Abstract
Chronic obstructive pulmonary disease is the 3rd leading cause of death worldwide, and the available treatments are unsatisfactory, resulting in a major economic burden. As cellular therapy is commonly used for lung disease, we investigated a treatment with CXCR4-overexpressing BMSCs in a COPD model. We extracted and purified Bone marrow mesenchymal stem cells (BMSCs) from SD rats. COPD apoptosis model was established by cigarette smoke exposure. BMSCs (1 × 106 cells per injection)were transplanted in vivo twice a month during model establishment, and alveolar rupture in the lung was assessed. Lung cell apoptosis was assessed by terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) analysis, and the concentrations of apoptotic proteins in the lungs were detected by Western blotting. We successfully isolated BMSCs and established CXCR4-overexpressing BMSCs. qRT‒PCR and Western blotting detection both reveal that CXCR4 mRNA level and protein both significantly higher expression in CXCR4-BMSCs than the pBABE-BMSCs. Continuous cigarette smoke exposure caused alveolar septal rupture: In the model group, the alveolar mean linear intercept in the first month was significantly lower than that in the third month (p < 0.05). In the third month, the alveolar mean linear intercept values of the control and CXCR4-BMSC groups were lower than those of the model group (control group p < 0.01, CXCR4-BMSC group p < 0.05), and TUNEL staining revealed that the apoptosis rates of the control and CXCR4-BMSC groups were significantly lower than those of the model group (p < 0.01). Furthermore, the levels of the apoptotic proteins cleaved caspase-8, cleaved caspase-3 and cleaved PARP-1 were higher in the model group than in the control group (p < 0.05) and significantly lower in the CXCR4-BMSC group than in the model group (p < 0.05). The transplantation of CXCR4-overexpressing BMSCs during COPD model generation significantly inhibited apoptosis via the extrinsic apoptosis pathway.
Graphical abstract
CXCR4 enhances the inhibitory effects of bone mesenchymal stem cells on lung cell apoptosis in a rat model of smoking-induced COPD

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is mostly caused by smoking,which destroys the alveolar septa and leads to emphysema through the inflammatory response, oxidative stress, and apoptosis pathways and other pathways [1]. Apoptosis is one of the main pathogenic pathways of COPD and contributes to the complete pathogenesis of COPD. Recent studies have shown that apoptosis is significantly increased in the lung tissue of COPD patients, and DNA fragmentation is the main cause of apoptosis [2, 3]. In addition, animal experiments have shown that the development of emphysema in a COPD model generated by exposure to cigarette smoke is related to cell apoptosis [4]. Clinical and basic trials have been conducted to investigate potential antiapoptotic therapies for COPD [5,6,7]. Alveolar cell apoptosis continues in COPD patients even after smoking cessation [8, 9]; thus, antiapoptotic therapy during the development of COPD is particularly important. To establish more effective treatments for COPD or delay its progression, further research on antiapoptotic therapies is needed.
The mortality rate of patients with COPD is increasing yearly because current therapies merely ameliorate the symptoms and do not delay disease progression [10,11,12]. Improving respiratory function by inhibiting cell apoptosis or repairing damaged lung tissue has become a major research direction, and led to focus on the transplantation of bone marrow mesenchymal stem cells (BMSCs) in vivo. BMSCs are cells of nonhematopoietic origin with the abilities to differentiate into multiple cell types and repair tissue [13]. Due to their low immunogenicity and immunoregulatory ability, BMSCs have been extensively used for in vivo transplantation into COPD models to explore their therapeutic effects [14]. The majority of research findings indicate that mesenchymal stem cells (MSCs) can be used to treat diseases via their anti-inflammatory effects, their promotion of tissue repair and regeneration and their antioxidative, antiapoptotic and immunomodulatory activities [15,16,17,18,19,20,21]. Some studies have shown that MSCs can inhibit apoptosis by reducing the expression of apoptotic proteins in tissue and secreting vascular endothelial growth factor [22].
The apoptotic pathways in cells are complex and include endogenous and exogenous pathways [23, 24]. Further research is needed to determine the antiapoptotic effects of BMSCs in COPD and their ability to delay the progression of this disease. A high concentration of SDF-1 can be detected in damaged organs, and BMSCs can be recruited and exert a therapeutic effect on damaged organs through the SDF-1 concentration gradient [25, 26]. CXC chemokine receptor 4 (CXCR4) is the cognate receptor of SDF-1, and BMSCs that overexpress CXCR4 can more effectively home to damaged tissue in vivo. Moreover, some studies have indicated that CXCR4 protein overexpression can enhance the paracrine effects of BMSCs [27,28,29,30].
In this study, a retrovirus transfection method was used to establish a CXCR4 overexpression model of BMSCs [31]. BMSCs were transplanted into in vivo rat models of COPD established by exposure to cigarette smoke [32, 33]. Many studies have explored the intervention effects of various measures after the construction of animal models. The aim of the present study was to determine whether BMSCs inhibit lung cell apoptosis during establishment of a rat model of COPD by exposure to cigarette smoke. Moreover, we investigated whether BMSCs can inhibit apoptosis in lung tissue and protect lung tissue from cigarette smoking-induced destruction by reducing the expression of cleaved caspase-3 through exogenous pathways and whether BMSCs overexpressing CXCR4 protein are more effective than normal BMSCs at inhibiting cell apoptosis via the exogenous apoptotic pathway.
Methods
Experimental design
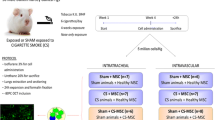
The trial design and study procedures are shown in Fig. 1.
Isolation and identification of BMSCs
Briefly, the hindlimbs were removed, and BMSCs were collected by flushing the marrow cavities with DMEM/F12 medium with 10% FBS and 1% penicillin–streptomycin. The whole bone marrow adherence method was used for the culture and isolation of primary BMSCs. BMSCs from the 3rd to 4th passages were identified based on their morphologic and immunophenotypic characteristics and differentiation ability.
Differentiation medium (Cyagen Biosciences, Guangzhou, China) was used to induce BMSCs to differentiate into osteoblasts, adipocytes and chondrocytes. For the induction of osteogenic and adipogenic differentiation, BMSCs were seeded onto six-well plates (Corning, Corning, NY, USA) at a density of 2.0 × 104 cells per well and cultured with BMSC Osteogenic Differentiation Basal Medium and Adipogenic Differentiation Basal Medium. To induce chondrogenic differentiation, 2.0 × 104 cells were pelleted in chondrogenic pellet cultures containing chondrogenic differentiation media. According to the manufacturer's instructions, BMSCs were cultured in differentiation media for 2–3 weeks, and the medium was refreshed every 3 days. At the end of the predetermined culture time, the results of osteogenic, adipogenic, and chondrogenic differentiation were determined by Alizarin red staining, Oil red O staining and Alcian blue staining, respectively.
