterial endotoxins, as produced by Vibrio spp., are known to generate oxygen-derived products and inflammation within mussels, with induced expression of MT to follow (Table S2). MT are increased for cellular protection, scavenging free radicals and acting as anti-inflammatory mediators (Ge et al. 2020).
Metabolites, genes, and stress proteins
Various metabolomic studies have identified metabolic changes associated with both endogenous (effect biomarkers) and exogenous (exposure biomarkers) metabolites during disease progression and Vibrio spp. infection (Table S3). Vibrio spp. exposure typically leads to significant disruptions in the host’s energy metabolism, osmotic regulation, oxidative stress responses, signalling pathways, and respiratory mechanisms. Recent metabolomic investigations of mussel immune responses to Vibrio spp. infections have revealed that specific metabolite biomarkers vary depending on factors such as the Vibrio strain (Ji et al. 2013; Liu et al. 2014b, c; Nguyen et al. 2018a), the different tissues (Nguyen et al. 2019b; Liu et al. 2014a), the sex of the host (Ellis et al. 2014; Liu et al. 2014c; Nguyen et al. 2018a), and environmental conditions (e.g. thermal stress, pollution) (François et al. 2015; Frizzo et al. 2021). Some of the affected metabolites in mussels following Vibrio spp. infections include glucose, glycine, betaine, homarine, threonine, alanine, aspartate, taurine, succinic acid, itaconic acid, and branched-chain amino acids (BCAAs). Amino acids, in particular, are crucial nutrients for pathogens and host defence mechanisms (Ren et al. 2018).
Vibrio spp. infections in mussels have been associated with significant alterations in amino acid and fatty acid metabolism, as well as protein synthesis related to immune functions. For instance, in Perna canaliculus infected with Vibrio sp. DO1 (V. coralliilyticus/neptunius–like isolate) (Nguyen et al. 2018a, 2018b, 2019b), itaconic acid was proposed as a potential biomarker, acting as an antimicrobial metabolite and supporting mussel immune functions (Nguyen and Alfaro 2019). The osmolyte function of amino acids in molluscs has been demonstrated where increases of hypotaurine, homarine, and glycine, and decreases dimethylglycine, taurine, and betaine were linked to osmotic stress induced by V. harveyi in M. galloprovincialis (Liu et al. 2014b). Also, alterations in metabolites such as glutamine, succinate, aspartate, glucose, ATP, homarine, and tyrosine indicated that V. anguillarum could induce disturbances in osmotic regulation and energy metabolism, along with cellular injury in M. galloprovincialis (Wu et al. 2013). Azizan et al. (2023c) found the haemolymph metabolome of mussels (Perna canaliculus) exposed to single bacterial species (Vibrio mediterranei, Photobacterium swingsii) and co-infection (a mixture of V. mediterranei and P. swingsii) showed response changes largely within energy metabolism. Mussels infected with V. mediterranei exhibited increased metabolites linked to the glutathione pathway, branched-chain amino acids, and others over time, supporting structural functions. When interpreting metabolite data related to Vibrio spp. infections, it is crucial to consider that detected metabolites may originate from the mussel response and bacterial metabolites. Moreover, metabolite profiles can be influenced by factors such as age, sex, and environmental conditions (Barber et al. 2019).
Fatty acids (lipids) are important for energy storage, development, growth, and survival, and have been classified as potential physiological indicators for evaluating pathogen (Vibrio spp.)–induced mortalities (Su et al. 2004), as seen in Perna canaliculus (Nguyen and Alfaro 2020; Ericson et al. 2022). Decreased concentrations of fatty acids, tridecanoic acid, myristic acid, palmitic acid, and linoleic acid were found in the haemolymph of P. canaliculus infected with Vibrio sp. DO1 potentially due to utilisation of energy sources required to stimulate an immune response against the infection (Nguyen et al. 2018a, 2018b, 2019b). In contrast, analyses of P. canaliculus hepatopancreases showed increases in the free fatty acids, as increased fatty acid synthesis was required to meet energy demands following infection (Nguyen et al. 2019b). The results listed in Table S3 are good starting points for summarising fatty acid biomarkers in mussels in response to Vibrio spp. infection, yet it should be considered that fatty acids are not exclusive to an organism (De Carvalho and Caramujo 2018), and are highly affected by diet (Zhukova 2019) and season (Silva et al. 2021), making the responses seen by mussels highly affected by various factors, not only the bacterial infection.
Transcriptome studies are crucial for understanding organism responses to diseases, stressors, and interventions at the molecular level, aiding in disease mechanisms, biomarker discovery, and therapeutic targets. RNA sequencing (RNA-seq) is commonly used, providing comprehensive insights into gene expression, alternative splicing, and novel transcripts, essential for understanding gene regulation and functional roles. In the Dong et al. (2017) study, haemocyte transcriptomes of Mytilus coruscus before and after V. alginnolyficus infection were analysed, revealing up- and down-regulated genes associated with immune pathways such as Toll-like receptor signalling and apoptosis.
Tanguy et al. (2018) investigated early immune responses in Mytilus edulis haemocytes challenged with V. splendidus LGP32, identifying various immune-related sequences and up-regulated transcripts associated with immune pathways. Li et al. (2024) focused on transcriptomic responses in M. unguiculatus infected with V. alginolyticus, revealing up-regulation of defence mechanisms and adaptive responses to pathogen invasion, including altered taste transduction pathways and increased expression of antimicrobial peptides. These findings shed light on Mytilus spp. innate immunity, potentially guiding strategies for disease management and sustainable culture practices.
Stress proteins protect and regenerate cells in response to stress and harmful conditions (Kroon et al. 2017). Generally, this group of proteins consists of heat shock proteins (HSPs), which responds to heat and other physical and chemical stressors, the glucose-regulated proteins (GRPs), which respond to oxygen or glucose deficiency, and the stressor-specific stress proteins, which include heme oxygenase proteins (Fabbri et al. 2008). HSPs, like HSP60, HSP70, and HSP90, are often used as general markers of stress and can respond to a wide range of environmental stresses. In studies of Vibrio infections in mussels, HSP70 family proteins are commonly examined, and their expression increases as the infection progresses. Two studies using real-time polymerase chain reaction (PCR) assays of samples from Mediterranean mussels exposed to V. anguillarum, observed that as infection progresses, this bacterium can produce more pronounced expression of HSP70 in mussel tissues (Cellura et al. 2006, 2007). In another study, the immune gene expressions in two mussel species (B. azoricus and M. galloprovincialis) were investigated after infection with Vibrio alginolyticus, V. anguillarum, and V. splendidus and a mixture of these Vibrio suspensions (Martins et al. 2014). The authors observed that the expression of HSP70 can vary between mussel species due to differences in DNA sequences and transcription timescales (Martins et al. 2014). Additionally, the conserved molecular chaperone, heat shock protein 90 (HSP90) involved in cell cycle control, organism development, and the regulation of cytosolic proteins, has been noted as a biomarker for infections of V. parahemolyticus in Mytilus coruscus (Liu et al. 2016). In M. galloprovincialis infected with V. harveyi, increased HSP90 was seen in gill and digestive gland samples, suggesting oxidative and immune stress responses due to the infection (Liu et al. 2014b). The same researchers also studied gender-specific metabolic changes in mussels challenged with V. harveyi (Liu et al. 2014c), with no differences detected in HSP90 between male and female mussels. Most recently, Castillo et al. (2017) investigated how ocean acidification (OA) and bacterial infection impact hsp70 and hsp90 gene expression in M. chilensis. The results revealed that the expression of the hsp90 gene is the only one that differs after exposure to elevated pCO2, with a significant down-regulation observed. Results of Hernroth et al. (2016) observed a response of M. edulis mussels under controlled CO2 conditions and exposure to V. parahaemolyticus, V. tubiashii, V. splendidus, and V. alginolyticus infections. They found that HSP70, along with several other peptides/proteins involved in marine bivalve immunity, was detected in the extracts, suggesting modulation of stress-related proteins due to hypercapnic acidosis following OA. HSPs may be a valuable biomarker as HSP genes and proteins are present in all organisms, yet caution should be applied when ascribing results to bacterial outcomes, as the expression of HSPs differs based on species. Within the same species, different tissues show different expression levels, sex and age plays a role in expression and the method of detection can also influence the results (De Jong et al. 2008).
Haematological and immunological parameters
Haematological parameters provide insight into the health and physiology of an organism (Kroon et al. 2017). These parameters can be measured through techniques such as cell counting, viability assessments, morphology examination, phagocytic activity assays, reactive oxygen species production, enzyme assays, flow cytometry, gene expression analysis, proteomics, metabolomics, and electron microscopy. In particular, haemocytes play a vital role in the innate immune system, by acting as defensive cells through tissue infiltration, aggregation, encapsulation, cytotoxic reactions, and phagocytosis of foreign particles (Rolton and Ragg 2020). Haemocytes of mussels can kill Vibrio spp. through phagocytosis, production of highly reactive molecules, as well as a number of antimicrobial peptides (AMPs), hydrolytic enzymes (Destoumieux-Garzón et al. 2020), and other immunological parameters (Azizan et al. 2023d), which will be discussed below and presented in Table S4.
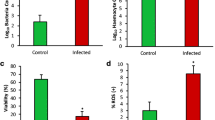
Total haemocyte count (THC)
THC is likely the most popular measure of haemocyte immune responses when investigating the effect of pathogens on mussels (Dalzochio et al. 2016). Several studies have shown that THC is affected by Vibrio spp. infections, showing patterns of increased and decreased THC responses. However, it should be kept in mind that THC results are variable depending on the nature of injected bacteria, the bivalve species, and the handling of the sample. For example, quantitative differences were reported when injecting M. galloprovincialis with either living or heat-killed V. splendidus LGP32 and V. anguillarum (Ciacci et al. 2009; Costa et al. 2009; Parisi et al. 2019). In P. canaliculus, increased THC was seen following 6 h of a Vibrio spp. infection (Nguyen et al. 2019b) and 3 days after exposure to Photobacterium swingsii infection (Azizan et al. 2023a), but decreased with combined P. swingsii infection and temperature stress (Azizan et al. 2023b). In a study by Laith et al. (2021) on P. viridis, the THC at the beginning of the experiment was higher than at other time points, suggesting that the challenged group did not regain natural immunity after 120 h of infection with V. alginolyticus. A decrease in THC was seen in M. edulis exposed to manganese (Mn) and when inoculated with the bacterium V. parahaemolyticus, likely contributing to the impaired haematopoiesis ability to combat the bacteria while further reinforcing the haemocytopenia (Oweson and Hernroth 2009). The monitoring of THC in infected mussels requires large sample numbers and rapid procedures when establishing biomarker values. As a result, the measurement of levels of haemolymph cells (haemocytes) can serve as a good biomarker as it is relatively easy to perform from the posterior adductor muscle (Eggermont et al. 2020), and more importantly, haemolymph sampling is non-destructive and can be performed on the same individual to increase experimental and statistical flexibility (Ford 1986).
