Abstract
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been growing swiftly worldwide. Patients with background chronic pulmonary inflammations such as asthma or chronic obstructive pulmonary diseases (COPD) are likely to be infected with this virus. Of note, there is an argument that COVID-19 can remain with serious complications like fibrosis or other pathological changes in the pulmonary tissue of patients with chronic diseases. Along with conventional medications, regenerative medicine, and cell-based therapy could be alternative approaches to compensate for organ loss or restore injured sites using different stem cell types. Owing to unique differentiation capacity and paracrine activity, these cells can accelerate the healing procedure. In this review article, we have tried to scrutinize different reports related to the harmful effects of SARS-CoV-2 on patients with asthma and COPD, as well as the possible therapeutic effects of stem cells in the alleviation of post-COVID-19 complications.
Video abstract
Similar content being viewed by others
Introduction
SARS-CoV-2, a β-type coronavirus, affects nearly all world population after appearing in Wuhan in November 2019 [1, 2]. It was suggested that COVID-19 contributes to extreme clinical symptoms mainly in patients with background metabolic disorders like diabetes and chronic respiratory diseases such as COPD and asthma. The main reason for this claim is the lack of normal function in lung tissue and the existence of chronic inflammation predisposes patients to exhibit subacute and acute forms of COVID-19 [3]. In two studies conducted by different research teams, it has been indicated that SARS-CoV-2 infection is more likely to lead to serious complications in asthmatic conditions and that these patients experience severe forms of COVID-19 with high-rate mortality [4, 5]. Likewise, the entry of pathogenic virus-like human rhinovirus exacerbates asthmatic conditions [6, 7].
Additionally, post-Covid-19 complications are another important issue in COPD and asthmatic patients. It is assumed that individuals affected by asthma and COPD experience serious pathological changes in their respiratory system notably the lungs even after recovery from COVID-19 [8]. In this regard, the restoration of normal lung function in asthmatic and COPD patients, which are affected by COVID-19, is the subject of debate. During the last decades, the application of stem cells and progress in regenerative medicine approaches has provided an alternative therapeutic platform in clinical settings. Considering the differentiation capacity and paracrine activity of stem cells, these cells have been applied for the restoration of injured tissues during several pathological conditions [9]. Here, we summarized reliable data about the effect of SARS-CoV-2 on COPD and asthmatic patients’ health and the possible impact of stem cells in the restoration and alleviation of post-COVID-19-related pathologies.
SARS-CoV-2 structure and COVID-19 pathogenesis
SARS-CoV-2, a causative agent of COVID-19, was reported first in Wuhan [10]. The human respiratory system is the main target of a virus with a high-morbidity rate compared to the previous forms of other coronaviruses [11]. Molecular investigations have shown that this virus is a new form of β-coronaviruses that was not previously identified in human. The viral nucleocapsid is surrounded by a lipid envelope which is armed with different protein types like a spike (S), envelope (E), and membrane (M) [8]. SARS-CoV-2 with positive-single strand RNA (+SSRNA) harbors several open reading frames (ORFs) to encode different viral proteins. ORF1a and 1b are located at the 5’ terminus and involved in the transcription of about 15 different proteins with non-structural (NSPs) and phosphatase (pp1a and b) activity (Fig. 1). NSPs mediate the formation of replication and transcription complex. Besides, the integrity of genome is preserved via NSPs by engaging certain enzymes. Envelop proteins such as M, S, and N are encoded by ORFs at 3’ terminus [12]. Other viral proteins like M and E participate in the shape and formation of the envelope [13].
Spike glycoproteins are highly glycosylated and composed of two subunits S1 and S2. These proteins are involved in the attachment of the virus to the host cell angiotensin-converting enzyme-2 (ACE-2) receptor and further entry into the cytosol [14]. S1 subunit exists in trimeric form and initiates virus attachment to ACE-2. Upon physical interaction between spike protein and ACE-2, S1 trimers are decomposed via the activity of cell membrane bonded serine protease (TMPRSS2) and Furin and further secreted into the extracellular matrix (ECM). The process of physical contact is continued via engaging the S2 subunit to progress a fusion step [15,16,17]. Also, integrins can be acted as alternative receptors for SARS-CoV-2 entry. These proteins can induce a conformational change in viral receptors [16]. Previous studies investigating the entry of SARS-CoV-2 entry into the target cells reported the critical role of the endosomal system in viral replication. The existence of pH-dependent proteases (Cathepsin B and L) inside endosomes can help prime S protein and fusion with the cell membrane [18, 19]. There is direct evidence for the participation of Basigin/CD147 and Neuropilin-1, heparan sulfate, and ADAM17 in SARS-CoV-2 fusion with the host cells [15, 20,21,22]. Within the cytosol, the released +SSRNA attaches to ribosomes to translate into RNA dependant RNA polymerase for the synthesis of polyproteins, and replications. Later, ORF1a and ORF1b are produced [23]. Following the completion of the translation process, vesicles originated from Golgi apparatus and endoplasmic reticulum Golgi intermediate compartment (ERGIC) transfer the viral components to vesicles to produce virions (Fig. 2) [12]. Once the virus infects the cells the pattern recognition receptors (PRRs) detect the viral genome.
The life cycle of SARS-CoV-2 in host cells; The life cycle of the virus begins with the binding of the S1 protein to the cellular receptor ACE2, followed by proteolytic cleavage that makes the S2 protein facilitate the fusion of the viral and cellular membranes. Within the cell, viral RNA replicates, followed by viral proteins translating from the RNA, followed by the assembly of viral proteins and genome within the endoplasmic reticulum and Golgi. Virions are eventually transported to the cell membrane by vesicles and released by budding from the cytoplasmic membrane
ACE-2 receptor is mainly localized at the apical surface of epithelial cells in pulmonary and gastrointestinal tracts, kidneys, and cardiac tissue [24]. It is believed that both ACE-2 receptor and TMPRSS2 can be detected almost in all cell types within the pulmonary tissue even in cells isolated from subsegmental bronchial branches [25]. The cellular distribution of TMPRSS2 is relatively equal in both lungs and subsegmental bronchial branches while the content of ACE-2 receptor is high in secretory cells [25]. The expression of the ACE-2 receptor and TMPRSS2 is high in type II alveolar cells (pneumocytes) compared to type I alveolar cells. In line with these statements, type II alveolar cells along with secretory cells are the main cellular targets for SARS-CoV-2 [13].