Surface markers (CD90, CD29, CD34, and CD45) of BMSCs were detected by flow cytometry. BMSCs were detached from T25 cell culture bottles (Corning, Corning, NY, USA) by trypsin and counted to adjust the initial cell density to 1 × 106 cells/mL with DMEM/F12 medium with 10% fetal bovine serum (FBS) and 1% penicillin‒streptomycin. The cells were added to an Eppendorf tube at 1 × 106 cells/tube and incubated in the dark for 30 min at 4 °C with the following monoclonal antibodies: CD90 antibody-FITC (EBioscience, USA), CD29 antibody-FITC (BioLegend, USA), CD34 antibody-PE (Santa Cruz, USA), CD45 antibody-PE (EBioscience, USA) and isotype controls for FITC and PE (both from BioLegend and Santa Cruz). After 30 min, the cells were washed three times with PBS and resuspended in 500 μL of PBS. A FACSCalibur system (BD Bioscience) was used to collect the BMSCs and analyze the expression of surface markers of BMSCs.
Establishment of stable CXCR4-overexpressing BMSCs
The sequence of rat CXCR4 (accession no. NM_022205.3) was obtained from the GenBank database. We designed cloning primers based on the rat CXCR4 sequence follows: forward, 5′-CACAGAATTCATGGAAATATACACTTCG-3′; reverse, 5′-CACAGTCGACTTAGCTGGAGTGAAAACTT-3′. The CXCR4 sequences were collected after RT‒PCR amplification and cloned into pBABE-puro plasmids using the EcoRI and SalI restriction enzyme sites. The ligation mixture was transformed into competent Trans5α cells (Transgen Biotech, USA) to generate the recombinant plasmid. Plasmid DNA was extracted from Trans5α cells, and sequence analysis confirmed that the inserted sequences were the same as the sequence of rat CXCR4 obtained from the GenBank database. For stable overexpression, a mixture of X-tremeGENE (Roche, USA) with the plasmid at a ratio of 1:3 was added to Dulbecco’s modified Eagle’s medium (DMEM) and added to 6-well plates for the transfection of Platinum-E (Plat-E) packaging cells with the retroviral packaging vector pBABE-puro-CXCR4 or pBABE-puro-null.
Eight hours after cotransfection, the medium was replaced with fresh medium, and the recombinant retroviral vectors were harvested after culture for 48 h. Cell debris was removed by filtration. Third-passage BMSCs were seeded in 6-well plates and transduced with the negative control retroviral vector pBABE-puro-null or the overexpressing retroviral vector pBABE-puro-CXCR4. The confluence of BMSCs before transduction was lower than 60%. The retroviral vector in DMEM was mixed with polybrene at a ratio of 3:7 (Sigma‒Aldrich, St. Louis, MO, USA) to obtain a final concentration of 4 ng/mL. After 8 h, the medium was replaced with DMEM, and the cells were cultured for 48 h. Subsequently, stably transfected cells were selected using culture medium containing puromycin (0.1 µg/µL).
Reverse transcription and real-time quantitative PCR (qRT‒PCR)
qRT‒PCR was used to detect the mRNA expression of CXCR4 for comparison between CXCR4-BMSCs and pBABE-BMSCs. PCR was performed with 5′-CCACAGAGTCAGAATCCTCAAG-3′ and 5′-GGTCAGTCTTTTAT ATCTGG GAAATG-3′ as the forward and reverse primers, respectively. The total RNA of the BMSCs was extracted using TRIzol reagent, and Genomic DNA Eraser was used for the removal of genomic DNA. According to the manufacturer’s instructions, a reverse transcription kit (TaKaRa, Japan) was used to transcribe the cDNA, and the SuperReal PreMix Plus SYBR Green kit (TaKaRa, Japan) was utilized for real-time qRT‒PCR with the following program: 95 °C for 30 s, 40 cycles of 95 °C for 10 s, 60 °C for 1 min, and 72 °C for 30 s, 95 °C for 1 min, 60 °C for 30 s, and 95 °C for 30 s. After normalization with the internal control 18S, the 2−ΔΔCq method was used to determine the relative CXCR4 mRNA expression levels for comparison between CXCR4-BMSCs and pBABE-BMSCs.
Establishment of the COPD rat model and cell therapy
A total of 64 healthy male SD rats (aged 3–4 weeks) were randomly divided into four groups (16 SD rats in each group): control group, CXCR4-BMSC treatment + cigarette smoke exposure group (CXCR4-BMSC group), pBABE-BMSC treatment + cigarette smoke exposure group (pBABE-BMSC group) and COPD model group (model group). We used the cigarette smoke exposure method for construction of the COPD rat models in this study. The animals in all of the groups except the control group were exposed to smoke from commercially available cigarettes. The rats in the CXCR4-BMSC, pBABE-BMSC and model groups were exposed to smoke from ten cigarettes (Coconut Tree brand, tobacco tar: 11 mg; nicotine smoke: 1.0 mg; flue gas of carbon monoxide: 13 mg) for 30 min/day.
The procedure for exposure to cigarette smoke was as follows: a plexiglas box was equally divided into two layers by a middle dividing board containing round vents. For cigarette smoke exposure, SD rats were placed in the upper layer, and ignited cigarettes were suspended in the lower layer. Two sets of five cigarettes were burned for approximately 15 min.
BMSCs were transplanted in vivo during the construction of the COPD rat models. BMSCs were intravenously injected twice a month. All intravenous injections were administered via the tail vein as follows: (1) rats in the CXCR4-BMSC group were injected via the tail vein with 1 mL of PBS containing 1 × 106 CXCR4-overexpressing BMSCs and (2) rats in the pBABE-BMSC group were injected via the tail vein with 1 mL of PBS containing 1 × 106 pBABE-puro-null-BMSCs) [34]. The animals in the control and model groups received injections of 1 mL of PBS via the tail vein.