Haemocyte characteristics
Haemocyte populations (granular, semi-granular, or agranular cells), often assessed through flow cytometry and visual observations also play an important role in bivalve susceptibility to pathogens (Destoumieux-Garzón et al. 2020). This was seen in M. galloprovincialis, where granular and semi-granular cells, harboured phagocytic activity, produced ROS and nitric oxide (NO) after being challenged with V. splendidus LGP32 and V. anguillarum (Ciacci et al. 2009). Furthermore, that study provided evidence that haemocyte populations decrease after Vibrio spp. infection, but restore after a recovery period (Ciacci et al. 2009). Other studies provide evidence that V. splendidus LGP32 and V. anguillarum infections cause the loss of haemocyte cell adhesion in mussels (Ciacci et al. 2010; Tanguy et al. 2013a), and also influence haemocyte motility (Sendra et al. 2020). Caution should be applied when aiming to use haemocytes as biomarkers, as haemocytes are involved in a wide range of physiological functions that may not be related to defence (e.g. metabolite transport, digestion, shell growth and repair, and repair of damaged tissue), therefore making them less specific as immunomarkers for Vibrio spp. responses (Dolar et al. 2020).
Pathogen-associated molecular patterns (PAMPs)
Haemocytes can also recognise pattern recognition receptors (PRRs), which are proteins capable of recognising molecules frequently found in pathogens [pathogen-associated molecular patterns (PAMPs)] to activate intracellular signalling pathways to finally trigger the synthesis of antimicrobial effectors. Bacterial challenges carried out with different Vibrio strains (V. alginolyticus, V. anguillarum, and V. splendidus) revealed distinct patterns of gene expression for most of the immune genes tested in Bathymodiolus azoricus and M. galloprovincialis (Martins et al. 2014). From that study, differences were found in two immune recognition genes [galectins and peptidoglycan recognition proteins (PGRPs)], supporting different recognition mechanisms and capacity to counteract bacterial challenges in the mussels (Martins et al. 2014). In addition, Toll-like receptors (TLRs) can also recognise PAMPs and initiate corresponding signalling transduction pathways via intermediators to activate a wide range of downstream immune factors, which eliminate invading pathogens (Saco et al. 2020). Several TLR genes that recognise V. anguillarum and V. parahaemolyticus were identified and up-regulated in Hyriopsis cumingii (Ren et al. 2013; Zhang et al. 2017) and M. coruscus (Xu et al. 2018). Although PAMPs may have limitations in terms of specificity, sensitivity, and the need for standardization, they still hold potential for being regularly used in disease monitoring and the development of vaccines. Areas of interest in PAMPs research include exploring novel PAMPs, investigating their role in non-infectious diseases, and understanding how the microbiome influences PAMPs and disease.
Antimicrobial peptides
Mussels are also rich in antimicrobial peptides (AMPs), such as defensin, mytilin, mytichitin-CB, myticusin-1, myticin, and mytimycin, which play a key role in immune defence processes (Rosani et al. 2011; Liao et al. 2013; Qin et al. 2014; Tanguy et al. 2018; Bouallegui 2019; Van Hung et al. 2019; Sendra et al. 2020). A majority of the AMPs identified from Mytilus spp. are cysteine-rich subgroups, except for the recently identified linear/α-helical family (Leoni et al. 2017; Bouallegui 2019). Although AMPs have been discovered mainly in Mytilus spp., the information on AMPs in Perna spp. remains largely unknown. As AMPs exhibit antibacterial activity against Vibrio spp., including V. splendidus, these peptides appear to have an important role in the immune defence of mussels against bacterial infection. However, further research is needed to fully validate the role of AMPs in the immune defence against Vibrio spp. and other bacterial pathogens in Perna spp. This is essential for advancing scientific knowledge, developing effective interventions, managing disease outbreaks, and conserving ecosystem health.
Phenoloxidase
Besides the AMP gene expression, another critical component of the immune system of bivalves, namely phenoloxidase activity (PO), has been examined in Vibrio sp. challenges (Luna-Acosta et al. 2017). An increase in PO detected in haemolymph, following V. anguillarum, V. harveyi, and V. coralliilyticus infections, has been seen in Mytilus edulis, Perna viridis, and Hyriopsis cumingii (Ren et al. 2013; Puspita and Hutabarat 2015; Van Hung et al. 2019). Typically, PO is a by-product of a complex cascade of reactions that includes melanization, wound healing, phagocytosis, and pathogen killing (Gerdol et al. 2018), making PO a good indicator of pathogen defence mechanisms in mussels (Muznebin et al. 2022) and potentially a useful biomarker for Vibrio spp. infections.
Lysosomal membrane stabilisation (LMS)
Lysosomal responses are promising, biomarkers of exposure, particularly to environmental perturbations and pathogen infections (Moore et al. 2006). Based on transmission electron microscopy (TEM) analyses of M. edulis, haemocytes infected with V. tapetis showed rapid ultracellular damage and lysosomal fusion, with morphological changes at the plasma membrane and cytoplasmic levels. However, no Vibrio spp. internalisation was observed in that study, indicating no intracellular degradation of bacteria (Balbi et al. 2019). Haemocyte lysosomal membrane stability (LMS) was evaluated as a marker of cellular stress in mussels induced by bacterial challenges (Balbi et al. 2019). Measured in both small and large granulocytes of M. galloprovincialis, cellular stress was confirmed following V. cholerae infection (Canesi et al. 2005). Furthermore, the measurement of LMS has been shown to be effective for comparing the cellular stress induced in vivo by heat-killed Gram ( +) and Gram ( −) bacteria, including V. anguillarum and V. splendidus (Ciacci et al. 2009). In a study by Canesi et al. (2010), it was demonstrated that both V. splendidus and V. anguillarum caused a decrease in the level of LMS in the digestive gland of M. galloprovincialis at all time points post-infection measured using neutral red retention (NRR) assay, which is also consistent with previous findings for V. cholerae (Canesi et al. 2005). Activation of different immune signalling pathways, including p38 MAPK, PKC, and PI-3 kinase, has been linked to decreases in LMS. The comparable impact on haemocyte LMS by both Vibrios could be explained by the fact that the NRR assay depends on adherent granular haemocytes, lacking differentiation among granulocyte sub-populations (Ciacci et al. 2010). Therefore, improving the assay to differentiate among granulocyte sub-populations would enhance its accuracy and effectiveness.
Apoptotic markers
The physiological and irreversible process of programmed cell death characterised by the fragmentation of DNA (Kroon et al. 2017) is implicated in many processes, such as tissue and organ development, homeostasis, and immune defence (Sokolova 2009; Gerdol et al. 2018). From the literature investigated, all studies reported, in Table S4, showed an increase in apoptosis in five different mussel species exposed to V. splendidus LGP32, V. anguillarum, V. aestuarianus 01/032, and other Vibrio species in field and laboratory studies (Tanguy et al. 2018; Parisi et al. 2019; Auguste et al. 2020; Lattos et al. 2021a). As such, apoptosis may be an appropriate biomarker, but further species-specific work is required to determine its suitability to assess mussel health. The proliferating cell nuclear antigen (PCNA), caspases (e.g. CASP8, CASP3, CASP7, and CASP6), and other components, such as the Fas-associated death domain (FADD), are commonly used biomarkers of apoptosis, as they are involved in DNA replication and in the activation and implementation of the apoptotic programme. These apoptotic markers were reported to be significantly up-regulated in M. edulis, M. galloprovincialis, B. azoricus, and Pinna nobilis following infection with V. splendidus LGP32, V. anguillarum, and other Vibrio species (Tanguy et al. 2013b; Parisi et al. 2019; Lattos et al. 2021a). In contrast, a study on haemocyte apoptosis (caspase 3/7) in P. canaliculus revealed infection with Vibrio sp. DO1 did not result in a change in the proportion of haemocytes producing ROS with temperature or injection treatments, nor induced apoptosis in mussels (Ericson et al. 2022). The research implies that apoptosis may be a sensitive biomarker tool to assess cellular stress in mussels, as well as consideration of potential confounding factors that may influence apoptotic processes in mussel cells.
Autophagic markers
Molecular autophagic markers have also been used in molluscan cells to identify cell injury and to remove unnecessary or dysfunctional components caused by a variety of environmental stressors and Vibrio sp. infections (Balbi et al. 2018). The autophagic process is a key regulator of innate immunity as it helps clear pathogens and regulates inflammation (Balbi et al. 2018). V. tapetis infection in M. galloprovincialis haemocytes led to rapid formation of large autophagosomes, and subsequent analysis showed increased LC3-II (microtubule-associated protein 1A/1B-light chain 3-II) expression and decreased levels of phosphorylated mammalian targets of rapamycin (mTor) and SQSTM1/p62 (sequestosome 1) (Balbi et al. 2018). Similarly, V. splendidus LGP32 induced PI3K (Phosphoinositide 3-kinase), Akt (Protein kinase B), and mTOR (Mammalian target of rapamycin) pathways in M. galloprovincialis haemocytes are part of a complex network associated with the JAK-STAT (Janus kinase-signal transducer and activator of transcription) signalling pathway that participates in immune response and the regulation of cell proliferation, autophagy, and apoptosis (Rey-Campos et al. 2019a). In Pinna nobilis co-infected with Vibrio bacteria and Haplosporidian parasites, autophagic markers, ubiquitin was increased, LC3 was unaffected, and SQSTM1/p62 was more affected by the Haplosporidian parasite (Lattos et al. 2021b). The use of autophagic markers in molluscan cells has provided insights into cell injury, environmental stress responses, and pathogen infections, although constraints in marker specificity and interpretation remain, promising for targeted therapeutic interventions.
