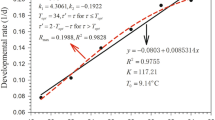
Abstract
The objective of this study was to investigate whether four spider mite species, Tetranychus ludeni, T. phaselus, T. piercei and T. truncatus, currently with insignificant economic impact, have the potential to achieve the same status as T. urticae, which until now has been considered as the most serious tetranychid pest species in orchards and greenhouses. We investigated the effect of temperature on development, survival and oviposition at 11 constant temperatures ranging from 15 to 40 °C at intervals of 2.5 °C and estimated demographic parameters, such as the intrinsic rate of natural increase (r m), for these five species at five constant temperatures. Developmental time from egg to adult (female and male) decreased with increasing temperature from 15 to 32.5 °C in all five species, but increased slightly at 35 °C or higher, especially in T. ludeni and T. urticae. Using linear and non-linear developmental rate models, the lower thermal thresholds for egg-to-adult (female and male) and egg-to-egg development were found to range from 9.8 to 11.7 and from 9.8 to 11.4 °C, respectively. The intrinsic optimal temperature (T Φ) ranged from 18.0 to 27.4 °C for egg-to-female adult and from 23.9 to 27.2 °C for egg-to-egg development. The oviposition period and adult longevity were strongly affected by temperature. The r m-values increased with increasing temperature from 15 to 30 or 35 °C in all five species. The highest r m-values at each temperature were 0.114 day−1 at 15 °C for T. ludeni, 0.199 day−1 at 20 °C for T. urticae, 0.314 day−1 at 25 °C for T. ludeni, 0.451 day−1 at 30 °C for T. ludeni and 0.433 day−1 at 35 °C for T. truncatus. The total fecundity, net reproductive rate (R 0) and r m of T. ludeni were higher than those of T. urticae at all temperatures. T. piercei and T. truncatus showed higher r m-values at 30 and 35 °C than T. urticae. The results indicate that the former three species are better adapted to hot weather than T. urticae and have a high potential to become serious pests.



Similar content being viewed by others
References
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Bolland HR, Gutierrez J, Flechtman CHW (1998) World catalogue of the spider mite family (Acari: Tetranychidae). Brill Academic, Leiden
Bonato O (1999) The effect of temperature on life history parameters of Tetranychus evansi (Acari: Tetranychidae). Exp Appl Acarol 23:11–19
Bounfour M, Tanigoshi LK (2001) Effect of temperature on development and demographic parameters of Tetranychus urticae and Eotetranychus carpini borealis (Acari: Tetranychidae). Ann Entomol Soc Am 94:400–404
Chao SRS, Lo PKC (1974) Biological studies of the spider mite, Tetranychus truncatus and its natural enemies. J Taiwan Agric Res 23:126–135
Chen CY, Chiu MC, Kuo MH (2013) Effect of warming with temperature oscillations on a low-latitude aphid, Aphis craccivora. Bull Entomol Res 103:406–413
Davies JT, Ireson JE, Allen GR (2009) Pre-adult development of Phytoseiulus persimilis on diets of Tetranychus urticae and Tetranychus lintearius: implications for the biological control of Ulex europaeus. Exp Appl Acarol 47:133–145
Estay SA, Lima M, Bozinovic F (2014) The role of temperature variability on insect performance and population dynamics in a warming world. Oikos 123:131–140
Fan LQ, Shi CL, Wang YP (2003) Developmental threshold temperature and the effective accumulated temperature of Tetranychus truncatus Ehara. Plant Prot 29(6):32–34
Ferrero M, de Moraes GJ, Kreiter S, Tixier MS, Knapp M (2007) Life tables of the predatory mite Phytoseiulus longipes feeding on Tetranychus evansi at four temperatures (Acari: Phytoseiidae, Tetranychidae). Exp Appl Acarol 41:45–53