Lung tissue harvesting
Every month, six rats from each group were anesthetized with 3% pentobarbital sodium (0.3 mL/100 g) administered intraperitoneally and then fixed on a foam board for harvesting of lung tissue. The harvested lung tissues were flushed with 10 mL of cold PBS to remove excess blood. The lower lobe of the right lung was fixed with paraformaldehyde (4%), and the other lung tissues were snap-frozen in liquid nitrogen and stored at − 80 °C until use.
Hematoxylin and eosin (H&E) staining and measurement of the alveolar mean linear intercept (MLI)
The lung tissues were fixed with paraformaldehyde (4%) for 12 h and embedded in paraffin. A rotary slicer was used to slice the lung tissue into 5-µm-thick sections, which were then stained with H&E. After H&E staining, the morphology of the lung tissue was observed and randomly photographed at ×200 magnification under a light microscope, and the resulting photographs were used to measure the alveolar MLI excluding the sections of bronchi, large bronchioli and blood vessels. Image-Pro Plus 6.0 software was used to measure the alveolar MLI. First, seven horizontal and eleven vertical lines were drawn on each photograph. Second, the number of alveolar septa on each line was counted. Third, the following equation was used to measure the alveolar MLI, which was calculated by dividing the length of the grid line by the number of intersections with alveolar walls [35].
Western blotting
The expression of apoptotic proteins in the lung tissue of each group and the expression of CXCR4 protein in BMSCs were detected by Western blotting. Total proteins from BMSCs and lung tissue were collected by lysis in RIPA lysis buffer containing a proteinase inhibitor. After a 30-min ice bath, the extracts were centrifuged at 12,000×g and 4 °C for 30 min, and the supernatants were collected. After the protein concentration was measured by BCA assay, the protein sample was mixed with SDS buffer at a ratio of 4:1 and boiled for 10 min. The proteins were separated by 12% SDS‒PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. PVDF membranes were blocked with TBST containing 5% nonfat milk at room temperature for 2 h and washed three times with TBST. Subsequently, the PVDF membranes were incubated with the following primary antibodies overnight: rabbit anti-rat CXCR4 (Abcam, 1:100), mouse anti-rat GAPDH (Abcam, 1:1000), rabbit anti-rat cleaved caspase-8 (Abcam, 1:1000), rabbit anti-rat cleaved caspase-3 (Abcam, 1:1000), rabbit anti-rat cleaved PARP-1 (Abcam, 1:1000), and rabbit anti-rat cleaved PARP-β-actin (Abcam, 1:1000). The PVDF membranes were washed three times with TBST and incubated with HRP-conjugated secondary antibodies (1:5000) for 2 h. A chemiluminescent detection system (Bio-Rad, USA) was used to detect the target protein, and the gray value was measured using ImageJ software.
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay
TUNEL staining was used to detect the apoptotic signal of lung cells. Lung tissue sections were incubated with proteinase K for 25 min at 37 °C and washed three times with PBS. After permeabilization with Triton X-100 for 20 min, the lung sections were incubated with a reaction mixture that contained TdT and dUTP (mixed at a ratio of 1:9) for 30 min at 37 °C. The sections were treated for 10 min in 3% hydrogen peroxide to quench endogenous peroxidase activity, incubated with Converter-POD solution, washed with PBS and stained with DAB. A cell with TUNEL signals within the DAB nuclear stain was deemed TUNEL-positive. The cells were randomly photographed at ×200 magnification under a light microscope. The numbers of cells in the alveoli and TUNEL-positive cells were counted using Mage-Pro Plus 6.0 software.Fields containing bronchi or large bronchioles were excluded from the analysis. The apoptosis rate was calculated as the number of apoptotic cells divided by the number of total cells [36].
Statistical analysis
Statistical analyses were performed using SPSS 17.0 statistical analysis software. All the data were tested for normality using the Shapiro‒Wilk test. All the data showed a normal distribution and are expressed as the means ± SDs. Student’s t test was used to determine the significance of differences between groups, and p < 0.05 was considered to indicate statistical significance.
Results
Morphological observations and induction of differentiation
The results confirmed that the 3rd-passage cultured cells were BMSCs (Fig. 2).Under microscope, the BMSCs exhibited a typical spindle shape, and they displayed a vortex shape once they reached 80–90% confluence. After induction with differentiation medium, the mineralization capacity of the osteoblasts was assessed by Alizarin red staining, with the bone-like nodules being stained red. The adipogenic differentiation of BMSCs was evidenced by red oil drops after Oil red O staining. Some BMSCs had differentiated into chondrogenic-like cells, and proteoglycans were stained blue with Alcian blue. Flow cytometric analysis of the surface markers of BMSCs showed that the positive rates of CD29 and CD90 were 97% and 97%, respectively; the positive rates of the isotype controls were 0.51% and 0.51%, respectively. In addition, the positive rates of CD45 and CD34 in BMSCs were 0.42% and 0.42%, respectively, and the positive rates of the isotype controls were 0.51% and 0.51%, respectively.
After separation and purification, the stem cells exhibited a typical spindle shape. Differentiation medium was used according to the protocol of Cyagen Biosciences, and the cells differentiated into osteoblasts, adipocytes and chondrocytes. The cells contained characteristic components of differentiated cells, such as bone-like nodules, oil drops and proteoglycans.Flow cytometric analysis of the surface markers of isolated cells revealed surface markers of BMSCs, with high expression of CD29 and CD90 and low expression of CD45 and CD34.
Overexpression of CXCR4 mRNA and protein in BMSCs
CXCR4 mRNA and protein expression in BMSCs after transfection was analyzed by qRT‒PCR and Western blotting. The qRT‒PCR results indicated that the CXCR4 mRNA level in the CXCR4-BMSC group was significantly higher than that in the pBABE-BMSC group, and the difference was significant (p < 0.001). Western blotting detection of CXCR4 protein expression in transfected BMSCs showed that the cells in the CXCR4-BMSC group exhibited significantly higher CXCR4 expression than those in the pBABE-BMSC group (p < 0.01) (Fig. 3).
After retrovirus transfection, the CXCR4 mRNA level in the CXCR4-BMSC group was significantly increased (*p < 0.001), and CXCR4 protein was stably expressed (*p < 0.01)
CXCR4-BMSC transplantation reduced the destruction of alveoli
Exposure to cigarette smoke can lead to inflammatory cell infiltration, goblet cell hyperplasia, and mucous gland hypertrophy of the lungs, and HE staining showed that the parenchymal wall of the alveoli thickened gradually with prolonged exposure to cigarette smoke. We also observed inflammatory exudates in the lung. The most prominent feature was alveolar septal rupture, with the alveoli ultimately fusing into pulmonary bullae, and the alveolar MLI gradually increased as the exposure time to cigarette smoke increased.