Inflammation is an investable procedure and is actively implicated during the infection of tissues with SARS-CoV-2. The close interaction between macrophages and SARS-CoV-2 can contribute to macrophage frustration. Then, the release of several cytokines by activated macrophages primes T lymphocytes such as T helper type 17 (Th17) cells. Recent works have revealed that a large number of factors like tumor necrosis factor-β (TNF-β), interleukins (IL)-1, -6, -8, and -21, monocyte chemoattractant protein-1 (MCP-1), CXCL10, and CCL2 are specifically present in the secretome released by activated macrophages [26]. Remarkably, these cytokines can lead to vasodilation and vascular permeability after leaking into the circulation. Importantly, weakening vascular integrity promotes plasma leakage into interstitial parenchyma and alveolar space, leading to an impaired gas exchange [27,28,29]. The activation of Toll-like receptors (TLR-3, and -7) can result in the detection and entry of SARS-CoV-2 into the target cells. Activated TLR-3 and -7 stimulate the phosphorylation of downstream effectors which activate NF-κB. The current understanding of NF-κB activity implies that this mechanism is required for the production of nuclear IFN and other inflammatory factors. An increase in type I IFN appears to eliminate internalized viruses. While the suppression of IFNs can trigger overproduction of cytokines and causes cytokine storm syndrome [30]. It is important to keep in mind that during this phenomenon an array of several cytokines like IL-1, -6, -17, -21, and -22, and TNF-α is released. In the latter infection stages, the elevation of these mediators recruits further neutrophils and macrophages via the modulation of CD4+ Th1cells activity. The continuity of these responses makes breathing difficult with remarkable hypoxemia and coughing [30, 31]. Local production of cytokines and continuous recruitment of immune cells into the infected sites lead to acute respiratory distress syndrome (ARDS) and organ failure.
Pathophysiology of COPD and asthma
Asthma
Asthma is a chronic inflammatory condition affecting both conducting and respiratory zones. In asthmatic patients, airway hyperresponsiveness and massive remodeling of lung parenchyma are the main pathological findings [32]. It has been shown that both genetic and environmental factors such as viruses, allergens, or occupational irritants have a role in this disease’s evolution [33, 34]. In individuals being exposed to these factors, airway inflammation is induced which is underlying asthma pathogenesis. Under normal conditions, the airway inflammatory response is tightly regulated by the balance between effector and regulatory immune cells [35]. With the onset of asthma, several immune cell types such as mast cells, eosinophils, Th2 cells, basophils, and platelets are abnormally recruited into target sites within the pulmonary niche (Fig. 3) [36,37,38,39]. The production and release of several chemokines and cytokines such as histamine, leukotrienes, prostanoids, kinins, and platelet-activating factors from immune cells can deteriorate the physiological behavior of epithelial cells, smooth muscle cells, vascular cells, and local dendritic cells. Along with these changes, the occurrence of massive pathological remodeling activates the dynamic growth of fibroblasts [35, 40,41,42]. In many cases, histological examination shows a dominant eosinophilic, neutrophilic, and/or mixed eosinophilic-neutrophilic inflammatory response. Based on the eosinophilic response, data suggest two asthmatic conditions including eosinophilic [high T2/T1 cell ratio and eosinophilic reaction] and non-eosinophilic [low T2 helper cells with a low number of eosinophils] types (Fig. 4) [43]. In eosinophilic asthma, Th2 cells secret certain cytokines such as thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 to recruit eosinophils [35, 43]. This form of asthma is clinically characterized by an early-stage allergic (atopic) reaction, leading to the dysregulation of the airway epithelial cell barrier. T cell-derived cytokines mainly TSLP can also stimulate local dendritic cells which in turn intensifies the eosinophilic reaction at the target sites [44, 45]. Further activation of dendritic cells can increase differentiation of lymphoblast toward Th2 cells via presenting antigens to Th1 cells [46]. In this scenario, Th2 and B cell activation can contribute to the production of IL-4, -5, and -13 [47, 48]. Based on these events, B cells can mature into plasma cells and produce a large content of IgE, leading to mast cell activation [47]. In contrast to eosinophilic (atopic) asthma, in non-eosinophilic form types, Th1 and Th17 cells along with neutrophils play a critical role in the progression of pathological changes. Data suggested the presence of different inflammatory factors such as IL‐1β, -6, ‐8, 17A/F, IFN‐γ, and TNF‐α [49]. The most prominent cellular event is likely a neutrophilic reaction and is seen in smokers and individuals with diabetic changes [43]. Activation of TLRs and release of IL-1β, -8, and TNF-α by Th1 and Th17 cells cause neutrophils recruited into the pulmonary niche [50]. The second type of non-eosinophilic asthma is known as paucigranulocytic asthma which is determined by the lack of changes in sputum or blood levels of eosinophils or neutrophils [51]. Data suggested the lack of prominent inflammation thus it is thought that this type of asthma closely correlates with dysfunction or abnormal morphology of smooth muscle cells, and nervous and vascular tissues [52, 53]. In case, inflammatory responses occur both neutrophilic and eosinophilic reactions (mixed granulocytic asthma) can be detected [54].
