Abstract
Background
We investigated the roles of stem cell factor (SCF)–c-kit and stromal derived factor-1 (SDF-1)–CXCR4 signaling axes in transmyocardial revascularization (TMR)-enhanced engraftment of transplanted bone marrow stem cells (BMSCs) in infarcted hearts.
Methods
3 weeks after LAD ligation, female Lewis rats underwent 10-channel needle-TMR, followed by daily IV injections of 1 million male donor BMSC for 5 days, either wild type (WT) or with knockdown (K/D) of c-kit or CXCR4, accomplished via a shRNA + plasmid in a lentiviral vector (N = 6/group).
Results
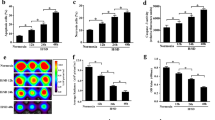
In our rat infarct model, 3 days after last BMSC injection, the number of BMSCs that homed into infarct was affected by both TMR and donor cell type, with greater BMSC engraftment with TMR and with WT BMSC (TMR, cell type, and interaction, P < 0.05). At 1 week, these differences persisted (TMR and cell type, P < 0.05). At 3 days, TMR significantly upregulated transcription of c-kit (TMR, p < 0.05), SCF (TMR and cell type, P < 0.05), CXCR4 (TMR and cell type, p < 0.05), and SDF-1 (TMR and cell type, P < 0.05). At 1 week, we saw similar declines in expression of c-kit (cell type, P < 0.05), SCF (TMR, P < 0.05), CXCR4 (TMR and cell type, P < 0.05), and SDF-1 (TMR, P < 0.05). At 1 week, TMR improved LV ejection fraction (LVEF) (N = 5) when WT BMSCs were infused, but knockdown of either c-kit or CXCR4 completely abrogated this TMR-mediated augmentation of BMSC reparative effect (TMR and cell type, P < 0.05).
Conclusions
Downregulation of either c-kit or CXCR4 in BMSC decreased engraftment of circulating BMSC and inhibited reparative effects of TMR.





Similar content being viewed by others
References
Williams, A. R., & Hare, J. M. (2011). Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation Research, 109(8), 923–940.
Yousef, Z. R., Redwood, S. R., & Marber, M. S. (2000). Postinfarction left ventricular remodeling: a pathophysiological and therapeutic review. Cardiovascular Drugs and Therapy, 14(3), 243–252.
Sutton, M. G. S. J., & Sharpe, N. (2000). Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation, 101(25), 2981–2988.
Wen, Z., Zheng, S., Zhou, C., Wang, J., & Wang, T. (2011). Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. Journal of Cellular and Molecular Medicine, 15(5), 1032–1043.
Rose, R. A., Jiang, H., Wang, X., Helke, S., Tsoporis, J. N., Gong, N., Keating, S. C. J., et al. (2008). Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells, 26(11), 2884–2892.
Shake, J. G., Gruber, P. J., Baumgartner, W. A., Senechal, G., Meyers, J., Redmond, M., Pittenger, M. F., et al. (2002). Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Annals of Thoracic Surgery, 73, 1919–1926.
Kim, S. H., Moon, H. H., Kim, H. A., Hwang, K. C., Lee, M., & Choi, D. (2009). Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Molecular Therapy, 19(4), 741–750.
Yau, T. M., Kim, C., Li, G., Zhang, Y., Weisel, R. D., & Li, R. K. (2005). Maximizing ventricular function with multimodal cell-based gene therapy. Circulation, 112(9 Suppl), I123–I128.
Yau, T. M., Kim, C., Li, G., Zhang, Y., Fazel, S., Spiegelstein, D., Weisel, R. D., et al. (2007). Enhanced angiogenesis with multimodal cell-based gene therapy. Annals of Thoracic Surgery, 83(3), 1110–1119.
Wollert, K. C., Meyer, G. P., Lotz, J., Ringes-Lichtenberg, S., Lippolt, P., Breidenbach, C., Fichtner, S., et al. (2004). Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet, 364(9429), 141–148.
Lunde, K., Solheim, S., Aakhus, S., Arnesen, H., Abdelnoor, M., & Forfang, K. (2005). Autologous stem cell transplantation in acute myocardial infarction: the ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scandinavian Cardiovascular Journal, 39(3), 150–158.
Dill, T., Schaechinger, V., Rolf, A., Mollmann, S., Thiele, H., Tillmanns, H., Assmus, B., et al. (2009). Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. American Heart Journal, 157(3), 541–547.