Statistical analysis indicated that the MLI in the third month (59.8750 ± 8.2148) was clearly higher than that in the first month (39.1049 ± 3.0874) and that the difference was significant (p < 0.05). Analysis of between-group differences in the third month revealed that the MLIs of the control group (34.4288 ± 1.5973) and the CXCR4-BMSC group(40.21932 ± 2.0722) were lower than that of the model group (59.8750 ± 8.2148); these differences were significant (control group vs. model group, p < 0.01; CXCR4-BMSC group vs. model group, p < 0.05). In addition, the alveolar MLI of the pBABE-BMSC group was lower than that of the model group, but the difference was not significant (p > 0.05) (Fig. 4).
In the COPD rat models, the CXCR4-overexpressing BMSCs slowed lung tissue destruction, and in the third month, the alveolar MLI of the CXCR4-BMSC group was significantly lower than that of the model group (*p < 0.05, **p < 0.01)
Treatment with BMSCs overexpressing CXCR4 significantly reduced TUNEL staining in lung tissue
Cell apoptosis in lung tissue collected in the third month was assessed by TUNEL analysis, and positive staining, which appeared tan or brown, was located in the nucleus. The apoptotic index of the model group was significantly higher than that of the control group (p < 0.01). In addition, transplanted BMSCs in vivo reduced the apoptotic index of lung tissue. The apoptotic index of the lung tissue collected from the CXCR4-BMSC group was significantly lower than that of the lung tissue collected from the model group (p < 0.01). Furthermore, the apoptotic index of lung tissue was lower in the pBABE-BMSC group than in the model group (p < 0.05) (Fig. 5).
Apoptotic cells in lung tissue were stained brown. The apoptosis rate was calculated as the number of apoptotic cells divided by the number of total cells, and the results were calculated as percentages and are presented as the means ± SDs. Significant differences are indicated (**p < 0.01 and *p < 0.05 versus the control group, pBABE-BMSC group, CXCR4-BMSC group and COPD model group)
Transplantation of BMSCs overexpressing CXCR4 decreased the expression of apoptotic proteins in lung tissue
Significant differences in the expression of apoptotic proteins were detected between the control group and the model group, and the difference gradually increased with increasing time of exposure to cigarette smoke (Fig. 6). The transplantation of BMSCs reduced apoptosis in lung tissue. In the third month, compared with the model group, the pBABE-BMSC group exhibited reduced expression of cleaved caspase-8 (p < 0.01), cleaved caspase-3 (p > 0.05) and cleaved PARP-1 (p > 0.05); no significant difference was found between these two groups in the first and second months. More significantly, the difference in expression of apoptotic proteins between the CXCR4-BMSC group and the model group was greater than that between the pBABE-BMSC and model groups. The difference in the expression of cleaved caspase-8 between these two groups was significant (p < 0.05) in the second month, but no significant difference in the expression of cleaved caspase-3 was detected. In the third month, the CXCR4-BMSC group exhibited significantly lower protein levels of cleaved caspase-8 (p < 0.01), cleaved caspase-3 and cleaved PARP-1 (p < 0.05) than the model group.The differences between the control and treated groups were as follows:The pBABE-BMSC group exhibited significantly higher protein levels of cleaved caspase-3 in the second (p < 0.05) and third months (p < 0.01) and significantly higher protein levels of cleaved caspase-8 in the third month (p < 0.05). The expression of apoptotic proteins was not significantly different between the CXCR4-BMSC group and the control group during the construction of the COPD apoptosis models.
Western blotting revealed a marked reduction in the expression of apoptotic proteins in the BMSC-treated rats, particularly in the rats treated with CXCR4-overexpressing BMSCs. The dynamics of the expression of apoptosis-related proteins in lung tissue during construction of the COPD rat models were observed. The expression of apoptosis-related proteins in the first, second and third months was detected, and the results showed that CXCR4-overexpressing BMSCs significantly inhibited the exogenous apoptosis pathway to reduce apoptosis in lung tissue, as shown by the reduced expression of cleaved caspase-8, cleaved caspase-3 and cleaved PARP-1. The data are presented as the means ± SDs. Significant differences are indicated (**p < 0.01 and *p < 0.05 versus the control group, pBABE-BMSC group, CXCR4-BMSC group and model group; Δp < 0.05 and ΔΔp < 0.01 versus the pBABE-BMSC group, CXCR4-BMSC group and control group).
Discussion
COPD remains a chronic disease with a high mortality rate, and the mortality rate is increasing yearly due to a lack of effective treatment [10,11,12]. Studies have confirmed that apoptosis is extremely important in the occurrence and development of COPD [2, 3], and anti-apoptotic therapy is considered a new strategy for COPD treatment. The anti-apoptoticeffect of BMSCsin the treatment of various diseases has been assessed [37,38,39], and the present study demonstrates the anti-apoptotic effect of BMSCs in the progression of COPD.
Cells obtained using the whole bone marrow adherence method have the potential for multidirectional differentiation and can be induced to differentiate into osteoblasts, adipocytes, and chondrocytes. Flow cytometric assays showed that the surface antigens of the cells conformed to the criteria defining BMSCs. The chemokine receptors of BMSCs play an important role in disease treatment, but the expression of chemokine receptors in BMSCs is reduced during isolation and purification [40]. In this study, CXCR4 overexpression in BMSCs was induced by retroviral transfection.
CXCR4 protein is a common G protein-coupled receptor on the cell surface; however, after several passages in vitro, BMSCs lose this receptor, which could be the most important cause of the decreases in their treatment effects [41].
To determine whether the promotion of CXCR-4 leads to stemness resistance in stem cells, we have not discovered experiments comparing the expression of the CXCR4 protein and stemness-related molecules among primary BMSCs, subcultured BMSCs, and CXCR4-overexpressing BMSCs. Some studies have revealed that overexpression of the CXCR-4 protein on the surface of BMSCs has no effect on the biological features or viability of the BMSCs themselves and that the expression of CXCR4 can maintain the stemness of stem cells [42, 43].