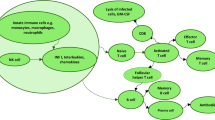
Schematic of eosinophilic and neutrophilic asthma immunopathology. In eosinophilic asthma, type 2 T helper cells secrete T cell-derived cytokines (TSLP, IL-25, and IL-33), which results in the recruiting of eosinophils. TSLP stimulates local dendritic cells, Activation of dendritic cells leads to the differentiation of lymphoblast toward type 2 T helper cells via presenting antigens to type 1 T helper cells. Type 2 T helper and B cells activation contribute to the production of IL-4, -5, and-13. B cells produce a large content of IgE, finally leading to mast cell activation. On the other hand, in non-eosinophilic asthma, the release of IL‐1β, -6, -8, -17, IFN‐γ, and TNF‐α by type 1 and 17 T helper cells cause neutrophils recruited into the pulmonary niche. Th T helper cells (0, 1, 2, and 17), TSLP Thymic stromal lymphopoietin, IL interleukin
COPD
Like asthma, COPD is a chronic inflammatory disease characterized by bronchitis, bronchi thickening, and emphysema [55, 56]. In response to inhaled particles, the injury and activation of epithelial cells lead to the infiltration of neutrophils and monocytes from blood into the pulmonary system. In collaboration with alveolar macrophages (dust cells), immune cells produce cytokines, chemokines, devastating enzymes, and free radicals (ROS) resulting in alveolar tissue remodeling (Fig. 5). Subsequent activation and recruitment of dendritic cells into the bronchi elevate in situ number of Th 1 and Th 17 cells. The addition of T cytotoxic lymphocytes (CD8+ cells) leads to the massive destruction of alveolar cells. It is proposed that the presence of autoantibodies in bronchoalveolar lavage of patients with COPD shows B cell activation [57, 58]. As a consequence, these changes activate compensatory responses which are linked to mucus hypersecretion, lung emphysema, pulmonary hypertension, and cor pulmonale [59]. An excessive mucin production promotes cell metaplasia in airway conduits. To be specific, the number of goblet cells increases in response to chronic conditions coincides with the hyperplasia of submucosal glands [60]. Phenotypic and morphological changes mediated by metaplasia can alter the length of cilia via a proteolytic process called ciliophagy [61]. As the number and length of cilia decreased, the mucus movement is interrupted through the pulmonary tract. Besides, the progress of metaplasia would exacerbate mucus entrapment [62]. This phenomenon can result in obstruction of airway conduits with a size of less than 2 mm. The loss of lung elasticity and emphysematous changes can accelerate the obstruction of airway conduits, leading to lung hyperinflation. The resulting high CO2 and low O2 concentrations in the blood of COPD patients contribute to hypercapnia and hypoxemia, respectively.
The COPD etiology is depicted in the diagram. In addition to activating epithelial cells, cigarette smoke and other environmental stimuli can also recruit neutrophils and macrophages from the bloodstream, which release a variety of chemotactic signals that recruit inflammatory cells to the lung. For instance, the CXC-chemokine receptor (CXCR) 2 is used by CXCL1 and CXCL8 to recruit neutrophils and monocytes. CD+ 8T cells can be recruited by CXCL 9, 10, and 11. The MMPs (2, 9, and 12), elastase, and Cathepsin K, L, and S that are involved in lung fibrosis and emphysema are also secreted by activated lung epithelial cells
Post-COVID complications in patients with background COPD and asthma
Whether background chronic pulmonary diseases can increase the possibility of COVID-19 in these patients is at the center of attention and needs further studies [63]. It has been shown that the occurrence of chronic pathological conditions such as COPD, asthma, smoking etc. can increase the severity and morbidity of COVID-19. These conditions enhance the susceptibility of respiratory conduit to viral infections such as SARS-COV-2. Even after recovery from COVID-19 infection specific pathologies such as fibrosis and massive tissue remodeling are mighty [64, 65]. In support of this notion, the regulation of receptors like ACE2- and CD147-related genes is induced in bronchial cells under chronic pathological conditions [63]. As above-mentioned, over-production of mucus in COPD patients can lead to respiratory distress and activation of inflammatory cascade which in turn contributes to the cytokine storm [66]. Besides, the normal activity of the mucociliary system is blunted in individuals with COPD, making these people more vulnerable to COVD-19 infection [67]. Despite this claim, some contradictory evidence has also been reported. Interestingly, some studies reported a low rate of COPD in COVID-19 patients [63]. One reason would be that the prolonged administration of corticosteroids and bronchodilators in COVID-19 patients can reduce the risk of COPD [63]. On the other hand, the hyperactivity of goblet cells and enhanced mucus secretion can be a physical barrier to reducing the accession of viral particles to the bronchial layer and alveolar cells [63]. Despite these facts, there is not enough proof for this hypothesis [68]. In addition to the remaining side effects of SARS-COV-2 infection in patients with COPD, also the mortality rate is significantly higher in these patients. The precise molecular and cellular mechanism supporting high-rate mortality in COPD patients after infection with SARS-COV-2 needs to be elucidated. Data suggest that COPD and such chronic conditions can increase the inflammatory burden, leading to significant mortality rates after infection with SARS-CoV-2 [69, 70]. The promotion of significant pathological changes under chronic conditions is linked to disruption of the mucociliary system and weakened function of alveolar macrophages [71, 72]. Even though, the number of alveolar macrophages increases in COPD patients. Lower contents of IRF-7, INF-α, and -β in COPD patients and smokers are the main cause of susceptibility to COVID-19 [73]. These factors are known to reduce SARS-CoV-2 replication inside the cytosol. In COPD patients, the activation of the ADAM17/EGFR axis can be initiated via the TLR signaling pathway. Both pro- and anti-inflammatory responses can be triggered via the activation of the ADAM17/EGFR axis. The production of CXCL8 is associated with neutrophil recruitment into the pulmonary niche. On the other hand, prolonged activation of the ADAM17/EGFR axis can cause anti-inflammation via the shedding of TNF-R2 [74]. Along with this claim, there is an abnormality in the function of adaptive systems such as B and T lymphocytes. Lower contents of polymeric immunoglobulin receptors also raise the possibility of IgA suppression and lack of appropriate viral clearance. The continuity of chronic immune responses can contribute to exhaust CD8+ lymphocytes and suppressed T regulatory cell function [73].