Omics
Immunological biomarkers related to illness can be effectively measured using omics tools, such as transcriptomics, proteomics, and metabolomics (Haddad et al., 2018). Transcriptional biomarkers linked to mussel health have identified a significant impact of a wide range of Vibrio spp. (e.g. V. splendidus, V. parahemolyticus, V. alginolyticus, V. alginnolyficus, V anguillarum) in response to stress, redox balance, metabolism, apoptotic processes, and immunity (Venier et al. 2011; Martins et al. 2014; Moreira et al. 2015; Dong et al. 2015, 2017; Tanguy et al. 2018; Rey-Campos et al. 2019a, b; Lori et al. 2020; Saco et al. 2020; Chen et al. 2021; Yang et al. 2021; Romero et al. 2022). Likewise, mussel proteomic biomarkers have allowed the detection of a network of protein changes due to oxidative stress and disturbances in energy metabolism when challenging mussels with Micrococcus luteus and V. anguillarum (Ji et al. 2013; Wu et al. 2013). Interestingly, protein biomarkers have been used to distinguish exposure and effect of Vibrio spp. infections between mussel species from very distinct natural habitats (Martins et al. 2014). As shown by Martins et al. (2014), Vibrio spp. (V. alginolyticus, V. anguillarum, or V. splendidus) infections in B. azoricus led to changes in protein sequences associated with metabolic pathways, energy production, and nutritional requirements, while infections in M. galloprovincialis affected putative protein functions linked to structural integrity, cellular maintenance, and signalling mechanisms. Recent proteomic analysis of Perna perna hepatopancreas challenged by Escherichia coli, Salmonella enterica, and Vibrio parahaemolyticus showed 597 significantly different proteins, with 343 down-regulated in VP-injected mussels, indicating immune suppression (Silva dos Santos et al. 2023). The identified proteins are key players in immune response pathways, providing insights into the interaction between the mussel immune system and bacteria, essential for coastal marine resource management. Overall, the application of proteomic studies to detect changes in protein biomarkers in mussel species exposed to Vibrio spp. could help to identify proteins involved in host–pathogen interactions. This approach enhances sensitivity in detecting stress responses to the studied Vibrio strains. However, the application of this protein biomarker needs substantial development to provide clear connections between other environmental stressors, exposure, changes in peptide abundances, and species specificity.
Biomarkers of effect
Biomarkers of effect relate to measurable alterations within tissues or body fluids of an organism, often linked to possible health impairment or disease (Dalzochio et al. 2016). For mussels infected with Vibrio spp., this most often links to histological assessments of mussel tissues by light, fluorescence, and/or electron microscopy as markers to assess the physical state (e.g. growth and health indices; the occurrence of vibriosis/cell necrosis, parasite, reproduction, and metabolic status) (Table S5) (Stentiford et al. 2005; Bignell et al. 2008). While histopathological methods of monitoring body health are extremely efficient, they only provide a morphological picture of alterations, which is discernible only at the final stages of pathology and cannot always define physiological disruption (Kumeiko et al. 2018). Additionally, they remain limited biomarkers based on only a few species of mollusc and lack sufficient detail for effective quantitative analyses. Often, semi-quantitative approaches are the preferred option, although they require well-trained researchers and extensive background knowledge (Costa et al. 2013). Histopathology of a range of mussel tissues following V. splendidus, Vibrio harveyi, V. coralliilyticus, V. tubiashii, V. mediterranei, and V. hispanicus, V. alginolyticus, V. splendidus ME9, and V. anguillarum NB10 exposure has been shown to result in an increase in histological alterations, including tissue necrosis, haemocyte infiltrations, and bacterial/parasitic colonisation when mussels are exposed to Vibrio spp., both in the laboratory and in the field. These effects were seen in M. edulis (Ben Cheikh et al. 2017; Wang et al. 2021), M. galloprovincialis (Parisi et al. 2019; Battistini et al. 2020), P. viridis (Laith et al. 2021), P. canaliculus (Kesarcodi-Watson et al. 2009a; Azizan et al. 2023a), and Pinna nobilis (Künili et al. 2021). Interestingly, all species at adult stage showed abnormalities to haemocytes and tissues (e.g. digestive gland, mantle, and gills) in response to Vibrio spp. infection (Ruiz et al. 2013; Ben Cheikh et al. 2017; Parisi et al. 2019; Battistini et al. 2020; Künili et al. 2021; Laith et al. 2021; Wang et al. 2021; Azizan et al. 2023a), while detachment of cilia cells and velum were uniquely described in mussel larvae (Kesarcodi-Watson et al. 2009a). Exposures with Vibrio spp. concentrations of 10−6 CFU/mL resulted in the vacuolisation, necrosis, and tissue separations in hepatopancreas and digestive glands, sloughing of tubule epithelial cells, karyomegaly, and hyperplasia (Laith et al. 2021). To complement histopathological findings, immunohistochemistry (IHC) can be used as an additional biomarker tool to examine the expression and localisation of immune-related proteins within the tissues of infected mussels that are known to be involved in mussel immune responses to Vibrio spp. infections. For example, researchers may use antibodies specific to proteins, such as the water channel proteins aquaporins and the Na+/K+ ATPase biomarker of osmoregulatory processes and the proliferating cell nuclear antigen, PCNA, and caspase-3 as biomarkers of apoptosis to detect their expression within the tissues (Parisi et al. 2019). Using IHC, Ben Cheikh et al. (2017) monitored the haemocytes exposed to V. splendidus–related strains by immunolabeling bacterial-like cells with full-length green fluorescent proteins (GFPs). The prominent changes in tissue structure seen in most studies investigated suggest that histopathological and IHC markers are suitable biomarkers for providing insight into the effect of Vibrio spp. on mussel tissues and cell health. Taken together, descriptive data obtained from histology and IHC assessment provide additional information to support other biomarkers in integrated data monitoring programs. These markers may be used as a tool for providing supporting information for measures (often combined with other biomarkers) that aim to assess historical exposure to, or effects of, pathogens (Bignell et al. 2008).
Biomarkers of susceptibility
Susceptibility biomarkers describe an organism’s capacity, based on genetic factors and alterations in receptors, to respond to exposure following contact with Vibrio spp. These types of biomarkers have been mostly investigated as bivalve immunomarkers and research is still evolving with regard to application to mussels. It has been shown that mussel susceptibility to specific Vibrio spp. (e.g. Splendidus clade, oyster pathogen) can be determined by host physiology (Charles et al. 2020b). Also, possible links between mussel and oyster susceptibility to Vibrio spp. infection and their microbiome profiles were made based on 16S rRNA gene–based analysis (Vezzulli et al. 2018). Moreover, studies have found the genus Vibrio (potential pathogenic species such as V. aestuarianus) constitutes a greater proportion of the microbiota in Crassostrea gigas compared to M. galloprovincialis, suggesting that oysters may offer better host environments (i.e. host-species intrinsic factors) than mussels for these bacteria to thrive. In addition, studies have suggested that shellfish innate immune memory or “immune priming” may help molluscs respond to pathogen infections (Rey-Campos et al. 2019a). Therefore, exposure to non-lethal doses of pathogens could provide molluscs with the ability to launch stronger immune responses during later phases of infection, potentially protecting mussels against Vibrio spp. infections. Exposure to V. splendidus in M. galloprovincialis mussels primed immune responses and induced changes in the expression of various genes, thereby enhancing the mussels’ ability to cope with infections (Rey-Campos et al. 2019a). Interestingly, successes in developing resistance to ostreid herpesvirus-1 (OsHV-1) are now being applied for resistance to Vibrio bacteria in oyster C. gigas, which have been implicated in summer mortality (de Lorgeril et al. 2018; Zhai et al. 2021). This work has demonstrated that Vibrio spp. resistance can be enhanced by genetic improvement and now can potentially be translated to mussels.
Constraints and potential of biomarker-based approaches
Despite the advancements in measurable responses to assess the relative condition of mussels following Vibrio spp. infections, connections between biomarker responses and baseline conditions (indicative of good health) are still needed. According to Waller and Cope (2019), establishing these connections involves three key steps. The first step involves selecting biomarkers or measures that yield the most useful information for mussel health assessment. Next, it is essential to determine what constitutes “healthy” or “normal” conditions in mussels, requiring characterisation of baseline health across different species, life stages, and geographic regions. The final step focuses on assessing changes in health characteristics from baselines due to disease, microbial outbreaks, or environmental stressors (Waller and Cope 2019). These steps aim to build a comprehensive biomarker database. Ideally, a biomarker would be transformed into a point-of-use test that mussel farmers, scientists, and decision-makers could effectively use in the field to monitor Vibrio spp. Ultimately, the high-throughput platforms and laborious sample preparation methods should be integrated into an affordable, hand-held tool that is easy to use and interpret by anyone (Pinu et al. 2019). Such devices are applied in other areas and can include lateral flow devices, dipstick approaches, or electrochemical detection (Trivedi et al. 2017). Yet, establishing reference intervals for test parameters under various conditions is crucial for developing reliable tools and interpreting results accurately (Geffré et al. 2009). Once a suspected biomarker with associated conditions has been identified, validation is required before it can truly be used as a biomarker (Hey et al. 2019). There have been many factors that have hindered biomarker validation, such as failure to disclose methodologies or replicate results, not having a clear plan for the biomarkers intended use, not understanding its form and function, not selecting adequate controls, mismatches with age, gender, tissue infection, etc. (Holland 2016).
Several variables (i.e. age, season, sex) can also influence biomarker reference intervals, necessitating a detailed description of the organism under investigation (Gutierrez et al. 2020). As summarised above, many of the biomarkers exhibited variable responses due to the mussel species, the type of tissue being analysed, sampling location and time, diet, exposure to chemical contaminants, temperature stress, life history, gender, and more (Fig. 4) (Moore et al. 2007). The natural ecosystem variations over time make it a substantial challenge to determine disturbances at an ecological level. The presence of other biological contaminants, atmospheric deposition of chemical contaminants, and global climate change likely mean there are no truly undisturbed ecosystems (Oliver and Fisher 1999; Hook et al. 2014). It is reasonable, however, for the investigated biomarker to be linked to adverse effects at the organism level (particularly effects biomarkers). Apart from biological variability, analytical variables can also influence the outcome of a biomarker. To ensure accurate and reproducible data among laboratories, it is necessary to create a standard protocol for sample collection, processing, and storage, as well as to verify the sensitivity and specificity of the biomarkers used (Moore et al. 2007). These variables are best confounded when collecting large populations of mussels representative of healthy and diseased populations (Moore et al. 2007; Hook et al. 2014; Brosset et al. 2021; Zare Jeddi et al. 2021). Therefore, better establishment of baseline concentrations of the population under investigation is essential (Oliver and Fisher 1999; Brosset et al. 2021), while also considering repeated evaluations from the same populations and locations, as well as the investigation of the environmental factors that affect the mussel traits (Oliver and Fisher 1999). In addition, more data relating to the mussel and environment should be reported with the measured outcomes to search for similarities and changes among studies.

Adapted from Moore et al. (2007)
Potential sources of variation identified in mussel-Vibrio studies for biomarker validation.