Fujibayashi Y, Kushida T (1992) Susceptibility of Panonychus ulmi (Koch) and Tetranychus urticae Koch to acaricides in the Tsugaru region, Aomori Prefecture. Ann Rept Plant Prot North Jpn 43:152–154 (in Japanese)
Goka K (1999) The effect of patch size and persistence of host plants on the development of acaricide resistance in the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). Exp Appl Acarol 23:419–427
Gotoh T (1997) Annual life cycle of populations of the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) in four Japanese pear orchards. Appl Entomol Zool 32:207–216
Gotoh T, Gomi K (2003) Life-history traits of the Kanzawa spider mite Tetranychus kanzawai (Acari: Tetranychidae). Appl Entomol Zool 38:7–14
Gotoh T, Suwa A, Kitashima Y, Rezk HA (2004) Developmental and reproductive performance of Tetranychus pueraricola Ehara and Gotoh (Acari: Tetranychidae) at four constant temperatures. Appl Entomol Zool 39:675–682
Gotoh T, Sugimoto N, Pallini A, Knapp M, Hernandez-Suarez E, Ferragut F, Ho CC, Migeon A, Navajas M, Nachman G (2010) Reproductive performance of seven strains of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) at five temperatures. Exp App Acarol 52:239–259
Ikemoto T (2005) Intrinsic optimum temperature for development of insects and mites. Environ Entomol 34:1377–1387
Ikemoto T (2008) Tropical malaria does not mean hot environments. J Med Entomol 45:963–969
Ikemoto T, Takai K (2000) A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ Entomol 29:671–682
Ikemoto T, Kurahashi I, Shi P-J (2013) Confidence interval of intrinsic optimum temperature estimated using thermodynamic SSI model. Insect Sci 20:420–428
Khanamani M, Fathipour Y, Hajiqanbar H (2013) Population growth response of Tetranychus urticae to eggplant quality: application of female age-specific and age-stage, two-sex life tables. Int J Acarol 39:638–648
Kiritani K (2013) Different effects of climate change on the population dynamics of insects. Appl Entomol Zool 48:97–104
Kishimoto H (2002) Species composition and seasonal occurrence of spider mites (Acri: Tetranychidae) and their predators in Japanese pear orchards with different agrochemical spraying programs. Appl Entomol Zool 37:603–615
Kondo A, Takafuji A (1985) Resource utilization pattern of two species of tetranychid mites (Acarina: Tetranychidae). Res Popul Ecol 27:145–157
Lawo JP, Lawo NC (2011) Misconceptions about the comparison of intrinsic rates of natural increase. J Appl Entomol 135:715–725
Magalhaes S, Fayard J, Janssen A, Carbonell D, Olivieri I (2007) Adaptation in a spider mite population after long-term evolution ona single host plant. J Evol Biol 20:2016–2027
Manly BFJ (1990) Stage-structured populations: sampling, analysis and simulation. Chapman & Hall, London, p 187
McCullagh P, Nelder JA (1989) Generalized linear models. Monographs on statistics and applied probability 37, 2nd edn. Chapman & Hall, Boca Raton
Migeon A, Dorkeld F (2006–2013) Spider mites web: a comprehensive database for the Tetranychidae. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 28 Feb 2014
Mizukoshi T, Goto M (2003) Control of cyclamen mite, Steniotarsonemus pallidus (Banks) (Acarina: Tarsonemidae), by pouring of hot water on strawberry. Ann Rept Plant Prot North Jpn 55:232–235 (in Japanese)
Nakagaki S (1980) Seasonal prevalence of spider mites in pear orchard with reference to analysis of population density. Bull Ibaraki Fruit Tree Res Stn 8:37–51 (in Japanese)
Okazaki K (2000) IPM program by multiple mating disruptor in apple orchard. Agric Tech 59:409–413 (in Japanese)
Pang BP, Zhou XR, Shi L, Mu HB (2004) Performance of Tetranychus truncatus Ehara (Acarina: Tetranychidae) reared with different host plants. Acta Entomol Sin 47:55–58
Riahi E, Nemati A, Shishehbor P, Saeidi Z (2011) Population growth parameters of the two-spotted spider mite, Tetranychus urticae, on three peach varieties in Iran. Acarologia 51:473–480