Quantitative transcriptomic analyses have revealed an apparent upregulation of ACE-2, TMPRSS2, and Furin in the pulmonary tissue of COPD and smokers. To this end, it is postulated that there is a close association between COVID-19 mortality and chronic respiratory diseases [75,76,77]. With regards to significant changes in the expression of key factors required for SARS-CoV-2 replication and deficient immune response, one can hypothesize that the occurrence of COVID-19 disease can cause more severe clinical manifestations and post-complications in COPD and smokers [78]. Results showed moderate to high persistent lung abnormalities in one out of third patients after infection with SARS and/or MERS [78]. Whether and how COVID-19 infection can cause similar outcomes in the target population needs further investigation. Evidence point to the high possibility of permanent pathological remodeling in the lung parenchyma, and chronic inflammation in COPD patients after infection with SARS-CoV-2, leading to chest pain, fatigue, and breathlessness [79]. Results have shown abnormal proliferation of local progenitors and goblet cells with concomitant ciliary dysfunction [80]. The lack of appropriate epithelialization rate and disruption of the mucociliary system cause microbial overload and vulnerability to viral infection. Endothelial layer damage and aberrant vascular remodeling reduce the number of recruited circulating stem cells into the pulmonary niche, resulting in local stem cell exhaustion, cellular senescence, and reduced regeneration capacity [81,82,83].
Based on previous data, asthmatic patients are also vulnerable to SARS-CoV-2 infection [84]. Patients with asthma experience deficiency in the innate immune system and anti-viral defense mechanism. These features intensifies cytokine storm in conditions associated with COVID-19 infection [85]. It has been confirmed that antiviral IFN response is significantly impaired in asthmatic conditions, which allows SARS-CoV-2 to easily enter target cells [86]. Like COPD, the onset of asthmatic condition up-regulates the expression of TMPRSS2 in human bronchial epithelial cells and increases SARS-CoV-2 infectivity due to acceleration of the spike protein cleavage [65]. Given the highly intricate nature of immune cell response within asthmatic parenchyma, the possible inhibitory effect of eosinophils and Th2 cell activity should be addressed regarding SARS-CoV-2 infection [87]. Clinical observations have confirmed the more severe form of COVID-19 disease in asthma patients [88]. These findings support the fact that stimulation of effectors associated with virus replication can overcome anti-inflammatory responses driven by recruited cells. In addition to immune cell exhaustion, findings direct to infection of these cells with viral particles. For example, CD147, also known as the Basigin protein, acts as a potent receptor for SARS-CoV-2 internalization in T lymphocytes and some epithelial cell types [65]. Other extracellular proteins such as Cyclophilins A and B, and CD44 can activate CD147 [89, 90]. It implies that the levels of CD147 and Cyclophilin B are elevated during asthmatic conditions [65]. Interestingly, it has been shown that Cyclophilin B is co-expressed with ACE-2 receptors under asthmatic conditions [65]. Molecular investigations have revealed an inverse correlation between ACE-2 receptor expression in the apical surface of epithelial cells and IL-4, -5, and -13 levels. In contrast, the elevation of these cytokines induces TMPRSS2 [91]. Compared to eosinophilic asthma, the levels of TMPRSS2, ACE-2 receptor, and furin are significantly higher in neutrophilic asthma [92]. Like COPD, it seems that background asthma makes patients vulnerable to post-COVID-19 symptoms such as shortness of breath [93].
Cell therapy in COPD and asthma
There are various approaches for the application of stem cells in varied pathological conditions. These cells can be directly used as naive cells or differentiated by-products after in vitro pre-treatments (Table 1). Differential self-renewality and differentiation capacity make stem cells unique cellular elements for the acceleration healing procedure (Table 2). From the aspect of differentiation capacity, stem cells include embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and mature types like mesenchymal stem cells (MSCs) [94,95,96]. ESCs can be isolated from the inner cell mass of blastocysts. These cells exhibit high-rate proliferation and bulk capacity to commit into three germ layers [97]. Currently, ethical criticism and the possibility of alloreactive immune cell activity have limited the extensive application of these cells in human subjects. These cells can be used as an allogenic cell source after differentiation into adult stem cell types [98, 99]. iPSCs, with stemness features similar to ESCs, are generated from somatic cells after genetic manipulation without human embryo samples [100]. These cells can help the resident tissue cells to heal the injured areas [100, 101]. In addition to the application of iPSCs as cell-based transplants, their secretory elements such as exosomes (Exo; nanovesicles with an average size of 40–150 nm) have been used for the alleviation and regeneration of injured myocardium because of angiogenic, anti-immunogenic, anti-fibrotic, and anti-apoptotic potential [102, 103]. MSCs are widely used, because of their easy expansion and non-invasive sampling [104]. Besides, these cells can be isolated from varied tissue types such as bone marrow, adipose tissue, umbilical cord, and amniotic fluid [105, 106]. Minimum levels of major histocompatibility complex (MHC-I and II), and other co-stimulatory factors with prominent immune regulatory features make MSCs the most accessible cell source for transplantation [107, 108]. Owing to differentiation capacity, and genomic stability, MSCs have been used for several pathological diseases [109, 110].
In the context of pulmonary diseases, stem cell therapy has led to the alleviation of several pathological conditions such as COPD and asthma in animal models [111]. Researchers have applied MSCs in several animal models of asthma types such as non-allergic, allergic, and cough-variant forms [112]. It was suggested that MSCs isolated from bone marrow, adipose tissue, umbilical cord blood, and placenta present a potential to diminish airway hyperresponsiveness and immune cell infiltration in animals with the acute model of asthma [113, 114]. It seems that the source of MSCs is important in achieving regenerative outcomes. For example, Abreu et al. claimed that bone marrow MSCs (BM-MSCs) have the superiority to reduce asthma pathologies such as fibrosis, eosinophilic reaction, and respiratory injury when compared to MSCs isolated from lung adipose tissue[115]. Systemic transplantation of BM-MSCs via tail vein reduced bronchiolar hypersensitivity, neutrophilic response, and Th17 cells cytokines in animal models [116].