A daunting challenge in the development of specific biomarkers for mussels-vibriosis comes to play when aiming to separate differences due to single and multiple microbial infections (Oliver and Fisher 1999; Newton and Cope 2006). For instance, Dolar et al. (2020) reported that bacterial and viral infections had different immune responses in the crustacean, Porcellio scaber. The authors showed that animals with a viral infection had significantly higher total haemocyte counts and higher proportions of three types of haemocytes compared to animals with bacterial infection. Research in microcosm experiments or exposure studies with host organisms is essential for evaluating their responses to a particular Vibrio pathogen or mixture of a polymicrobial infection. This research is crucial for developing biomarkers tailored to specific infectious agents (Waller and Cope 2019). Rapid and straightforward methods for detecting Vibrio spp. (or other infectious agents) include molecular techniques such as portable PCR devices or Loop-Mediated Isothermal Amplification (LAMP) tests (Augustine et al. 2020). These methods swiftly detect and measure Vibrio DNA or RNA in the field, enabling prompt responses to outbreaks or environmental contamination, minimising risks, and improving management strategies. Additionally, enzyme-linked immunosorbent assays (ELISAs), immunochromatographic tests, and lateral flow assays are commonly used for detecting Vibrio antigens, antibodies, or other biomarkers response, offering simplicity and speed in identifying exposure to these pathogens and understanding the host response to them (Stewart et al. 2017).
Compared to individual platforms alone, integrating data from various platforms could identify a biomarker panel with better sensitivity and specificity (Hook et al. 2014). There are a variety of multi-biomarkers that can be used together as a model for a biomonitoring programme, including oxidative stress, immunological markers, pathological alteration, metabolites, genes, and proteins (as suggested in Table 1 based on evidence presented in Tables S1 - S5). Several studies have shown a practical application of the use of multi-biomarkers in management plans to indicate the presence of pathogenic Vibrio spp. and their detrimental effects on mussels. Matozzo et al. (2018) utilised advanced statistical analysis (MANOVA analysis) on an array of effect biomarker responses in haemocyte and in gills and digestive gland, alongside pathological alterations, and microbiological analyses, to correlate both exposure and effects of stressors. When integrated, these datasets have been successfully used to forecast stressful conditions, routinely measured, bimonthly, from four rearing sites in the Gulf of La Spezia. It has been suggested that the health status of farmed mussels (M. galloprovincialis) deteriorates with time, resulting in them becoming susceptible to pathogenic bacteria, viruses, and protozoa (Matozzo et al. 2018). Shellfish management could be made more proactive by following this type of study, which can inform managers on population dynamics, provide early-warning signals, and provide information about what is driving the changes.
Diagnosis is another important area in mussel health management, that could potentially benefit from omics and bioinformatics technologies. A rapid and accurate diagnosis of pathogens is crucial for the management of diseases and the study of infections and immune responses in hosts. Most recently, it was observed that dual RNA-seq, in which transcriptional biomarkers in both the pathogen and the host were analysed simultaneously can provide direct insight into the host–pathogen interaction as opposed to traditional approaches (e.g. microarrays or reverse transcription PCR). While this biomarker technique is still in its infancy and has yet to be investigated in Vibrio-mussel interactions, it has the potential to define and refine host-related factors related to pathogen virulence determinants, thereby identifying biomarkers relevant to pathogen-specific illness and disease outcomes (Westermann et al. 2012; Nuss et al. 2017).
Conclusions
Mussel biomarkers have become increasingly important in assessing the impact of bacteriological contamination on aquatic environments. In this review, we provide an overview of oxidative stress, haematological and immunological, metabolite and protein, histological and immunohistochemical biomarkers to compile and discuss the available literature regarding the use of mussel species to indicate the effect of different strains of Vibrio spp. The data presented in this work show that Vibrio spp. can be detrimental to mussels and can provide scientific evidence about the biological effects of bacteriological contamination on these animals. The existing knowledge on mussel biomarkers, as applied in the environmental assessments, highlights both strengths and limitations. Mussel biomarkers present notable advantages, including their capability to quantify the availability of bacteriological contaminants, integrate the multifaceted effects of polymicrobial mixtures, facilitate a mechanistic understanding of impact, and act as a surrogate indicator of physiological “health” or “harm”. Additionally, they offer utility in assessing the process of recovery from environmental stressors.
However, there are limitations to consider. Some biomarkers are non-specific and may be affected by environmental or biotic factors, while others are chemically influenced. Additionally, some biomarkers provide transient responses and could be destructive to organisms. Moreover, they may not be sufficiently sensitive at low exposure levels, and long-term data sets are often required, necessitating a significant investment.
Prioritising research efforts to validate mussel biomarkers under field conditions, biobanking baseline information, and improving the selection and deployment of relevant bioassays are essential steps in strengthening ecological risk assessment and management strategies. A single biomarker may not be sufficient to monitor the effect of mussels. However, a multiple-biomarker approach is highly recommended for optimal use in biological monitoring programs to evaluate the risk of diverse strains of Vibrio spp. The combination of biomarkers can provide a comprehensive picture and better overview for studying the effects of Vibrio spp. and to eliminate the interference of other biotic and abiotic interference in the biomarker responses. Therefore, the appropriate combination of biomarkers as listed in Table 1 is recommended to assess the effect of Vibrio spp. on mussels. Additional research should investigate how these biomarkers respond over the long term to various bacterial contaminants. This research is essential for their integration into diagnostic tools assessing the condition of the marine environment and predicting ecotoxicological risks. Incorporating field experience would also greatly enhance this effort. Moreover, it is clear that most research on mussel biomarkers has been developed under laboratory conditions and efforts are being made to validate the effectiveness of these biomarkers under field conditions to facilitate the comparison and interpretation of biomarker responses as valuable tools for ecological risk assessment. Biobanking of baseline information on the response of mussels to Vibrio spp. infections will also be required to develop effective animal health strategies and policies and increase confidence in the basis for risk management interventions. Building on this concept of mussel biomarkers to Vibrio spp. through well-reported research and validation steps will be key to strengthening efforts towards Vibrio spp. management and monitoring in mussels.
Data availability
No datasets were generated or analysed during the current study.
References
Amiard JC, Caquet T, Lagadic L (2000) Use of biomarkers for environmental quality assessment. Technique et documentation, Laviosier, Paris, pp 55–157
Amin AKMR, Tanaka M, Al-saari N, Feng G, Mino S, Ogura Y, Hayashi T, Meirelles PM, Thompson FL, Gomez-Gil B, Sawabe T, Sawabe T (2017) Thaumasiovibrio occultus gen. nov. sp. nov. and Thaumasiovibrio subtropicus sp. nov. within the family Vibrionaceae, isolated from coral reef seawater off Ishigaki Island, Japan. Syst Appl Microbiol 40:290–296
Ashrafudoulla M, Mizan MFR, Park H, Byun K-H, Lee N, Park SH, Ha S-D (2019) Genetic relationship, virulence factors, drug resistance profile and biofilm formation ability of Vibrio parahaemolyticus isolated from mussel. Front Microbiol 10:513
Auguste M, Balbi T, Ciacci C, Canonico B, Papa S, Borello A, Vezzulli L, Canesi L (2020) Shift in immune parameters after repeated exposure to nanoplastics in the marine bivalve Mytilus. Front Immunol 11:426
Augustine R, Hasan A, Das S, Ahmed R, Mori Y, Notomi T, Kevadiya BD, Thakor AS (2020) Loop-mediated isothermal amplification (LAMP): a rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology 9:182
Azizan A, Alfaro AC, Venter L, Jaramillo D, Bestbier M, Foxwell J, Bennet P, Young T (2023a) Quantification of pathogenic Photobacterium swingsii and characterisation of disease progression in the New Zealand Greenshell™ mussel, Perna canaliculus. J Invertebr Pathol 19:e108065
Azizan A, Alfaro AC, Venter L, Zhang JJ, Ericson JA, Young T, Delorme NJ, Ragg NLC (2023b) Interactive effects of elevated temperature and Photobacterium swingsii infection on the survival and immune response of marine mussels (Perna canaliculus). Mar Environ Res 196:e106392
Azizan A, Carter J, Alfaro AC, Venter L, Young T, Sharma SS, Chen T (2023c) Investigating the effect of bacterial co-infections on juvenile and adult, green-lipped mussels (Perna canaliculus). J World Aquacult Soc 55:386–403