Perin, E. C., Willerson, J. T., Pepine, C. J., Henry, T. D., Ellis, S. G., Zhao, D. X., Silva, G. V., et al. (2012). Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA, 307(16), 1717–1726.
Hare, J. M., Fishman, J. E., Gerstenblith, G., DiFede Velazquez, D. L., Zambrano, J. P., Suncion, V. Y., Tracy, M., et al. (2012). Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA, 308(22), 2369–2379.
Hou, D., Youssef, E.A., Brinton, T.J., Zhang, P., Rogers, P., Price, E.T., et al. (2005). Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation, 112[suppl I], I-150-I-156.
Gyongyosi, M., Blanco, J., Marian, T., Tron, L., Petnehazy, O., Petrasi, Z., et al. (2008). Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circulation. Cardiovascular Imaging, 1, 94–103.
Allen, K. B., Kelly, J., Borkon, A. M., Stuart, R. S., Daon, E., Pak, A. F., Zorn, G. L., et al. (2008). Transmyocardial laser revascularization: from randomized trials to clinical practice. A review of techniques, evidence-based outcomes, and future directions. Anesthesiology Clinics, 26(3), 501–519.
Estvold, S. K., Mordini, F., Zhou, Y., Yu, Z. X., Sachdev, V., Arai, A., & Horvath, K. A. (2010). Does laser type impact myocardial function following transmyocardial laser revascularization. Lasers in Surgery and Medicine, 42(10), 746–751.
Horvath, K. A., Belkind, N., Wu, I., Greene, R., Doukas, J., Lomasney, J. W., McPherson, D. D., et al. (2001). Functional comparison of transmyocardial revascularization by mechanical and laser means. Annals of Thoracic Surgery, 72(6), 1997–2002.
Al-Sheikh, T., Allen, K. B., Straka, S. P., Heimansohn, D. A., Fain, R. L., Hutchins, G. D., Sawada, S. G., et al. (1999). Cardiac sympathetic denervation after transmyocardial laser revascularization. Circulation, 100(2), 135–140.
Cooley, D. A., Frazier, O. H., Kadipasaoglu, K. A., Pehlivanoglu, S., Shannon, R. L., & Angelini, P. (1994). Transmyocardial laser revascularization. Anatomic evidence of long-term channel patency. Texas Heart Institute Journal, 21(3), 220–224.
Patel, A. N., Spadaccio, C., Kuzman, M., Park, E., Fischer, D. W., Stice, S. L., et al. (2007). Improved cell survival in infarcted myocardium using a novel combination transmyocardial laser and cell delivery system. Cell Transplantation, 16(9), 899–905.
Zhang, G. W., Liu, X. C., Li-Ling, J., Luan, Y., Ying, Y. N., Wu, X. S., et al. (2011). Mechanisms of the protective effects of BMSCs promoted by TMDR with heparanizied bFGF-incorporated stent in pig model of acute myocardial ischemia. Journal of Cellular and Molecular Medicine, 15(5), 1075–1086.
Yang, Z., Wu, Y., Zhang, H., Jin, P., Wang, W., Hou, J., et al. (2011). Low-level laser irradiation alters cardiac cytokine expression following acute myocardial infarction: a potential mechanism for laser therapy. Photomedicine and Laser Surgery, 29(6), 391–398.
Spiegelstein, D., Kim, C., Zhang, Y., Li, G., Weisel, R. D., Li, R. K., & Yau, T. M. (2007). Combined transmyocardial revascularization and cell-based angiogenic gene therapy increases transplanted cell survival. American Journal of Physiology Heart and Circulatory Physiology, 293(6), H3311–H3316.
Shahzad, U., Li, G., Zhang, Y., & Yau, T. M. (2012). Transmyocardial revascularization induces mesenchymal stem cell engraftment in infarcted hearts. Annals of Thoracic Surgery, 94(2), 556–562.
Sordi, V., Malosio, M. L., Marcheesi, F., Mercalli, A., Melzi, R., Giordano, T., et al. (2005). Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood, 106(2), 419–427.
Zhang, G. W., Wen, T., Gu, T. X., Li-Ling, J., Wang, C., Zhao, Y., Liu, J., et al. (2012). Transmyocardial drilling revascularization combined with heparinized bFGF-incorporating stent activates resident cardiac stem cells via SDF-1/CXCR4 axis. Experimental Cell Research, 318(4), 391–399.