Like asthma, numerous studies have examined the therapeutic effects of MSCs in COPD. A meta-analysis study revealed that both intravascular and intratracheal administration of MSCs can be in favor of blunting acute pulmonary inflammation and reduction of apoptotic changes in animals [117]. Intravenous injection of MSCs can promote abnormal vascularization induced by emphysematous changes in animal models [118]. In a similar work, MSCs ceased the production of pro-inflammatory cytokines and increased epithelial growth factor, accelerating the regeneration procedure with two side activities [119]. In a clinical trial study, intravenous injection of allogenic MSCs in COPD patients diminished acute phase protein levels such as C-reactive protein up to 1 month after transplantation without significant differences in pulmonary function tests [107, 120, 121]. Despite the beneficial properties of MSCs in pulmonary diseases, there is still uncertainty about their application as the therapeutic choice in the clinical setting.
Stem cells in COPD and asthmatic patients with COVID-19 post-complication
As above-mentioned, COPD and asthma patients are vulnerable to infection with SARS-CoV-2 and its post-complication indicated persistent lung injury. Despite these descriptions, stem cell therapy might be a choice for the alleviation and promotion of the healing process in these patients. Of note, in an experiment iPSC-derived type 2 alveolar cells were administrated via the systemic route in a mouse model of hyperoxia, a condition that can be seen in severe COVID patients. These cells successfully diminished hyperoxia-induced alveolar injury. The main reason for the application of iPSC after differentiation to type 2 alveolar cells is that this strategy would decrease the possibility of teratoma development and immune cell infiltration [122]. In another study, direct administration of iPSCs and a step-wise differentiation procedure restored the function of the mucociliary system via differentiation into multiciliated cells [123]. Takuji and co-workers produced functional alveolar macrophages from iPSCs to re-establish immune function within the lung parenchyma [124]. In a similar work, Happle et al. used iPSC-derived macrophages in a mouse model of pulmonary alveolar proteinosis. Results indicated the reduction of alveolar proteinosis, surfactant protein D ratio, and broncho-alveolar turbidity [125]. However, there are no specific clinical trials associated with the direct application of iPSC-derived macrophages and/or goblet cells in COVID-19 cases. Based on data from animal models and in vitro studies, it is assumed that the administration of differentiated cell types via different injection routes may bring regenerative outcomes. Due to the persistent and massive fibrosis in pulmonary parenchyma of severe COVID-19 survivors [126,127,128], the application of stem cells and/or mature cell types is directed to inhibition of fibrosis, in case the cell therapy is the only choice. In support of this notion, iPSC-derived type 2 alveolar cells alleviated bleomycin-induced fibrosis in a rat model via the inhibition of TGF-β and α-smooth muscle actin [129]. Likewise, these cells are potent enough to suppress epithelial-mesenchymal transition induced by TGF-β [130].
More recently, MSCs and Exo have been applied to patients with SARS-CoV-2, leading to hopeful results (Table 3). Intravenously injected MSCs decreased lung inflammation, improved clinical symptoms, and returned oxygen saturation to normal levels [131]. In phase 1 clinical trial study, Wharton's jelly MSCs exhibited prominent anti-inflammatory features with the elevation of IL-10 and stromal cell-derived factor-1. Besides, the levels of pro-inflammatory cytokines were reached near-to-normal levels. Despite the existence of promising outcomes, headache in one of the patients has been reported which alleviated without any pharmaceutical intervention [132, 133].
Conclusion
The SARS-CoV-2 virus can cause extreme complications either during the infection or after remediation in COPD and asthmatic patients. It is logical to hypothesize that post-COVID complications can be more probable and last for a long time in these groups of patients. However, stem cell-based regenerative medicine with the capacity to recreate cells and organs can be helpful to compensate for the destruction of their lungs. More studies are mandatory to address the beneficial effects of stem cell therapies in patients with chronic pulmonary diseases affected by viral infections.
Availability of data and materials
Not applicable.
Abbreviations
- +SSRNA:
-
Positive-single strand ribonucleic acid
- ACE-2:
-
Angiotensin-converting enzyme-2
- ADAM17:
-
A Disintegrin and Metalloprotease 17
- ARDS:
-
Acute respiratory distress syndrome
- BM-MSCs:
-
Bone marrow-derived mesenchymal stem cells
- CCL2:
-
C–C Motif Ligand 2
- CD147:
-
Cluster of differentiation-147
- CD4+:
-
Cluster of differentiation-4+
- CO2:
-
Carbon dioxide
- COPD:
-
Chronic obstructive pulmonary disease
- COVID-19:
-
Coronavirus-19
- CXCL10:
-
C-X-C Motif Chemokine Ligand 10
- E:
-
Envelope
- ECM:
-
Extracellular matrix
- EGFR:
-
Epidermal growth factor receptor
- ERGIC:
-
Endoplasmic Reticulum Golgi Intermediate Compartment
- ESCs:
-
Embryonic stem cells
- HRV:
-
Human rhinovirus
- IFNs:
-
Interferons
- IgA:
-
Immunoglobulin A
- ILs:
-
Interleukins
- iPSCs:
-
Induced pluripotent stem cells
- IRF-7:
-
Interferon Regulatory Factor-7
- M:
-
Membrane
- MCP-1:
-
Monocyte Chemoattractant Protein-1
- MERS:
-
Middle East Respiratory Syndrome
- MHC:
-
Major Histocompatibility Complex
- MSCs:
-
Mesenchymal stem cells
- NF-ƙB:
-
Nuclear Factor Kappa Light Chain Enhancer of Activated B cells
- NSPs:
-
Non-structural phosphatase
- O2:
-
Oxygen
- ORFs:
-
Open reading frames
- PRRs:
-
Pattern recognition receptors
- ROS:
-
Reactive oxygen species
- S:
-
Spike
- SARS:
-
Severe acute respiratory syndrome
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- TGF:
-
Tumor growth factor
- Th17:
-
T helper 17
- TLRs:
-
Toll-like receptors
- TMPRSS2:
-
Transmembrane Protease Serine 2
- TNF:
-
Tumor necrosis factor
- TSLP:
-
Thymic stromal lymphopoietin
References
Bagheri HS, Karimipour M, Heidarzadeh M, Rajabi H, Sokullu E, Rahbarghazi R. Does the global outbreak of COVID-19 or other viral diseases threaten the stem cell reservoir inside the body? Stem Cell Rev Rep. 2021;17(1):214–30.