Smoking is the primary pathogenic factor of COPD. In this study, SD rat models of COPD were constructed via exposure to cigarette smoke. After rat exposure to cigarette smoke, a significant increase in the MLI was observed along with alveolar enlargement and parenchymal wall destruction, and the destruction of the parenchymal wall increased gradually with increasing exposure time to cigarette smoke. The TUNEL assay showed that the destruction of alveoli was associated with cell apoptosis. Moreover, cigarette smoke exposure caused DNA fragmentation and resulted in apoptosis; thus, the expression of apoptosis-related proteins in the lung was significantly increased. The analysis of apoptosis-related proteins revealed that cigarette smoke induced the cleavage of caspase-8 by TNF-related apoptosis-inducing ligand and that caspase-3 could be cleaved by cleaved caspase-8. Furthermore, we detected DNA breaks in the nucleus, which might have been due to increased expression of cleaved PARP-1 protein, and the trend in the expression of cleaved PARP-1 was similar to the trend in the apoptosis rate in lung tissue determined by TUNEL assays. All of these results showed that the expression of apoptosis-related proteins and DNA fragmentation were increased in the COPD models. Exposure to cigarette smoke induced alveolar septal rupture and caused expansion of the alveolar cavity, which might have been related to the DNA breaks in the nucleus and eventually led to the apoptosis of lung tissue cells.
The transplantation of BMSCs in vivo is often used for the treatment of lung disease, and transplantation via the tail vein is a common transplantation method used with rat models. In this study, BMSCs were transplanted in vivo via the tail vein during the generation of SD rat models of COPD. Histological observations and MLI measurements showed that BMSCs transplanted in vivo via the tail vein reduced alveolar septal rupture and alveolar cavity expansion, and the effects became increasingly obvious with increasing treatment time.
Moreover, the results from the TUNEL assay of lung tissue showed that the transplantation of BMSCs in vivo via the tail vein reduced apoptosis in lung tissue by reducing the DNA breaks in the nucleus, which eventually delayed the progression of COPD. Cigarette smoke can induce cell apoptosis in lung tissue through various pathways, and the results described above showed that the transplantation of BMSCs in vivo reduced cell apoptosis; this reduction may have been related to the reductions in nuclear DNA breakage.
Apoptosis has long been considered essential for normal development, acting to remove superfluous or damaged cells resulting from high proliferation and shaping tissues by removing unwanted cells [44, 45]. Some studies have demonstrated persistent apoptosis in the lung tissues of nonsmoking healthy people; this persistent apoptosis is related to DNA breaks in the nucleus [46]. In this study, we discovered that the control group showed cell apoptosis in lung tissue, and the expression of apoptotic proteins indicated that the extrinsic apoptotic pathway played an important role in apoptosis in the control group and resulted in DNA breakage in the nucleus.
To further explore the therapeutic effect of BMSC transplantation in the SD rat model of COPD, we detected the expression of apoptosis-related proteins in lung tissues and compared it among the various groups. Analysis of these proteins in the third month showed that BMSC transplantation reduced the expression levels of cleaved PARP-1 and nuclear DNA breakage compared with those in the model group. Moreover, the expression of cleaved caspase-8 and cleaved caspase-3 was substantially reduced in the BMSC transplantation groups compared with the model group. These results indicated that the transplantation of BMSCs in vivo via the tail vein inhibited the exogenous apoptotic pathway to reduce cell apoptosis by decreasing DNA fragmentation in the nucleus and that BMSCs overexpressing CXCR4 yielded better therapeutic outcomes than pBABE-BMSCs.
Apoptosis can be divided into early apoptosis and advanced apoptosis, and proapoptotic proteins can be activated at the early apoptosis stage [47, 48]. The results revealed that BMSCs can inhibit early apoptosis in lung tissue by inhibiting the exogenous apoptotic pathway.
In our comparison between the control group and the treated groups, we discovered that the expression of apoptosis-related proteins had already increased in the first month. As the time of exposure to cigarette smoke increased, we continued to observe differences in the expression of apoptosis-related proteins between the control and treatment groups, which became more pronounced in the second and third months, especially between the control group and the pBABE-BMSC group. These results revealed that the transplantation of BMSCs in vivo inhibited the progression of COPD by reducing apoptosis but did not allow lung function to recover under persistent exposure to cigarette smoke.
The treatment initially yielded no effect, but a clear effect was detected at approximately the third month of therapy. This finding was attributed to the accumulation of cigarette smoke in the circulatory system during construction of the apoptosis model, which impacted the therapeutic effect of BMSCs. For example, nicotine interacts with BMSCs via choline receptors and affects BMSC migration, differentiation, and paracrine signaling [49,50,51,52]. After cell transplantation, the exposure to cigarette smoke was continued, which affected the activity and therapeutic effect of BMSCs. The expression of apoptosis-related proteins in lung tissue did not significantly differ among the groups at the early stage of BMSC transplantation therapy because of the effect of cigarette smoke.
The results of this experiment showed that the transplantation of BMSCs overexpressing CXCR4 protein significantly inhibited apoptosis during the course of COPD. The use of MSCs to treat lung diseases has been researched extensively because of their prominent role in immune modulation; their ability to migrate to injury sites; their secretion of growth factors, anti-inflammatory cytokines, and extracellular vesicles as well as their activities mediated through differentiation and cell–cell junction [53,54,55,56].
However, further study is needed to elucidate the exact mechanism through which BMSCs inhibit apoptosis or reduce alveolar damage. BMSCs transplanted via the tail vein can accumulate in the pulmonary circulatory system and participate in the treatment of lung diseases [57, 58]. The local expression of SDF-1 increases after lung tissue damage, and BMSCs overexpressing CXCR4 can be recruited to lung tissue much more easily via the SDF‐1/CXCR4 axis. Previous research on the therapeutic effect of BMSCs overexpressing CXCR4 has focused on homing to the site of injury through the CXCR4/SDF-1 axis [27, 30].
The homing of BMSCs to the lung tissue had been confirmed, but they stay in the lung for only a short time. In a previous experiment, the abundance of MSCs in the lung was obviously decreased at 1 h after transplantation, and most had accumulated in the liver within 24 h [59]. Most studies on the homing of stem cells have focused on the differentiation of stem cells into damaged tissue cells, but there have been reports of increased stem cell death at the transplant site. In addition, due to the short residence time of stem cells in lung tissues, we believe that stem cells reduce tissue damage in the development of COPD by other methods in addition to homing and differentiating [60].
All the evidence to date suggests that MSCs inhibit apoptosis not only by homing to the lung but also by other activities. Recent studies have revealed the effects of paracrine signaling in BMSCs, such as angiogenesis stimulation and the adjustment of inflammatory conditions and immune responses in animal models of pulmonary diseases [55, 56]. Exosomes are important for cell-to-cell communication, and BMSCs can transfer their cargo to remote sites by exosomes to treat diseases. Exosomes play an important paracrine role in BMSCs.