Azizan A, Venter L, Alfaro AC (2023d) A review on green-lipped mussel, Perna canaliculus immunology: the drivers, virulence factors, advances, and applications. N Z J Mar Freshw Res 1– 45
Balbi T, Fabbri R, Cortese K, Smerilli A, Ciacci C, Grande C, Vezzulli L, Pruzzo C, Canesi L (2013) Interactions between Mytilus galloprovincialis hemocytes and the bivalve pathogens Vibrio aestuarianus 01/032 and Vibrio splendidus LGP32. Fish Shellfish Immunol 35:1906–1915
Balbi T, Cortese K, Ciacci C, Bellese G, Vezzulli L, Pruzzo C, Canesi L (2018) Autophagic processes in Mytilus galloprovincialis hemocytes: effects of Vibrio tapetis. Fish Shellfish Immunol 73:66–74
Balbi T, Auguste M, Cortese K, Montagna M, Borello A, Pruzzo C, Vezzulli L, Canesi L (2019) Responses of Mytilus galloprovincialis to challenge with the emerging marine pathogen Vibrio coralliilyticus. Fish Shellfish Immunol 84:352–360
Bao M, Huo L, Wu J, Ge D, Lv Z, Chi C, Liao Z, Liu H (2018) A novel biomarker for marine environmental pollution of CAT from Mytilus coruscus. Mar Pollut Bull 127:717–725
Barber D, Villaseñor A, Escribese MM (2019) Metabolomics strategies to discover new biomarkers associated to severe allergic phenotypes. Asia Pac Allergy 9:e37
Battistini R, Varello K, Listorti V, Zambon M, Arcangeli G, Bozzetta E, Francese DR, Ercolini C, Serracca L (2020) Microbiological and histological analysis for the evaluation of farmed mussels (Mytilus galloprovincialis) health status, in coastal areas of Italy. Pathogens 9:395
Ben Cheikh Y, Travers M-A, Morga B, Godfrin Y, Rioult D, Le Foll F (2016) First evidence for a Vibrio strain pathogenic to Mytilus edulis altering hemocyte immune capacities. Dev Comp Immunol 57:107–119
Ben Cheikh Y, Travers M-A, Le Foll F (2017) Infection dynamics of a V. splendidus strain pathogenic to Mytilus edulis: in vivo and in vitro interactions with hemocytes. Fish Shellfish Immunol 70:515–523
Bignell JP, Dodge MJ, Feist SW, Lyons B, Martin PD, Taylor NGH, Stone D, Travalent L, Stentiford GD (2008) Mussel histopathology: effects of season, disease and species. Aquat Biol 2:1–15
Bigot-Clivot A, La Carbona S, Cazeaux C, Durand L, Géba E, Le Foll F, Xuereb B, Chalghmi H, Dubey JP, Bastien F (2022) Blue mussel (Mytilus edulis)—a bioindicator of marine water contamination by protozoa: laboratory and in situ approaches. J Appl Microbiol 132:736–746
Bouallegui Y (2019) Immunity in mussels: an overview of molecular components and mechanisms with a focus on the functional defenses. Fish Shellfish Immunol 89:158–169
Brooks S, Lyons B, Goodsir F, Bignell J, Thain J (2009) Biomarker responses in mussels, an integrated approach to biological effects measurements. J Toxicol Environ Health - A: Curr Issues 72:196–208
Brosset P, Cooke SJ, Schull Q, Trenkel VM, Soudant P, Lebigre C (2021) Physiological biomarkers and fisheries management. Rev Fish Biol Fisheries 31:797–819
Canesi L, Betti M, Ciacci C, Lorusso LC, Gallo G, Pruzzo C (2005) Interactions between Mytilus haemocytes and different strains of Escherichia coli and Vibrio cholerae O1 El Tor: Role of kinase-mediated signalling. Cell Microbiol 7:667–674
Canesi L, Barmo C, Fabbri R, Ciacci C, Vergani L, Roch P, Gallo G (2010) Effects of Vibrio challenge on digestive gland biomarkers and antioxidant gene expression in Mytilus galloprovincialis. Comp Biochem Physiol - C Toxicol Pharmacol 152:399–406
Castillo N, Saavedra LM, Vargas CA, Gallardo-Escárate C, Détrée C (2017) Ocean acidification and pathogen exposure modulate the immune response of the edible mussel Mytilus chilensis. Fish Shellfish Immunol 70:149–155
Cellura C, Toubiana M, Parrinello N, Roch P (2006) HSP70 gene expression in Mytilus galloprovincialis hemocytes is triggered by moderate heat shock and Vibrio anguillarum, but not by V. splendidus or Micrococcus lysodeikticus. Dev Comp Immunol 30:984–997
Cellura C, Toubiana M, Parrinello N, Roch P (2007) Specific expression of antimicrobial peptide and HSP70 genes in response to heat-shock and several bacterial challenges in mussels. Fish Shellfish Immunol 22:340–350
Chahouri A, Yacoubi B, Moukrim A, Banaoui A (2022) Integration assay of bacteriological risks in marine environment using Salmonella spp and multimarker response in the bivalve Donax trunculus: novel biomonitoring approach. Chemosphere 297:134149
Charles M, Bernard I, Villalba A, Oden E, Burioli EAV, Allain G, Trancart S, Bouchart V, Houssin M (2020a) High mortality of mussels in northern Brittany – evaluation of the involvement of pathogens, pathological conditions and pollutants. J Invertebr Pathol 170:107308
Charles M, Trancart S, Oden E, Houssin M (2020b) Experimental infection of Mytilus edulis by two Vibrio splendidus-related strains: determination of pathogenicity level of strains and influence of the origin and annual cycle of mussels on their sensitivity. J Fish Dis 43:9–21
Chen H, Wang M, Zhang H, Wang H, Zhou L, Zhong Z, Cao L, Lian C, Sun Y, Li C (2021) microRNAs facilitate comprehensive responses of Bathymodiolinae mussel against symbiotic and nonsymbiotic bacteria stimulation. Fish Shellfish Immunol 119:420–431
Chimalapati S, Lafrance AE, Chen L, Orth K (2020) Vibrio parahaemolyticus: basic techniques for growth, genetic manipulation, and analysis of virulence factors. Curr Protoc Microbiol 59:e131
Ciacci C, Citterio B, Betti M, Canonico B, Roch P, Canesi L (2009) Functional differential immune responses of Mytilus galloprovincialis to bacterial challenge. Comp Biochem Physiol - B Biochem Mol Biol 153:365–371
Ciacci C, Betti M, Canonico B, Citterio B, Roch P, Canesi L (2010) Specificity of anti-Vibrio immune response through p38 MAPK and PKC activation in the hemocytes of the mussel Mytilus galloprovincialis. J Invertebr Pathol 105:49–55
Costa MM, Prado-Alvarez M, Gestal C, Li H, Roch P, Novoa B, Figueras A (2009) Functional and molecular immune response of Mediterranean mussel (Mytilus galloprovincialis) haemocytes against pathogen-associated molecular patterns and bacteria. Fish Shellfish Immunol 26:515–523
Costa PM, Carreira S, Costa MH, Caeiro S (2013) Development of histopathological indices in a commercial marine bivalve (Ruditapes decussatus) to determine environmental quality. Aquat Toxicol 126:442–454
Dallarés S, Carrasco N, Álvarez-Muñoz D, Rambla-Alegre M, Solé M (2018) Multibiomarker biomonitoring approach using three bivalve species in the Ebro Delta (Catalonia, Spain). Environ Sci Pollut Res 25:36745–36758
Dalzochio T, Rodrigues GZP, Petry IE, Gehlen G, Da Silva LB (2016) The use of biomarkers to assess the health of aquatic ecosystems in Brazil: a review. Int Aquat Res 8:283–298
De Carvalho CC, Caramujo MJ (2018) The various roles of fatty acids. Molecules 23:2583
de Jong L, Moreau X, Thiéry A (2008) Expression of heat shock proteins as biomarker tool in aquatic invertebrates: actual knowledge and ongoing developments for the early detection of environmental changes and ecological risks. In: Morel E, Vincent C (eds) Heat-Shock Proteins: New Research. Nova Publishers, pp 375–392
de Lorgeril J, Lucasson A, Petton B, Toulza E, Montagnani C, Clerissi C, Vidal-Dupiol J, Chaparro C, Galinier R, Escoubas JM, Haffner P, Dégremont L, Charrière GM, Lafont M, Delort A, Vergnes A, Chiarello M, Faury N, Rubio T, Leroy MA, Pérignon A, Régler D, Morga B, Alunno-Bruscia M, Boudry P, Le Roux F, Destoumieux-Garzόn D, Gueguen Y, Mitta G (2018) Immune-suppression by OsHV-1 viral infection causes fatal bacteraemia in Pacific oysters. Nat Commun 9:4215
de Souza Valente C, Wan AHL (2021) Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J Invertebr Pathol 181:107527
Delorme NJ, Venter L, Rolton A, Ericson JA (2021) Integrating animal health and stress assessment tools using the green-lipped mussel Perna canaliculus as a case study. J Shellfish Res 40:93–112
Deretic V, Saitoh T, Akira S (2013) Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13:722–737
Destoumieux-Garzón D, Canesi L, Oyanedel D, Travers MA, Charrière GM, Pruzzo C, Vezzulli L (2020) Vibrio–bivalve interactions in health and disease. Environ Microbiol 22:4323–4341
Dolar A, Mayall C, Drobne D, Kokalj AJ (2020) Modulations of immune parameters caused by bacterial and viral infections in the terrestrial crustacean Porcellio scaber: implications for potential markers in environmental research. Dev Comp Immunol 113:103789
Domeneghetti S, Varotto L, Civettini M, Rosani U, Stauder M, Pretto T, Pezzati E, Arcangeli G, Turolla E, Pallavicini A, Venier P (2014) Mortality occurrence and pathogen detection in Crassostrea gigas and Mytilus galloprovincialis close-growing in shallow waters (Goro lagoon, Italy). Fish Shellfish Immunol 41:37–44
Dong HT, Nguyen VV, Phiwsaiya K, Gangnonngiw W, Withyachumnarnkul B, Rodkhum C, Senapin S (2015) Concurrent infections of Flavobacterium columnare and Edwardsiella ictaluri in striped catfish, Pangasianodon hypophthalmus in Thailand. Aquaculture 448:142–150
Dong W, Chen Y, Lu W, Wu B, Qi P (2017) Transcriptome analysis of Mytilus coruscus hemocytes in response to Vibrio alginnolyficus infection. Fish Shellfish Immunol 70:560–567
Eggermont M, Bossier P, Pande GSJ, Delahaut V, Rayhan AM, Gupta N, Islam SS, Yumo E, Nevejan N, Sorgeloos P, Gomez-Gil B, Defoirdt T (2017) Isolation of Vibrionaceae from wild blue mussel (Mytilus edulis) adults and their impact on blue mussel larviculture. FEMS Microbiol Ecol 93:1–11
Eggermont M, Cornillie P, Dierick M, Adriaens D, Nevejan N, Bossier P, Van Den Broeck W, Sorgeloos P, Defoirdt T, Declercq AM (2020) The blue mussel inside: 3D visualization and description of the vascular-related anatomy of Mytilus edulis to unravel hemolymph extraction. Sci Rep 10:6773
Ellis RP, Spicer JI, Byrne JJ, Sommer U, Viant MR, White DA, Widdicombe S (2014) 1H NMR metabolomics reveals contrasting response by male and female mussels exposed to reduced seawater pH, increased temperature, and a pathogen. Environ Sci Technol 48:7044–7052