Gianotti R, et al. COVID-19-related dermatosis in November 2019: could this case be Italy’s patient zero. Br J Dermatol. 2021;184(5):970–1.
Marhl M, et al. Diabetes and metabolic syndrome as risk factors for COVID-19. Diabetes Metab Syndr. 2020;14(4):671–7.
Leung JM, et al. Covid-19 and COPD. 2020. 56(2).
Alqahtani JS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0233147.
Busse WW, Lemanske RF Jr, Gern JEJ. Role of viral respiratory infections in asthma and asthma exacerbations. The Lancet. 2010;376(9743):826–34.
Abrams EM, W’t Jong G, Yang CLJC. Asthma and COVID-19. CMAJ. 2020;192(20):551.
Bagheri HS, et al. Does the global outbreak of COVID-19 or other viral diseases threaten the stem cell reservoir inside the body? Stem Cell Rev Rep. 2021;17(1):214–30.
Wang Y, et al. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012;6:66.
Sanyaolu A, et al. Comorbidity and its impact on patients with COVID-19. SN Comp Clin Med. 2020;66:1–8.
Cao XJ. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–70.
V’kovski P, et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–70.
Carcaterra M, Caruso C. Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: a physio-pathological theory. Med Hypoth. 2021;146:110412–110412.
Wrapp D, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3.
Heurich A, et al. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–307.
Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020.181(2):271–80.e8.
Zheng Y-Y, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–60.
Simmons G, et al. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci. 2005;102(33):11876–81.
Padmanabhan P, Desikan R, Dixit NM. Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARS-CoV-2 infection. PLoS Comput Biol. 2020;16(12):100–8461.
Wang K, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. Biorxiv. 2020;6:66.
Cantuti-Castelvetri L, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–60.
Clausen TM, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043–57.e15.
Sigrist CJ, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res. 2020;177:104759.
Guo Y-J, et al. ACE2 overexpression inhibits angiotensin II-induced monocyte chemoattractant protein-1 expression in macrophages. Arch Med Res. 2008;39(2):149–54.
Mollica V, Rizzo A, Massari F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020;16(27):2029–33.
Li G, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–32.
Guan W, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Wu C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020;180(7):934–43.
De Wit E, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–34.
Rodriguez IJ, et al. Human immune response to SARS-CoV-2: What is known? A scoping review. Infection. 2020;24(3):26–35.
Moore JB, June CHJS. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–4.
Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469–85.
Eyerich S, et al. New biological treatments for asthma and skin allergies. Allergy. 2020;75(3):546–60.
Cockcroft DW. Environmental causes of asthma. In: Seminars in respiratory and critical care medicine. Thieme Medical Publishers; 2018.
Arora P, Ansari S. Role of various mediators in inflammation of asthmatic airways. Asthma-Biological Evidences; 2019.
Bousquet J, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–9.
Suzuki Y, et al. Airway basophils are increased and activated in eosinophilic asthma. Allergy. 2017;72(10):1532–9.
Herd C, Page C. Pulmonary immune cells in health and disease: platelets. Eur Respir J. 1994;7(6):1145–60.
Willart M, Lambrecht B. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy. 2009;39(1):12–9.
Woodruff PG, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169(9):1001–6.
Ingram JL, et al. Airway fibroblasts in asthma manifest an invasive phenotype. Am J Respir Crit Care Med. 2011;183(12):1625–32.
Gaurav R, Agrawal DK. Clinical view on the importance of dendritic cells in asthma. Expert Rev Clin Immunol. 2013;9(10):899–919.
Kuruvilla ME, Lee F, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–33.
Salazar F, Ghaemmaghami A. Allergen recognition by innate immune cells: critical role of dendritic and epithelial cells. Front Immunol. 2013;4:356.
Liu YJ. TSLP in epithelial cell and dendritic cell cross talk. Adv Immunol. 2009;101:1–25.
Maazi H, et al. Role of plasmacytoid dendritic cell subsets in allergic asthma. Allergy. 2013;68(6):695–701.
Chang HS, et al. Neutrophilic inflammation in asthma: mechanisms and therapeutic considerations. Expert Rev Respir Med. 2017;11(1):29–40.
Bonecchi R, Iellem G, Bordignon PP, Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34.
Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy. 2020;75(2):311–25.
Yang IA, et al. The role of Toll-like receptors and related receptors of the innate immune system in asthma. Curr Opin Allergy Clin Immunol. 2006;6(1):23–8.
Demarche S, et al. Detailed analysis of sputum and systemic inflammation in asthma phenotypes: are paucigranulocytic asthmatics really non-inflammatory? BMC Pulm Med. 2016;16(1):1–13.
Tliba O, Panettieri RA Jr. Paucigranulocytic asthma: uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol. 2019;143(4):1287–94.
Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med. 2018;197(1):22–37.
Svenningsen S, Nair P. Asthma endotypes and an overview of targeted therapy for asthma. Front Med. 2017;4:158.
Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. The Lancet. 2007;370(9589):765–73.
Viegi G, et al. Definition, epidemiology and natural history of COPD. Eur Respir J. 2007;30(5):993–1013.
Barbu C, Iordache M, Man M. Inflammation in COPD: pathogenesis, local and systemic effects. Rom J Morphol Embryol. 2011;52(1):21–7.
Moldoveanu B, et al. Inflammatory mechanisms in the lung. J Inflamm Res. 2009;2:1.