During COPD development, exosomes are also produced by immune cells in lung tissue and play a role in the interaction of immune cells and in inducing the apoptosis of lung tissue cells. The paracrine effects of stem cells, in which exosomes can modulate immune responses, have been shown to reduce the inflammatory response in lung tissue diseases, but there is still much to learn regarding the anti-apoptotic mechanism. Building a COPD model requires long-term exposure to cigarette smoke, and the activity of stem cells will be further reduced because of exposure to this continuously harsh environment, but secreted exosomes will have a sustained therapeutic effect regardless of environmental conditions. Clinical studies on the therapeutic effect of stem cell-derived exosomes in COPD have shown that stem cell-derived exosomes can alleviate post-COVID-19 complications by reducing the inflammatory response [61, 62]. BMSCs overexpressing CXCR4 can exert enhanced paracrine effects and inhibit apoptosis by secreting cell growth factors, and these effects are believed to be related to SDF-1-induced CXCR4 internalization [63].
This experiment showed that the transplantation of BMSCs overexpressing CXCR4 protein during COPD model establishment can inhibit apoptosis via extrinsic pathways and thereby delay the progression of COPD. However, the mechanisms underlying the antiapoptotic effects of CXCR4-overexpressing BMSCs need to be further elucidated.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Safitri W, Martini S, Artanti KD, Li CY (2021) Smoking from a younger age is the dominant factor in the incidence of chronic obstructive pulmonary disease: case-control study. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18116047
Aoshiba K, Zhou F, Tsuji T, Nagai A (2012) DNA damage as a molecular link in the pathogenesis of COPD in smokers. Eur Respir J 39(6):1368–1376. https://doi.org/10.1183/09031936.00050211
Nitta NA, Sato T, Komura M, Yoshikawa H, Suzuki Y, Mitsui A et al (2022) Exposure to the heated tobacco product IQOS generates apoptosis-mediated pulmonary emphysema in murine lungs. Am J Physiol Lung Cell Mol Physiol. https://doi.org/10.1152/ajplung.00215.2021
Sun X, Feng X, Zheng D, Li A, Li C, Li S, Zhao Z (2019) Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo. Clin Sci (Lond) 133(13):1523–1536. https://doi.org/10.1042/cs20190331
Paschalaki K, Rossios C, Pericleous C, MacLeod M, Rothery S, Donaldson GC et al (2022) Inhaled corticosteroids reduce senescence in endothelial progenitor cells from patients with COPD. Thorax. https://doi.org/10.1136/thoraxjnl-2020-216807
Zhou Q, Zhang L, Sun Y, Xie M, Lin J (2020) Clinical value of N-acetylcysteine combined with terbutaline sulfate in elderly patients with chronic obstructive pulmonary disease and its effect on apoptosis/anti-apoptosis mechanism. Ann Palliat Med 9(5):3393–3401. https://doi.org/10.21037/apm-20-1605
Hodge S, Hodge G, Holmes M, Reynolds PN (2005) Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur Respir J 25(3):447–454. https://doi.org/10.1183/09031936.05.00077604
Strulovici-Barel Y, Staudt MR, Krause A, Gordon C, Tilley AE, Harvey BG et al (2016) Persistence of circulating endothelial microparticles in COPD despite smoking cessation. Thorax 71(12):1137–1144. https://doi.org/10.1136/thoraxjnl-2015-208274
Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E et al (2015) Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 5(2):020415. https://doi.org/10.7189/jogh.05-020415
Ferraro M, Di Vincenzo S, Dino P, Bucchieri S, Cipollina C, Gjomarkaj M, Pace E (2019) Budesonide, Aclidinium and Formoterol in combination limit inflammaging processes in bronchial epithelial cells exposed to cigarette smoke. Exp Gerontol 118:78–87. https://doi.org/10.1016/j.exger.2019.01.016
Teng C, Jeng L, Shyu W (2018) Role of insulin-like growth factor 1 receptor signaling in stem cell stemness and therapeutic efficacy. Cell Transpl 27(9):1313–1319. https://doi.org/10.1177/0963689718779777
El Haddad N, Heathcote D, Moore R, Yang S, Azzi J, Mfarrej B et al (2011) Mesenchymal stem cells express serine protease inhibitor to evade the host immune response. Blood 117(4):1176–1183. https://doi.org/10.1182/blood-2010-06-287979
Guan R, Yao H, Li Z, Qian J, Yuan L, Cai Z et al (2021) Sodium tanshinone IIA sulfonate attenuates cigarette smoke extract-induced mitochondrial dysfunction, oxidative stress and apoptosis in alveolar epithelial cells by enhancing SIRT1 pathway. Toxicol Sci. https://doi.org/10.1093/toxsci/kfab087
Yang S, Wu H, Zhao J, Wu X, Zhao J, Ning Q et al (2014) Feasibility of 8-OHdG formation and hOGG1 induction in PBMCs for assessing oxidative DNA damage in the lung of COPD patients. Respirology (Carlton, Vic.) 19(8):1183–1190. https://doi.org/10.1111/resp.12378
English K, Ryan J, Tobin L, Murphy M, Barry F, Mahon B (2009) Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 156(1):149–160. https://doi.org/10.1111/j.1365-2249.2009.03874.x
Hyvärinen K, Holopainen M, Skirdenko V, Ruhanen H, Lehenkari P, Korhonen M et al (2018) Mesenchymal stromal cells and their extracellular vesicles enhance the anti-inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)-23 and IL-22. Front Immunol 9:771. https://doi.org/10.3389/fimmu.2018.00771
Lim J, Ryu D, Lee S, Park G, Min C (2017) Mesenchymal stem cells (MSCs) attenuate cutaneous sclerodermatous graft-versus-host disease (Scl-GVHD) through inhibition of immune cell infiltration in a mouse model. J Invest Dermatol 137(9):1895–1904. https://doi.org/10.1016/j.jid.2017.02.986
Gu Y, Zhang Y, Bi Y, Liu J, Tan B, Gong M et al (2015) Mesenchymal stem cells suppress neuronal apoptosis and decrease IL-10 release via the TLR2/NFκB pathway in rats with hypoxic-ischemic brain damage. Mol Brain 8(1):65. https://doi.org/10.1186/s13041-015-0157-3