Ericson JA, Venter L, Welford MRV, Kumanan K, Alfaro AC, Ragg NLC (2022) Effects of seawater temperature and acute Vibrio sp. challenge on the haemolymph immune and metabolic responses of adult mussels (Perna canaliculus). Fish Shellfish Immunol 128:664–675
Fabbri E, Valbonesi P, Franzellitti S (2008) HSP expression in bivalves. Invertebr Surviv J 5:135–161
Ford SE (1986) Effect of repeated hemolymph sampling on growth, mortality, hemolymph protein and parasitism of oysters, Crassostrea virginica. Comp Biochem Physiol A: Physiol 85:465–470
François G, Mélanie D, Marlène F, Michel F (2015) Effects of a municipal effluent on the freshwater mussel Elliptio complanata following challenge with Vibrio anguillarum. J Environ Sci (china) 37:91–99
Fridovich I (1973) Superoxide dismutase. Annu Rev Biochem 44:147–159
Frizzo R, Bortoletto E, Riello T, Leanza L, Schievano E, Venier P, Mammi S (2021) NMR metabolite profiles of the bivalve mollusc Mytilus galloprovincialis before and after immune stimulation with Vibrio splendidus. Front Mol Biosci 8:1–14
García-García E, Prado-Álvarez M, Novoa B, Figueras A, Rosales C (2008) Immune responses of mussel hemocyte subpopulations are differentially regulated by enzymes of the PI 3-K, PKC, and ERK kinase families. Dev Comp Immunol 32:637–653
Ge D, Zhang L, Long Z, Chi C, Liu H (2020) A novel biomarker for marine environmental pollution: a metallothionein from Mytilus coruscus. Aquacult Rep 17:100364
Geba E, Aubert D, Durand L, Escotte S, La Carbona S, Cazeaux C, Bonnard I, Bastien F, Ladeiro MP, Dubey JP (2020) Use of the bivalve Dreissena polymorpha as a biomonitoring tool to reflect the protozoan load in freshwater bodies. Water Res 170:115297
Geffré A, Friedrichs K, Harr K, Concordet D, Trumel C, Braun JP (2009) Reference values: a review. Vet Clin Pathol 38:288–298
Gerdol M, Gomez-Chiarri M, Castillo MG, Figueras A, Fiorito G, Moreira R, Novoa B, Pallavicini A, Ponte G, Roumbedakis K, Venier P, Vasta GR (2018) Immunity in molluscs: recognition and effector mechanisms, with a focus on bivalvia. In: Cooper EL (ed) Adv Comp Immunol, pp 225–341
Geret F, Manduzio H, Company R, Leboulenger F, Bebianno MJ, Danger JM (2004) Molecular cloning of superoxide dismutase (Cu/Zn-SOD) from aquatic molluscs. Mar Environ Res 58:619–623
Gestal C, Roch P, Renault T, Pallavicini A, Paillard C, Novoa B, Oubella R, Venier P, Figueras A (2008) Study of diseases and the immune system of bivalves using molecular biology and genomics. Rev Fish Sci 16:133–156
Goh SG, Bayen S, Burger D, Kelly BC, Han P, Babovic V, Gin KY-H (2017) Occurrence and distribution of bacteria indicators, chemical tracers and pathogenic vibrios in Singapore coastal waters. Mar Pollut Bull 114:627–634
Gutierrez AM, Bassols A, Soler L, Pineiro M (2020) Editorial: Factors influencing biomarker range intervals in farm animals. Front Vet Sci 7:587741
Haddad N, Johnson N, Kathariou S, Métris A, Phister T, Pielaat A, Tassou C, Wells-Bennik M H J, Zwietering M H (2018) Next generation microbiological risk assessment—Potential of omics data for hazard characterisation. Int J Food Microbiol 287:28–39
Hagger JA, Jones MB, Leonard DP, Owen R, Galloway TS (2006) Biomarkers and integrated environmental risk assessment: are there more questions than answers? Integr Environ Assess Manag: Int J 2:312–329
Harrison J, Nelson K, Morcrette H, Morcrette C, Preston J, Helmer L, Titball RW, Butler CS, Wagley S (2022) The increased prevalence of Vibrio species and the first reporting of Vibrio jasicida and Vibrio rotiferianus at UK shellfish sites. Water Res 211:117942
Hernroth B, Baden S, Tassidis H, Hörnaeus K, Guillemant J, Bergström Lind S, Bergquist J (2016) Impact of ocean acidification on antimicrobial activity in gills of the blue mussel (Mytilus edulis). Fish Shellfish Immunol 55:452–459
Hey SP, D’Andrea E, Jung EH, Tessema F, Luo J, Gyawali B, Kesselheim AS (2019) Challenges and opportunities for biomarker validation. J Law Med Ethics 47:357–361
Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA (2013) Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 1:483–491
Holland RL (2016) What makes a good biomarker? Adv Precis Med 1:66–77
Holt E, Miller S (2010) Bioindicators: using organisms to measure environmental impacts. Nat Educ Knowl 3:8
Hook SE, Gallagher EP, Batley GE (2014) The role of biomarkers in the assessment of aquatic ecosystem health. Integr Environ Assess Manag 10:327–341
Islam SS, Zhang S, Eggermont M, Bruto M, Le Roux F, Defoirdt T (2022) The impact of the multichannel quorum sensing systems of Vibrio tasmaniensis and Vibrio crassostreae on virulence towards blue mussel (Mytilus edulis) larvae. Aquaculture 547:737414
Ji C, Wu H, Wei L, Zhao J, Wang Q, Lu H (2013) Responses of Mytilus galloprovincialis to bacterial challenges by metabolomics and proteomics. Fish Shellfish Immunol 35:489–498
Kamel N, Burgeot T, Banni M, Chalghaf M, Devin S, Minier C, Boussetta H (2014) Effects of increasing temperatures on biomarker responses and accumulation of hazardous substances in rope mussels (Mytilus galloprovincialis) from Bizerte lagoon. Environ Sci Pollut Res 21:6108–6123
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2009a) Two pathogens of Greenshell™ mussel larvae, Perna canaliculus : Vibrio splendidus and a V. coralliilyticus/neptunius -like isolate. J Fish Dis 32:499–507
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson LF (2009b) Challenge of New Zealand Greenshell™ mussel Perna canaliculus larvae using two Vibrio pathogens: a hatchery study. Dis Aquat Org 86:15–20
Kroon F, Streten C, Harries S (2017) A protocol for identifying suitable biomarkers to assess fish health: a systematic review. PLoS ONE 12:e0174762
Kumeiko VV, Sokolnikova YN, Grinchenko AV, Mokrina MS, Kniazkina MI (2018) Immune state correlates with histopathological level and reveals molluscan health in populations of Modiolus kurilensis by integral health index (IHI). J Invertebr Pathol 154:42–57
Künili İE, Ertürk Gürkan S, Aksu A, Turgay E, Çakir F, Gürkan M, Altinağaç U (2021) Mass mortality in endangered fan mussels Pinna nobilis (Linnaeus 1758) caused by co-infection of Haplosporidium pinnae and multiple Vibrio infection in Çanakkale Strait, Turkey. Biomarkers 26:450–461
Laitano MV, Fernández-Gimenez AV (2016) Are mussels always the best bioindicators? Comparative study on biochemical responses of three marine invertebrate species to chronic port pollution. Bull Environ Contam Toxicol 97:50–55
Laith AA, Ros-Amira MK, Sheikh HI, Effendy AWM, Najiah M (2021) Histopathological and immunological changes in green mussel, Perna viridis, challenged with Vibrio alginolyticus. Fish Shellfish Immunol 118:169–179
Lane HS, Brosnahan CL, Poulin R (2022) Aquatic disease in New Zealand: synthesis and future directions. NZ J Mar Freshwat Res 56:1–42
Langston W, Chesman B, Burt G, Hill C (2007) Review of biomarkers, bioassays and their potential use in monitoring the Fal and Helford SAC. Proj Rep Mar Biol Assoc Plymouth 1–68
Lattos A, Bitchava K, Giantsis IA, Theodorou JA, Batargias C, Michaelidis B (2021a) The implication of vibrio bacteria in the winter mortalities of the critically endangered Pinna nobilis. Microorganisms 9:1–15
Lattos A, Feidantsis K, Georgoulis I, Giantsis IA, Karagiannis D, Theodorou JA, Staikou A, Michaelidis B (2021b) Pathophysiological responses of Pinna nobilis individuals enlightens the etiology of mass mortality situation in the mediterranean populations. Cells 10:2838
Le Roux F, Blokesch M (2018) Eco-evolutionary dynamics linked to horizontal gene transfer in Vibrios. Annu Rev Microbiol 72:89–110
Le Roux F, Wegner KM, Polz MF (2016) Oysters and Vibrios as a model for disease dynamics in wild animals. Trends Microbiol 24:568–580
Leoni G, De Poli A, Mardirossian M, Gambato S, Florian F, Venier P, Wilson DN, Tossi A, Pallavicini A, Gerdol M (2017) Myticalins: a novel multigenic family of linear, cationic antimicrobial peptides from marine mussels (Mytilus spp.). Mar Drugs 15:261
Leprêtre M, Geffard A, Palos Ladeiro M, Dedourge-Geffard O, David E, Delahaut L, Bonnard I, Barjhoux I, Nicolaï M, Noury P, Espeyte A, Chaumot A, Degli-Esposti D, Geffard O, Lopes C (2022) Determination of biomarkers threshold values and illustration of their use for the diagnostic in large-scale freshwater biomonitoring surveys. Environ Sci Eur 34:115
Li H, Zhao J, Li Y, Dong Z, Lin S, Guo B, Qi P (2024) Transcriptome analysis reveals tissue-specific responses of Mytilus unguiculatus to Vibrio alginolyticus infection. Fish Shellfish Immunol 144:109301
Li M, Penner GB, Hernandez-Sanabria E, Oba M, Guan LL (2009) Effects of sampling location and time, and host animal on assessment of bacterial diversity and fermentation parameters in the bovine rumen. J Appl Microbiol 107:1924–1934
Li S, Alfaro AC, Nguyen TV, Young T, Lulijwa R (2020) An integrated omics approach to investigate summer mortality of New Zealand Greenshell™ mussels. Metabolomics 16:1–16
Liao Z, Wang XC, Liu HH, Fan MH, Sun JJ, Shen W (2013) Molecular characterization of a novel antimicrobial peptide from Mytilus coruscus. Fish Shellfish Immunol 34:610–616
Liu X, Zhao J, Wu H, Wang Q (2013) Metabolomic analysis revealed the differential responses in two pedigrees of clam Ruditapes philippinarum towards Vibrio harveyi challenge. Fish Shellfish Immunol 35:1969–1975
Liu HH, He JY, Chi CF, Shao J (2014a) Differential HSP70 expression in Mytilus coruscus under various stressors. Gene 543:166–173
Liu X, Ji C, Zhao J, Wang Q, Li F, Wu H (2014b) Metabolic profiling of the tissue-specific responses in mussel Mytilus galloprovincialis towards Vibrio harveyi challenge. Fish Shellfish Immunol 39:372–377
Liu X, Sun H, Wang Y, Ma M, Zhang Y (2014c) Gender-specific metabolic responses in hepatopancreas of mussel Mytilus galloprovincialis challenged by Vibrio harveyi. Fish Shellfish Immunol 40:407–413