Kim EK. Pathophysiology of COPD. In: COPD2017; Springer. p. 57–63.
Lai H, Rogers DF. New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: targeting intracellular signaling pathways. J Aerosol Med Pulm Drug Deliv. 2010;23(4):219–31.
Cloonan SM, et al. “Ciliophagy” The consumption of cilia components by autophagy. Autophagy. 2014;10(3):532–4.
Tilley AE, et al. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379–406.
Yong SJ. Diseased lungs may hinder COVID-19 development: a possible reason for the low prevalence of COPD in COVID-19 patients. Med Hypoth. 2021;153: 110628.
Adeloye D, et al. The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med. 2021;9(12):1467–78.
Radzikowska U, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75(11):2829–45.
Khan MA, et al. Cytokine storm and mucus hypersecretion in COVID-19: review of mechanisms. J Inflamm Res. 2021;14:175.
Olloquequi J. COVID-19 Susceptibility in chronic obstructive pulmonary disease. Eur J Clin Invest. 2020;50(10): e13382.
Schultze A, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8(11):1106–20.
Kurai D, et al. Virus-induced exacerbations in asthma and COPD. Front Microbiol. 2013;4:293.
Miravitlles M, Anzueto A. Chronic respiratory infection in patients with chronic obstructive pulmonary disease: What is the role of antibiotics? Int J Mol Sci. 2017;18(7):1344.
Barnes PJ. Alveolar macrophages in chronic obstructive pulmonary disease (COPD). Cell Mol Biol. 2004;50:OL627-37.
Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014;5:435.
Polverino F, Kheradmand F. COVID-19, COPD, and AECOPD: immunological, epidemiological, and clinical aspects. Front Med. 2021;7:1121.
Zipeto D, et al. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front Immunol. 2020;11:66.
Smith JC, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev Cell. 2020;53(5):514-529e3.
Sharif-Askari NS, et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1–6.
Cai G, et al. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;201(12):1557–9.
Halpin DM, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36.
Darley DR, et al. Persistent symptoms up to four months after community and hospital-managed SARS-CoV-2 infection. Med J Aust. 2021;6:66.
Ghosh M, et al. Exhaustion of airway basal progenitor cells in early and established chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(7):885–96.
Mercado N, Ito K, Barnes PJJT. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015;70(5):482–9.
Tura-Ceide O, et al. Progenitor cell mobilisation and recruitment in pulmonary arteries in chronic obstructive pulmonary disease. Respir Res. 2019;20(1):1–9.
MacNee WJ. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans. 2009;37(4):819–23.
Lin L, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection—a review of immune changes in patients with viral pneumonia. Emerg Microb Infect. 2020;9(1):727–32.
Liuzzo Scorpo M, Ferrante G, LaGrutta S. An overview of asthma and COVID-19: protective factors against SARS-COV-2 in pediatric patients. Front Pediatr. 2021;9:661–206.
Matsumoto K, Saito H. Does asthma affect morbidity or severity of COVID-19? J Allergy Clin Immunol. 2020;146(1):55–7.
Liu S, et al. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59:78–88.
Skevaki C, et al. Asthma-associated risk for COVID-19 development. J Allergy Clin Immunol. 2020;146(6):1295–301.
Wang K, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):1–10.
Yurchenko V, et al. CD147 is a signaling receptor for cyclophilin B. Biochem Biophys Res Commun. 2001;288(4):786–8.
Kimura H, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146(1):80-88.e8.
Kermani N, et al. Airway expression of SARS-CoV-2 receptor, ACE2, and proteases, TMPRSS2 and furin, in severe asthma. medRxiv. 2020;6:66.
Fernández-de-Las-Peñas C, et al. Similar prevalence of long-term post-COVID symptoms in patients with asthma: a case-control study. JInfect. 2021;6:66.
Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos Trans R Soc B Biol Sci. 2010;365(1537):155–63.
McCulloch EA, Till JE. Perspectives on the properties of stem cells. Nat Med. 2005;11(10):1026–8.
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22.
Martello G, Smith A. The nature of embryonic stem cells. Annu Rev Cell Dev Biol. 2014;30:647–75.
Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–55.
Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85(2):635–78.
Ye L, Swingen C, Zhang J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr Cardiol Rev. 2013;9(1):63–72.
Okano H, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112(3):523–33.
Nishikawa S-I, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9(9):725–9.
Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–42.
Khaksar M, et al. High glucose condition limited the angiogenic/cardiogenic capacity of murine cardiac progenitor cells in in vitro and in vivo milieu. Cell Biochem Funct. 2018;36(7):346–56.
Rajabi H, et al. Current status of used protocols for mesenchymal stem cell differentiation: a focus on insulin producing, osteoblast-like and neural cells. Curr Stem Cell Res Ther. 2019;14(7):570–8.
Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481–92.
D’Agostino B, et al. Mesenchymal stem cell therapy for the treatment of chronic obstructive pulmonary disease. Expert Opin Biol Ther. 2010;10(5):681–7.
Harrell CR, et al. Mesenchymal stem cell-based therapy of inflammatory lung diseases: current understanding and future perspectives. Stem Cells Int. 2019;6:66.
Rajabi H, Aslani S, Rahbarghazi R. Level of miR-101a and miR-107 in human adipose mesenchymal stem cells committed to insulin-producing cells. Int J Mol Cell Med. 2021;10(1):68.
Aslani S, et al. Dynamic of miRNA-101a-3p and miRNA-200a during induction of osteoblast differentiation in adipose-derived mesenchymal stem cells. Int J Mol Cell Med. 2020;9(2):140.
Mirershadi F, et al. Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Res Ther. 2020;11(1):400.
Ahmadi M, et al. Contributory anti-inflammatory effects of mesenchymal stem cells, not conditioned media, on ovalbumin-induced asthmatic changes in male rats. Inflammation. 2016;39(6):1960–71.