Ashman, L. K. (1999). The biology of stem cell factor and its receptor C-kit. International Journal of Biochemistry and Cell Biology, 31, 1037–1051.
Gagari, E., Rand, M. K., Tayari, L., Vastardis, H., Sharma, P., Hauschka, P. V., & Damoulis, P. D. (2006). Expression of stem cell factor and its receptor, c-kit, in human oral mesenchymal cells. European Journal of Oral Sciences, 114(5), 409–415.
Carson, W. E., Haldar, S., Baiocchi, R. A., Croce, C. M., & Caligiuri, M. A. (1994). The c-kit ligand suppresses apoptosis of human natural killer cells through the upregulation of bcl-2. Proceedings of the National Academy of Sciences of the United States of America, 91, 7553–7557.
Ghadge, S. K., Mühlstedt, S., Özcelik, C., & Bader, M. (2011). SDF-1α as a therapeutic stem cell homing factor in myocardial infarction. Pharmacology and Therapeutics, 129(1), 97–108.
Kucia, M., Reca, R., Miekus, K., Wanzeck, J., Wojakowski, W., Janowska-Wieczorek, A., Ratajczak, J., et al. (2005). Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells, 23(7), 879–894.
Petit, I., Jin, D., & Rafii, S. (2007). The SDF-1–CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends in Immunology, 28(7), 299–307.
Wang, Y., Deng, Y., & Zhou, G. Q. (2008). SDF-1α/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Research, 1195, 104–112.
Teichholz, L. E., Kreulen, T., Herman, M. V., & Gorlin, R. (1976). Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. American Journal of Cardiology, 37, 7–11.
Wandt, B., Bojo, L., Tolagen, K., & Wranne, B. (1999). Echocardiographic assessment of ejection fraction in left ventricular hypertrophy. Heart, 82, 192–198.
Luo, Y., Zhao, X., Zhou, X., Ji, W., Zhang, L., Luo, T., Liu, H., et al. (2013). Short-term intermittent administration of CXCR4 antagonist AMD3100 facilitates myocardial repair in experimental myocardial infarction. Acta Biochimica et Biophysica Sinica, 45(7), 561–569.
Chen, Z., Pan, X., Yao, Y., Yan, F., Chen, L., Huang, R., & Ma, M. (2014). Regulation of c-kit + progenitor cells by stromal cell derived factor-1α in adult murine heart. Heart, Lung & Circulation, 23(1), 75–81.
Chen, Z., Pan, X., Yao, Y., Yan, F., Chen, L., Huang, R., & Ma, G. (2013). Epigenetic regulation of cardiac progenitor cells marker c-kit by stromal cell derived factor-1α. PLoS One, 8(7), e69134.
Cheng, M., Zhou, J., Wu, M., Boriboun, C., Thorne, T., Liu, T., Xiang, Z., et al. (2010). CXCR4-mediated bone marrow progenitor cell maintenance and mobilization are modulated by c-kit activity. Circulation Research, 107(9), 1083–1093.
Koenig, A., Yakisan, E., Reuter, M., Huang, M., Sykora, K., Corbacioglu, S., & Welte, K. (1994). Differential regulation of stem cell factor mRNA expression in human endothelial cells by bacterial pathogens: an in vitro model of inflammation. Blood, 83(10), 2836–2843.
Koenig, A., Corbacioglu, S., Ballmaier, M., & Welte, K. (1997). Downregulation of c-kit expression in human endothelial cells by inflammatory stimuli. Blood, 90(1), 148–15.
Potapova, I. A., Brink, P. R., Cohen, I. S., & Doronin, S. V. (2008). Culturuing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. Journal of Biological Chemistry, 283, 13100–13107.
Acknowledgments
TMY holds the Angelo & Lorenza DeGasperis Chair in Cardiovascular Surgery Research.
Conflict of Interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahzad, U., Li, G., Zhang, Y. et al. Transmyocardial Revascularization Enhances Bone Marrow Stem Cell Engraftment in Infarcted Hearts Through SCF—C-kit and SDF-1—CXCR4 Signaling Axes. Stem Cell Rev and Rep 11, 332–346 (2015). https://doi.org/10.1007/s12015-014-9571-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9571-7