Liu H, He J, Zhao R, Chi C, Bao Y (2015) A novel biomarker for marine environmental pollution of pi-class glutathione S-transferase from Mytilus coruscus. Ecotoxicol Environ Saf 118:47–54
Liu H, Wu J, Xu M, He J (2016) A novel biomarker for marine environmental pollution of HSP90 from Mytilus coruscus. Mar Pollut Bull 111:428–434
Lomartire S, Marques JC, Gonçalves AM (2021) Biomarkers based tools to assess environmental and chemical stressors in aquatic systems. Ecol Ind 122:107207
Lori S, Rovere GD, Ezzat L, Smits M, Ferraresso SS, Babbucci M, Marin MG, Masiero L, Fabrello J, Garro E, Carraro L, Cardazzo B, Patarnello T, Matozzo V, Bargelloni L, Milan M (2020) The effects of glyphosate and AMPA on the mediterranean mussel Mytilus galloprovincialis and its microbiota. Environ Res 182:108984
Lucy FE, Graczyk TK, Tamang L, Miraflor A, Minchin D (2008) Biomonitoring of surface and coastal water for Cryptosporidium, Giardia, and human-virulent microsporidia using molluscan shellfish. Parasitol Res 103:1369–1375
Luna-Acosta A, Breitwieser M, Renault T, Thomas-Guyon H (2017) Recent findings on phenoloxidases in bivalves. Mar Pollut Bull 122:5–16
Lupo C, Bougeard S, Le Bihan V, Blin JL, Allain G, Azéma P, Benoit F, Béchemin C, Bernard I, Blachier P, Brieau L, Danion M, Garcia A, Gervasoni E, Glize P, Lainé A, Lapègue S, Mablouké C, Poirier L, Raymond JC, Treilles M, Chauvin C, Le Bouquin S (2021) Mortality of marine mussels Mytilus edulis and M. galloprovincialis: systematic literature review of risk factors and recommendations for future research. Rev Aquacult 13:504–536
Martins E, Figueras A, Novoa B, Santos RS, Moreira R, Bettencourt R (2014) Comparative study of immune responses in the deep-sea hydrothermal vent mussel Bathymodiolus azoricus and the shallow-water mussel Mytilus galloprovincialis challenged with Vibrio bacteria. Fish Shellfish Immunol 40:485–499
Matozzo V, Ercolini C, Serracca L, Battistini R, Rossini I, Granato G, Quaglieri E, Perolo A, Finos L, Arcangeli G, Bertotto D, Radaelli G, Chollet B, Arzul I, Quaglio F (2018) Assessing the health status of farmed mussels (Mytilus galloprovincialis) through histological, microbiological and biomarker analyses. J Invertebr Pathol 153:165–179
Mcfall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, Hentschel U, King N, Kjelleberg S, Knoll AH, Kremer N, Mazmanian SK, Metcalf JL, Nealson K, Pierce NE, Rawls JF, Reid A, Ruby EG, Rumpho M, Sanders JG, Tautz D, Wernegreen JJ (2013) Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci 110:3229–3236
Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263:17205–17208
Moore MN, Icarus Allen J, Mcveigh A (2006) Environmental prognostics: an integrated model supporting lysosomal stress responses as predictive biomarkers of animal health status. Mar Environ Res 61:278–304
Moore RE, Kirwan J, Doherty MK, Whitfield PD (2007) Biomarker discovery in animal health and disease: the application of post-genomic technologies. Biomarker Insights 2:185–196
Moreira R, Pereiro P, Canchaya C, Posada D, Figueras A, Novoa B (2015) RNA-Seq in Mytilus galloprovincialis: comparative transcriptomics and expression profiles among different tissues. BMC Genomics 16:728
Muznebin F, Alfaro AC, Venter L, Young T (2022) Acute thermal stress and endotoxin exposure modulate metabolism and immunity in marine mussels (Perna canaliculus). J Therm Biol 110:103327
Najwaa MN, Danielb AMD, Amin K, Effendya A (2015) Detection of virulence genes in Vibrio alginolyticus isolated from green mussel. Perna Viridis J Teknol 77:19–23
Nandi A, Yan LJ, Jana CK, Das N (2019) Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid Med Cell Longev 2019:9613090
Newton T, Cope W (2006) Biomarker responses of unionid mussels to environmental contaminants. In: Farris JL (ed) Freshwater bivalve ecotoxicology. CRC Press, Boca Raton, pp 257–284
Nguyen TV, Alfaro AC (2019) Targeted metabolomics to investigate antimicrobial activity of itaconic acid in marine molluscs. Metabolomics 15:97
Nguyen TV, Alfaro AC (2020) Metabolomics investigation of summer mortality in New Zealand Greenshell™ mussels (Perna canaliculus). Fish Shellfish Immunol 106:783–791
Nguyen TV, Alfaro AC, Merien F, Young T, Grandiosa R (2018a) Metabolic and immunological responses of male and female new Zealand Greenshell™ mussels (Perna canaliculus) infected with Vibrio sp. J Invertebr Pathol 157:80–89
Nguyen TV, Alfaro AC, Young T, Ravi S, Merien F (2018b) Metabolomics study of immune responses of New Zealand Greenshell™ mussels (Perna canaliculus) infected with pathogenic Vibrio sp. Mar Biotechnol 20:396–409
Nguyen TV, Alfaro AC, Merien F, Young T (2019a) In vitro study of apoptosis in mussel (Perna canaliculus) haemocytes induced by lipopolysaccharide. Aquaculture 503:8–15
Nguyen TV, Alfaro AC, Young T, Merien F (2019b) Tissue-specific immune responses to Vibrio sp. infection in mussels (Perna canaliculus ): a metabolomics approach. Aquaculture 500:118–125
Nuss AM, Beckstette M, Pimenova M, Schmühl C, Opitz W, Pisano F, Heroven AK, Dersch P (2017) Tissue dual RNA-seq allows fast discovery of infection-specific functions and riboregulators shaping host–pathogen transcriptomes. Proc Natl Acad Sci 114:E791–E800
Oliver LM, Fisher WS (1999) Appraisal of prospective bivalve immunomarkers. Biomarkers 4:510–530
Ong HMG, Zhong Y, Hu C, Ong KH, Khor WC, Schlundt J, Aung KT (2023) Antimicrobial resistance risk assessment of Vibrio parahaemolyticus isolated from farmed Green mussels in Singapore. Microorganisms 11:1498
Oweson C, Hernroth B (2009) A comparative study on the influence of manganese on the bactericidal response of marine invertebrates. Fish Shellfish Immunol 27:500–507
Paillard C, Le Roux F, Borrego JJ (2004) Bacterial disease in marine bivalves, a review of recent studies: trends and evolution. Aquat Living Resour 17:477–498
Pain S, Devin S, Parant M (2007) Biomarker versus environmental factors: seasonal variations and modelling multixenobiotic defence (MXD) transport activity in transplanted zebra mussels. Sci Total Environ 373:103–112
Parisi MG, Maisano M, Cappello T, Oliva S, Mauceri A, Toubiana M, Cammarata M (2019) Responses of marine mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) after infection with the pathogen Vibrio splendidus. Comp Biochem Physiol C: Toxicol Pharmacol 221:1–9
Parry HE, Pipe RK (2004) Interactive effects of temperature and copper on immunocompetence and disease susceptibility in mussels (Mytilus edulis). Aquat Toxicol 69:311–325
Pinu FR, Goldansaz SA, Jaine J (2019) Translational metabolomics: current challenges and future opportunities. Metabolites 9:108
Potasman I, Paz A, Odeh M (2002) Infectious outbreaks associated with bivalve shellfish consumption: a worldwide perspective. Clin Infect Dis 35:921–928
Prabhakaran K, Nagarajan R, Merlin Franco F, Anand Kumar A (2017) Biomonitoring of Malaysian aquatic environments: a review of status and prospects. Ecohydrol Hydrobiol 17:134–147
Puspita M, Hutabarat J , Widowati I (2015) Effects of temperature in the phenoloxidase activity of Perna viridis challenged with Vibrio harveyi (Working paper). Diponegoro University 1– 9
Qin CL, Huang W, Zhou SQ, Wang XC, Liu HH, Fan MH, Wang RX, Gao P, Liao Z (2014) Characterization of a novel antimicrobial peptide with chiting-biding domain from Mytilus coruscus. Fish Shellfish Immunol 41:362–370
Qureshi A, Niazi JH (2020) Biosensors for detecting viral and bacterial infections using host biomarkers: a review. Analyst 145:7825–7848
Ren Q, Sun RR, Zhao XF, Wang JX (2009) A selenium-dependent glutathione peroxidase (Se-GPx) and two glutathione S-transferases (GSTs) from Chinese shrimp (Fenneropenaeus chinensis). Comp Biochem Physiol - C Toxicol Pharmacol 149:613–623
Ren Q, Zhong X, Yin SW, Hao FY, Hui KM, Zhang Z, Zhang CY, Yu XQ, Wang W (2013) The first Toll receptor from the triangle-shell pearl mussel Hyriopsis cumingii. Fish Shellfish Immunol 34:1287–1293
Ren W, Rajendran R, Zhao Y, Tan B, Wu G, Bazer FW, Zhu G, Peng Y, Huang X, Deng J, Yin Y (2018) Amino acids as mediators of metabolic cross talk between host and pathogen. Front Immunol 9:1–13
Rey-Campos M, Moreira R, Gerdol M, Pallavicini A, Novoa B, Figueras A (2019a) Immune tolerance in Mytilus galloprovincialis hemocytes after repeated contact with Vibrio splendidus. Front Immunol 10:1–15
Rey-Campos M, Moreira R, Valenzuela-Muñoz V, Gallardo-Escárate C, Novoa B, Figueras A (2019b) High individual variability in the transcriptomic response of Mediterranean mussels to Vibrio reveals the involvement of myticins in tissue injury. Sci Rep 9:1–15
Richard JC, Campbell LJ, Leis EM, Agbalog RE, Dunn CD, Waller DL, Knowles S, Putnam JG, Goldberg TL (2021) Mussel mass mortality and the microbiome: evidence for shifts in the bacterial microbiome of a declining freshwater bivalve. Microorganisms 9:1976
Rolton A, Ragg NLC (2020) Green-lipped mussel (Perna canaliculus) hemocytes: a flow cytometric study of sampling effects, sub-populations and immune-related functions. Fish Shellfish Immunol 103:181–189
Romero A, Costa M, Forn-Cuni G, Balseiro P, Chamorro R, Dios S, Figueras A, Novoa B (2014) Occurrence, seasonality and infectivity of Vibrio strains in natural populations of mussels Mytilus galloprovincialis. Dis Aquat Organ 108:149–163
Romero A, Novoa B, Figueras A (2022) Genomic and transcriptomic identification of the cathepsin superfamily in the Mediterranean mussel Mytilus galloprovincialis. Dev Comp Immunol 127:104286
Rosani U, Varotto L, Rossi A, Roch P, Novoa B, Figueras A, Pallavicini A, Venier P (2011) Massively parallel amplicon sequencing reveals isotype-specific variability of antimicrobial peptide transcripts in Mytilus galloprovincialis. PLoS ONE 6:e26680