Markel T, Crafts T, Jensen A, Hunsberger E, Yoder M (2015) Human mesenchymal stromal cells decrease mortality after intestinal ischemia and reperfusion injury. J Surg Res 199(1):56–66. https://doi.org/10.1016/j.jss.2015.06.060
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove C, Bovenkerk J et al (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109(10):1292–1298. https://doi.org/10.1161/01.Cir.0000121425.42966.F1
Gong H, Cheng W, Wang Y (2019) Tumor necrosis factor-related apoptosis-inducing ligand inhibits the growth and aggressiveness of colon carcinoma via the exogenous apoptosis signaling pathway. Exp Ther Med 17(1):41–50. https://doi.org/10.3892/etm.2018.6901
Huang X, Ou C, Shu Y, Wang Y, Gong S, Luo R et al (2021) A self-sustained nanoplatform reverses TRAIL-resistance of pancreatic cancer through coactivating of exogenous and endogenous apoptotic pathway. Biomaterials 272:120795. https://doi.org/10.1016/j.biomaterials.2021.120795
Vagima Y, Lapid K, Kollet O, Goichberg P, Alon R, Lapidot T (2011) Pathways implicated in stem cell migration: the SDF-1/CXCR4 axis. Methods Mol Biol (Clifton, N.J.) 750:277–289. https://doi.org/10.1007/978-1-61779-145-1_19
Saito Y, Shimada M, Utsunomiya T, Ikemoto T, Yamada S, Morine Y et al (2014) Homing effect of adipose-derived stem cells to the injured liver: the shift of stromal cell-derived factor 1 expressions. J Hepatobiliary Pancreat Sci 21(12):873–880. https://doi.org/10.1002/jhbp.147
Wang Y, Fu W, Zhang S, He X, Liu Z, Gao D, Xu T (2014) CXCR-7 receptor promotes SDF-1α-induced migration of bone marrow mesenchymal stem cells in the transient cerebral ischemia/reperfusion rat hippocampus. Brain Res 1575:78–86. https://doi.org/10.1016/j.brainres.2014.05.035
Wang Z, Wang Y, Wang Z, Gutkind J, Wang Z, Wang F et al (2015) Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells (Dayton, Ohio) 33(2):456–467. https://doi.org/10.1002/stem.1878
Shahzad U, Li G, Zhang Y, Li R, Rao V, Yau T (2015) Transmyocardial revascularization enhances bone marrow stem cell engraftment in infarcted hearts through SCF-C-kit and SDF-1-CXCR4 signaling axes. Stem Cell Rev Rep 11(2):332–346. https://doi.org/10.1007/s12015-014-9571-7
Yang J, Zhang N, Wang H, Gao P, Yang Q, Wen Q (2015) CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem 290(4):1994–2006. https://doi.org/10.1074/jbc.M114.605063
Chen H, Zhu H, Chu Y, Xu F, Liu Y, Tang B et al (2014) Construction of mouse VCAM-1 expression vector and establishment of stably transfected MSC line C3H10T1/2. Zhongguo Shi Yan Xue Ye Xue Za Zhi 22(5):1396–1401. https://doi.org/10.7534/j.issn.1009-2137.2014.05.041
Li Y, Li S, Li J, Deng L, Tian Y, Jiang S et al (2012) A rat model for stable chronic obstructive pulmonary disease induced by cigarette smoke inhalation and repetitive bacterial infection. Biol Pharm Bull 35(10):1752–1760. https://doi.org/10.1248/bpb.b12-00407
Lin J, Xu F, Wang G, Kong L, Luo Q, Lv Y et al (2016) Paeoniflorin attenuated oxidative stress in rat COPD model induced by cigarette smoke. Evid-Based Complement Altern Med: eCAM 2016:1698379. https://doi.org/10.1155/2016/1698379
Zhang E, Yang Y, Chen S, Peng C, Lavin M, Yeo A et al (2018) Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/β-catenin signaling in rats. Stem Cell Res Ther 9(1):311. https://doi.org/10.1186/s13287-018-1045-4
Cervilha DAB, Ito JT, Lourenço JD, Olivo CR, Saraiva-Romanholo BM, Volpini RA et al (2019) The Th17/Treg cytokine imbalance in chronic obstructive pulmonary disease exacerbation in an animal model of cigarette smoke exposure and lipopolysaccharide challenge association. Sci Rep 9(1):1921. https://doi.org/10.1038/s41598-019-38600-z
Mimae T, Hagiyama M, Inoue T, Yoneshige A, Kato T, Okada M et al (2014) Increased ectodomain shedding of lung epithelial cell adhesion molecule 1 as a cause of increased alveolar cell apoptosis in emphysema. Thorax 69(3):223–231. https://doi.org/10.1136/thoraxjnl-2013-203867
Rabe K, Hurd S, Anzueto A, Barnes P, Buist S, Calverley P et al (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176(6):532–555. https://doi.org/10.1164/rccm.200703-456SO
Gogebakan B, Bayraktar R, Ulaslı M, Oztuzcu S, Tasdemir D, Bayram H (2014) The role of bronchial epithelial cell apoptosis in the pathogenesis of COPD. Mol Biol Rep 41(8):5321–5327. https://doi.org/10.1007/s11033-014-3403-3
Zhao Q, Hao C, Wei J, Huang R, Li C, Yao W (2021) Bone marrow-derived mesenchymal stem cells attenuate silica-induced pulmonary fibrosis by inhibiting apoptosis and pyroptosis but not autophagy in rats. Ecotoxicol Environ Saf 216:112181. https://doi.org/10.1016/j.ecoenv.2021.112181
Xu TB, Li L, Luo XD, Lin H (2017) BMSCs protect against liver injury via suppressing hepatocyte apoptosis and activating TGF-β1/Bax singling pathway. Biomed Pharmacother 96:1395–1402. https://doi.org/10.1016/j.biopha.2017.11.023
Zhang CS, Shao K, Liu CW, Li CJ, Yu BT (2019) Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci 23(15):6691–6699. https://doi.org/10.26355/eurrev_201908_18560
Rombouts W, Ploemacher R (2003) Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17(1):160–170. https://doi.org/10.1038/sj.leu.2402763
Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE et al (2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104(9):2643–2645. https://doi.org/10.1182/blood-2004-02-0526
Chen Z, Chen Q, Du H, Xu L, Wan J (2018) Mesenchymal stem cells and CXC chemokine receptor 4 overexpression improved the therapeutic effect on colitis via mucosa repair. Exp Ther Med 16(2):821–829. https://doi.org/10.3892/etm.2018.6233
Ho SY, Ling TY, Lin HY, Liou JT, Liu FC, Chen IC et al (2017) SDF-1/CXCR4 signaling maintains stemness signature in mouse neural stem/progenitor cells. Stem Cells Int 2017:2493752. https://doi.org/10.1155/2017/2493752