Yu X, et al. A narrative review of research advances in mesenchymal stem cell therapy for asthma. Ann Transl Med. 2020;8(21):66.
Mirershadi F, et al. Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Res Ther. 2020;11(1):1–12.
Abreu SC, et al. Mechanisms of cellular therapy in respiratory diseases. Intensive Care Med. 2011;37(9):1421–31.
Cruz FF, Rocco PRM. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev Respir Med. 2020;14(1):31–9.
Liu X, Fang Q, Kim H. Preclinical studies of mesenchymal stem cell (MSC) administration in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. PLoS ONE. 2016;11(6): e0157099.
Glassberg MK, et al. Stem cell therapy for copd: Hope and exploitation. Chest. 2021;160(4):1271–81.
Abreu SC, et al. Mesenchymal stromal cell-derived extracellular vesicles in lung diseases: current status and perspectives. Front Cell Dev Biol. 2021;9:97.
Kokturk N, et al. Stem cell therapy in chronic obstructive pulmonary disease. How far is it to the clinic? Am J Stem Cells. 2018;7(3):56.
Balkissoon R. Stem cell therapy for COPD: where are we? Chronic Obstruct Pulmon Dis J COPD Found. 2018;5(2):148.
Shafa M, et al. Human induced pluripotent stem cell–derived lung progenitor and alveolar epithelial cells attenuate hyperoxia-induced lung injury. Cytotherapy. 2018;20(1):108–25.
Firth AL, et al. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc Natl Acad Sci. 2014;111(17):E1723–30.
Suzuki T, et al. Use of induced pluripotent stem cells to recapitulate pulmonary alveolar proteinosis pathogenesis. Am J Respir Crit Care Med. 2014;189(2):183–93.
Happle C, et al. Pulmonary transplantation of human induced pluripotent stem cell–derived macrophages ameliorates pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2018;198(3):350–60.
Zhou S, et al. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. Ajr Am J Roentgenol. 2020;214(6):1287–94.
Pan Y, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30(6):3306–9.
Stewart CA, et al. Lung cancer models reveal SARS-CoV-2-induced EMT contributes to COVID-19 pathophysiology. BioRxiv. 2021;6:66.
Alvarez-Palomo B, et al. Induced pluripotent stem cell-derived lung alveolar epithelial type II cells reduce damage in bleomycin-induced lung fibrosis. Stem Cell Res Ther. 2020;11:1–12.
Zhou Y, et al. Induced pluripotent stem cells inhibit bleomycin-induced pulmonary fibrosis in mice through suppressing TGF-β1/Smad-mediated epithelial to mesenchymal transition. Front Pharmacol. 2016;7:430.
Shu L, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):1–11.
Saleh M, et al. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther. 2021;12(1):1–13.
Lanzoni G, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–73.
Shroff G. A review on stem cell therapy for multiple sclerosis: special focus on human embryonic stem cells. Stem Cells Clon Adv Appl. 2018;11:1.
Adamiak M, et al. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ Res. 2018;122(2):296–309.
Nelson TJ, et al. Stem cell platforms for regenerative medicine. Clin Transl Sci. 2009;2(3):222–7.
Lee T-L, et al. Regulating the stem cell industry: needs and responsibilities. Bull World Health Organ. 2017;95(9):663.
El-Badawy A, El-Badri N. Clinical efficacy of stem cell therapy for diabetes mellitus: a meta-analysis. PLoS ONE. 2016;11(4): e0151938.
Wernig M, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci. 2008;105(15):5856–61.
Chen J, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–86.
González MA, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheumatism. 2009;60(4):1006–19.
Gazdic M, et al. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep. 2015;11(2):280–7.
Sun L, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27(6):1421–32.
Ortiz LA, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci. 2007;104(26):11002–7.
Danielyan L, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuven Res. 2011;14(1):3–16.
Sakaida I, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40(6):1304–11.
Hatzistergos KE, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–22.
Chen L, et al. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS ONE. 2009;4(9): e7119.
Roddy GW, et al. Action at a distance: Systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cells. 2011;29(10):1572–9.
Yañez R, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24(11):2582–91.
Goodwin M, et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29(7):1137–48.
Li Q, Chen X, Li J. Marrow-derived mesenchymal stem cells regulate the inflammatory response and repair alveolar type II epithelial cells in acute lung injury of rats. J Int Med Res. 2020;48(4):0300060520909027.
Leeman KT, et al. Mesenchymal stem cells increase alveolar differentiation in lung progenitor organoid cultures. Sci Rep. 2019;9(1):1–10.
Kruk DM, et al. Paracrine regulation of alveolar epithelial damage and repair responses by human lung-resident mesenchymal stromal cells. Cells. 2021;10(11):2860.
Selvasandran K, et al. A tumor necrosis factor-α and hypoxia-induced secretome therapy for myocardial repair. Ann Thorac Surg. 2018;105(3):715–23.
Yao Y, et al. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res Ther. 2019;10(1):1–17.
Yang H, et al. Human induced pluripotent stem cell-derived mesenchymal stem cells promote healing via TNF-α-stimulated gene-6 in inflammatory bowel disease models. Cell Death Dis. 2019;10(10):1–16.
Xu J, et al. Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics. 2020;10(26):12204.
Zhang B, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–68.
Acknowledgements
All authors would thank the Koç University Translational Medicine Research Center (KUTTAM) and Stem Cell Research Center staff for guidance and help.
Funding
This study was supported by a grant from Koç University Translational Medicine Research Center (KUTTAM).
Author information
Authors and Affiliations
Contributions
H.R., D. M., N. K., G.T.A, S.K.K, S. E., O.K, collected data, reviewed literature, and prepared the draft. H.B. and R.R. read and edited the final manuscript and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rajabi, H., Mortazavi, D., Konyalilar, N. et al. Forthcoming complications in recovered COVID-19 patients with COPD and asthma; possible therapeutic opportunities. Cell Commun Signal 20, 173 (2022). https://doi.org/10.1186/s12964-022-00982-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-022-00982-5