Ruiz Y, Suárez P, Alonso A, Longo E, San Juan F (2013) Mutagenicity test using Vibrio harveyi in the assesment of water quality from mussel farms. Water Res 47:2742–2756
Ryan PB, Burke TA, Cohen Hubal EA, Cura JJ, Mckone TE (2007) Using biomarkers to inform cumulative risk assessment. Environ Health Perspect 115:833–840
Saco A, Rey-Campos M, Novoa B, Figueras A (2020) Transcriptomic response of mussel gills after a Vibrio splendidus infection demonstrates their role in the immune response. Front Immunol 11:1–18
Sawabe T, Ogura Y, Matsumura Y, Feng G, Amin AR, Mino S, Nakagawa S, Sawabe T, Kumar R, Fukui Y, Satomi M, Matsushima R, Thompson FL, Gomez-Gil B, Christen R, Maruyama F, Kurokawa K, Hayashi T (2013) Updating the Vibrio clades defined by multilocus sequence phylogeny: proposal of eight new clades, and the description of Vibrio tritonius sp. nov. Front Microbiol 4:414
Sendra M, Saco A, Rey-Campos M, Novoa B, Figueras A (2020) Immune-responsive gene 1 (IRG1) and dimethyl itaconate are involved in the mussel immune response. Fish Shellfish Immunol 106:645–655
Sigel A, Sigel H, K O Sigel R (2015) Metallothioneins and related chelators. In: Sigel H, Sigel A, Sigel RKO (eds) Metallothioneins and related chelators. De Gruyter
Silva DC, Neto JM, Nunes C, Gonçalves FJ, Coimbra MA, Marques JC, Gonçalves AM (2021) Assessment of seasonal and spatial variations in the nutritional content of six edible marine bivalve species by the response of a set of integrated biomarkers. Ecol Ind 124:107378
Silva dos Santos F, Neves RAF, Bernay B, Krepsky N, Teixeira VL, Artigaud S (2023) The first use of LC-MS/MS proteomic approach in the brown mussel Perna perna after bacterial challenge: searching for key proteins on immune response. Fish Shellfish Immunol 134:108622
Sokolova IM (2009) Apoptosis in molluscan immune defense. Invertebr Surviv J 6:49–58
Stabili L, Acquaviva MI, Cavallo RA (2005) Mytilus galloprovincialis filter feeding on the bacterial community in a Mediterranean coastal area (Northern Ionian Sea, Italy). Water Res 39:469–477
Stentiford GD, Viant MR, Ward DG, Johnson PJ, Martin A, Wenbin W, Cooper HJ, Lyons BP, Feist SW (2005) Liver tumors in wild flatfish: a histopathological, proteomic, and metabolomic study. OMICS 9:281–299
Stewart LD, Tort N, Meakin P, Argudo JM, Nzuma R, Reid N, Delahay RJ, Ashford R, Montgomery WI, Grant IR (2017) Development of a novel immunochromatographic lateral flow assay specific for Mycobacterium bovis cells and its application in combination with immunomagnetic separation to test badger faeces. BMC Vet Res 13:131
Su JH, Chang MC, Lee YS, Tseng IC, Chuang YC (2004) Cloning and characterization of the lipase and lipase activator protein from Vibrio vulnificus CKM-1. Biochim Biophys Acta (BBA) - Gene Struct Expr 1678:7–13
Takemura AF, Chien DM, Polz MF (2014) Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front Microbiol 5:1–26
Tanguy M, Mckenna P, Gauthier-Clerc S, Pellerin J, Danger JM, Siah A (2013a) Functional and molecular responses in Mytilus edulis hemocytes exposed to bacteria, Vibrio splendidus. Dev Comp Immunol 39:419–429
Tanguy M, Mckenna P, Gauthier-Clerc S, Pellerin J, Danger JM, Siah A (2013b) Sequence analysis of a normalized cDNA library of Mytilus edulis hemocytes exposed to Vibrio splendidus LGP32 strain. Results Immunol 3:40–50
Tanguy M, Gauthier-Clerc S, Pellerin J, Danger JM, Siah A (2018) The immune response of Mytilus edulis hemocytes exposed to Vibrio splendidus LGP32 strain: a transcriptomic attempt at identifying molecular actors. Fish Shellfish Immunol 74:268–280
Thompson J, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, Polz MF (2004) Diversity and dynamics of a North Atlantic coastal Vibrio community. Appl Environ Microbiol 70:4103–4110
Travers M-A, Boettcher Miller K, Roque A, Friedman CS (2015) Bacterial diseases in marine bivalves. J Invertebr Pathol 131:11–31
Trivedi DK, Hollywood KA, Goodacre R (2017) Metabolomics for the masses: the future of metabolomics in a personalized world. New Horiz Transl Med 3:294–305
Van Hung N, De Schryver P, Dung NV, Nevejan N, Bossier P (2019) Ralstonia eutropha, containing high poly-β-hydroxybutyrate levels, regulates the immune response in mussel larvae challenged with Vibrio coralliilyticus. Fish Shellfish Immunol 84:196–203
Venier P, Varotto L, Rosani U, Millino C, Celegato B, Bernante F, Lanfranchi G, Novoa B, Roch P, Figueras A, Pallavicini A (2011) Insights into the innate immunity of the Mediterranean mussel Mytilus galloprovincialis. BMC Genomics 12:1–19
Vezzulli L, Colwell RR, Pruzzo C (2013) Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microb Ecol 65:817–825
Vezzulli L, Stagnaro L, Grande C, Tassistro G, Canesi L, Pruzzo C (2018) Comparative 16SrDNA gene-based microbiota profiles of the Pacific Oyster (Crassostrea gigas) and the Mediterranean Mussel (Mytilus galloprovincialis) from a shellfish farm (Ligurian Sea, Italy). Microb Ecol 75:495–504
Vidal-Liñán L, Bellas J, Salgueiro-González N, Muniategui S, Beiras R (2015) Bioaccumulation of 4-nonylphenol and effects on biomarkers, acetylcholinesterase, glutathione-S-transferase and glutathione peroxidase, in Mytilus galloprovincialis mussel gills. Environ Pollut 200:133–139
Waller DL, Cope WG (2019) The status of mussel health assessment and a path forward. Freshwat Mollusk Biol Conserv 22:26
Wang M, Su X, Li Y, Jun Z, Li T (2010) Cloning and expression of the Mn-SOD gene from Phascolosoma esculenta. Fish Shellfish Immunol 29:759–764
Wang L, Qiu L, Zhou Z, Song L (2013a) Research progress on the mollusc immunity in China. Dev Comp Immunol 39:2–10
Wang Q, Yuan Z, Wu H, Liu F, Zhao J (2013b) Molecular characterization of a manganese superoxide dismutase and copper/zinc superoxide dismutase from the mussel Mytilus galloprovincialis. Fish Shellfish Immunol 34:1345–1351
Wang D, Mbewe N, De Bels L, Couck L, Van Stappen G, Van den Broeck W, Nevejan N (2021) Pathogenesis of experimental vibriosis in blue mussel (Mytilus edulis ) larvae based on accurate positioning of GFP-tagged Vibrio strains and histopathological and ultrastructural changes of the host. Aquaculture 535:e736347
Westermann AJ, Gorski SA, Vogel J (2012) Dual RNA-seq of pathogen and host. Nat Rev Microbiol 10:618–630
World Health Organization (1993) Biomarkers and risk assessment: concepts and principles. Environmental health criteria, vol 155. WHO, Geneva, pp 82
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492
Wu H, Ji C, Wei L, Zhao J, Lu H (2013) Proteomic and metabolomic responses in hepatopancreas of Mytilus galloprovincialis challenged by Micrococcus luteus and Vibrio anguillarum. J Proteomics 94:54–67
Wu J, Bao M, Ge D, Huo L, Lv Z, Chi C, Liao Z, Liu H (2017) The expression of superoxide dismutase in Mytilus coruscus under various stressors. Fish Shellfish Immunol 70:361–371
Xu M, Wu J, Ge D, Wu C, Chi C, Lv Z, Liao Z, Liu H (2018) A novel toll-like receptor from Mytilus coruscus is induced in response to stress. Fish Shellfish Immunol 78:331–337
Yang H, Xu Z, Guo B, Zhang X, Liao Z, Qi P, Yan X (2021) Integrated analysis of miRNAome and transcriptome reveals miRNA-mRNA network regulation in Vibrio alginolyticus infected thick shell mussel Mytilus coruscus. Mol Immunol 132:217–226
Zaghloul A, Saber M, Gadow S, Awad F (2020) Biological indicators for pollution detection in terrestrial and aquatic ecosystems. Bull Natl Res Centre 44:1–11
Zare Jeddi M, Hopf NB, Viegas S, Price AB, Paini A, van Thriel C, Benfenati E, Ndaw S, Bessems J, Behnisch PA, Leng G, Duca RC, Verhagen H, Cubadda F, Brennan L, Ali I, David A, Mustieles V, Fernandez MF, Louro H, Pasanen-Kase R (2021) Towards a systematic use of effect biomarkers in population and occupational biomonitoring. Environ Int 146:106257
Zhai S, Yang B, Zhang F, Li Q, Liu S (2021) Estimation of genetic parameters for resistance to Vibrio alginolyticus infection in the Pacific oyster (Crassostrea gigas). Aquaculture 538:e736545
Zhang W, Li C (2021) Virulence mechanisms of vibrios belonging to the Splendidus clade as aquaculture pathogens, from case studies and genome data. Rev Aquac 13:2004–2026
Zhang HW, Huang Y, Man X, Wang Y, Hui KM, Yin SW, Zhang XW (2017) HcToll3 was involved in anti-Vibrio defense in freshwater pearl mussel, Hyriopsis cumingii. Fish Shellfish Immunol 63:189–195
Zhang R, Zhang LL, Ye X, Tian YY, Sun CF, Lu MX, Bai JJ (2013) Transcriptome profiling and digital gene expression analysis of Nile tilapia (Oreochromis niloticus) infected by Streptococcus agalactiae. Mol Biol Rep 40:5657–5668
Zhao J, Li C, Chen A, Li L, Su X, Li T (2010a) Molecular characterization of a novel big defensin from clam Venerupis philippinarum. PLoS ONE 5:e13480
Zhao J, Qiu L, Ning X, Chen A, Wu H, Li C (2010b) Cloning and characterization of an invertebrate type lysozyme from Venerupis philippinarum. Comp Biochem Physiol - B Biochem Mol Biol 156:56–60
Zhukova NV (2019) Fatty acids of marine mollusks: impact of diet, bacterial symbiosis and biosynthetic potential. Biomolecules 9:857
Acknowledgements
We are thankful to the Aquaculture Biotechnology Research Group at AUT for the ongoing support. We want to acknowledge the anonymous reviewer whose revisions improved this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by the Aquaculture Health Strategies to Maximise Productivity and Security programme and the Shellfish Aquaculture Research Platform, funded by the New Zealand Ministry for Business, Innovation and Employment (contract Nos: CAWX1707 & CAWX1801).
Author information
Authors and Affiliations
Contributions
AA and LV wrote the main manuscript and ACA edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azizan, A., Venter, L. & Alfaro, A.C. Biomarkers of mussel exposure to Vibrionaceae: A review. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01531-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01531-2