Opferman J, Korsmeyer S (2003) Apoptosis in the development and maintenance of the immune system. Nat Immunol 4(5):410–415. https://doi.org/10.1038/ni0503-410
Madden S, Donovan M, Cotter T (2007) Key apoptosis regulating proteins are down-regulated during postnatal tissue development. Int J Dev Biol 51(5):415–423. https://doi.org/10.1387/ijdb.062263sm
Xue H, Xie B, Xu N, Li H, Chen Q, Xie W, Wang H (2021) Etanercept protected against cigarette smoke extract-induced inflammation and apoptosis of human pulmonary artery endothelial cells via regulating TNFR1. Int J Chron Obstruct Pulmon Dis 16:1329–1345. https://doi.org/10.2147/copd.S295580
Duncan-Lewis C, Hartenian E, King V, Glaunsinger B (2021) Cytoplasmic mRNA decay represses RNA polymerase II transcription during early apoptosis. Elife. https://doi.org/10.7554/eLife.58342
Khalil C, Chahine J, Haykal T, Al Hageh C, Rizk S, Khnayzer R (2021) E-cigarette aerosol induced cytotoxicity, DNA damages and late apoptosis in dynamically exposed A549 cells. Chemosphere 263:127874. https://doi.org/10.1016/j.chemosphere.2020.127874
Benowitz N, Hukkanen J, Jacob P (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 192:29–60. https://doi.org/10.1007/978-3-540-69248-5_2
Zeng H, Qin Y, Chen H, Bu Q, Li Y, Zhong Q et al (2014) Effects of nicotine on proliferation and survival in human umbilical cord mesenchymal stem cells. J Biochem Mol Toxicol 28(4):181–189. https://doi.org/10.1002/jbt.21551
Zhou Y, Gan Y, Taylor H (2011) Cigarette smoke inhibits recruitment of bone-marrow-derived stem cells to the uterus. Reprod Toxicol (Elmsford, N.Y.) 31(2):123–127. https://doi.org/10.1016/j.reprotox.2010.10.007
Wahl E, Schenck T, Machens H, Egaña J (2016) Acute stimulation of mesenchymal stem cells with cigarette smoke extract affects their migration, differentiation, and paracrine potential. Sci Rep 6:22957. https://doi.org/10.1038/srep22957
Sdrimas K, Kourembanas S (2014) MSC microvesicles for the treatment of lung disease: a new paradigm for cell-free therapy. Antioxid Redox Signal 21(13):1905–1915. https://doi.org/10.1089/ars.2013.5784
Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T et al (2014) Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol 306(11):L975-985. https://doi.org/10.1152/ajplung.00242.2013
Zhu H, Xiong Y, Xia Y, Zhang R, Tian D, Wang T et al (2017) Therapeutic effects of human umbilical cord-derived mesenchymal stem cells in acute lung injury mice. Sci Rep 7:39889. https://doi.org/10.1038/srep39889
Gupta N, Su X, Popov B, Lee J, Serikov V, Matthay M (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol (Baltimore, Md.: 1950) 179(3):1855–1863. https://doi.org/10.4049/jimmunol.179.3.1855
Lee R, Pulin A, Seo M, Kota D, Ylostalo J, Larson B et al (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5(1):54–63. https://doi.org/10.1016/j.stem.2009.05.003
Yang Y, Li Y, Wang Y, Ruan G, Tian C, Wang Q et al (2021) The effects of BMMSC treatment on lung tissue degeneration in elderly macaques. Stem Cell Res Ther 12(1):156. https://doi.org/10.1186/s13287-021-02201-3
Eggenhofer E, Benseler V, Kroemer A, Popp F, Geissler E, Schlitt H et al (2012) Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 3:297. https://doi.org/10.3389/fimmu.2012.00297
Mirershadi F, Ahmadi M, Rezabakhsh A, Rajabi H, Rahbarghazi R, Keyhanmanesh R (2020) Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Res Ther 11(1):400. https://doi.org/10.1186/s13287-020-01921-2
Rajabi H, Konyalilar N, Erkan S, Mortazavi D, Korkunc SK, Kayalar O et al (2022) Emerging role of exosomes in the pathology of chronic obstructive pulmonary diseases; destructive and therapeutic properties. Stem Cell Res Ther 13(1):144. https://doi.org/10.1186/s13287-022-02820-4
Rajabi H, Mortazavi D, Konyalilar N, Aksoy GT, Erkan S, Korkunc SK et al (2022) Forthcoming complications in recovered COVID-19 patients with COPD and asthma; possible therapeutic opportunities. Cell Commun Signal 20(1):173. https://doi.org/10.1186/s12964-022-00982-5
Wu S, Li Y, Huang W, Cai W, Liang J, Paul C et al (2017) Paracrine effect of CXCR4-overexpressing mesenchymal stem cells on ischemic heart injury. Cell Biochem Funct 35(2):113–123. https://doi.org/10.1002/cbf.3254
Acknowledgements
We would like to thank Huimin Liu for the help with the manuscript preparation.
Funding
This work was supported in part by the Guangdong Province Financial Technology Research and Development, Promotion and Application Special Fund Project (Yue Cai Gong [2013] No. 401).
Author information
Authors and Affiliations
Contributions
JG contributed to the conception and design of the study; the provision of study materials; the collection, analysis and interpretation of the data; and the writing of the manuscript. YL contributed to the provision of study materials. JC and HS contributed to the data collection and analysis. HL contributed to the writing of the manuscript, specifically its review and editing, project administration, funding acquisition, and supervision. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Consent to participate
Not applicable.
Consent for publication
All participants have approved this article for publication.
Ethical approval
This experiment was approved by the Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China (SYXK2017-0124).
Research involving human and animal participants
The animals used in this experiment were specific pathogen-free (SPF) Sprague‒Dawley (SD) rats purchased from Guangdong Medical Laboratory Animal Center.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, J., Liang, Y., Chen, J. et al. CXCR4 enhances the inhibitory effects of bone mesenchymal stem cells on lung cell apoptosis in a rat model of smoking-induced COPD. Apoptosis 28, 639–652 (2023). https://doi.org/10.1007/s10495-022-01800-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01800-6