Riahi E, Shishehbor P, Nemati AR, Saeidi Z (2013) Temperature effects on development and life table parameters of Tetranychus urticae (Acari: Tetranychidae). J Agric Sci Technol 15:661–672
Sabelis MW (1981) Biological control of two-spotted spider mites using phytoseiid predators. Part I. Modelling the predator–prey interaction at the individual level. Agricultural Research Reports, PUDOC, Wageningen, p 242
Sabelis MW (1985) Reproductive strategies. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control. Elsevier, Amsterdam, pp 265–278
Sabelis MW (1991) Life-history evolution of spider mites. In: Schuster R, Murphy PW (eds) The acari. Reproduction, development and life-history strategies. Chapman & Hall, London, pp 23–49
Saito Y (1979) Comparative studies on life histories of three species of spider mites (Acarina: Tetranychidae). Appl Entomol Zool 14:83–94
Sakunwarin S, Chandrapatya A, Baker GT (2003) Biology and life table of the cassava mite, Tetranychus truncatus Ehara (Acari: Tetranychidae). Syst Appl Acarol 8:13–24
SAS (2006) SAS Enterprise Guide 4.1. SAS Institute Inc. SAS Campus Drive, Cary NC 27513
Scranton K, Stavrinides M, Mills NJ, de Valpine P (2013) Small-scale intraspecific life history variation in herbivorous spider mites (Tetranychus pacificus) is associated with host plant cultivar. PLoS One 8(9):e72980
Shi P-J, Ikemoto T, Egami C, Sun Y, Ge F (2011) A modified program for estimating the parameters of the SSI model. Environ Entomol 40:462–469
Snell TW (1978) Fecundity, developmental time, and population growth rate. Oecologia 32:119–125
Takafuji A (1980) Population dynamics of some phytophagous mites and their predators on goldenrod Solidago altissima L. I. Seasonal trends in population numbers and spatial distributions. Res Popul Ecol 21:197–216
Takafuji A, Kamibayashi M (1984) Life cycle of a non-diapausing population of the two-spotted spider mite, Tetranychus urticae Koch in a pear orchard. Res Popul Ecol 26:113–123
Takafuji A, Yokotsuka T, Goka K, Kishimoto H (1996) Ecological performance of the spider mite, Tetranychus okinawanus Ehara (Acari, Tetranychidae), a species newly described from Okinawa Islands (1). J Acarol Soc Jpn 5:75–81
Takafuji A, Ozawa A, Nemoto H, Gotoh T (2000) Spider mites of Japan: their biology and control. Exp Appl Acarol 24:319–335
Trichilo PJ, Wilson LT (1993) An ecosystem analysis of spider mite outbreaks: physiological stimulation or natural enemy suppression. Exp Appl Acarol 17:291–314
Uchida M (1982) Ecological studies on the abundance and diapauses of spider mites and the damage caused by the spider mites in Japanese pear orchards. Spec Bull Tottori Fru Tree Exp Stn 2:1–63 (in Japanese)
Ullah MS, Moriya D, Badii MH, Nachman G, Gotoh T (2011) A comparative study of development and demographic parameters of Tetranychus merganser and Tetranychus kanzawai (Acari: Tetranychidae) at different temperatures. Exp Appl Acarol 54:1–19
Van de Vrie M, McMurtry JA, Huffaker CB (1972) Ecology of tetranychid mites and their natural enemies: a review III. Biology, ecology, and pest status, and host–plant relations of tetranychids. Hilgardia 41:343–432
Wrensch DL (1985) Reproductive parameters. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control. Elsevier, Amsterdam, pp 165–170
Wyatt IJ, White PF (1977) Simple estimation of intrinsic increase rates for aphids and tetranychid mites. J Appl Ecol 14:757–766
Acknowledgments
The authors are grateful to Dr. T. Ikemoto for providing us R statistical software, and to Dr. Y. Kitashima and Mr. R. Sugawara, Ibaraki University, for their kind help in this research.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
See Table 6.
Appendix 2
See Table 7.
Appendix 3
See Table 8.
Appendix 4
See Table 9.
Rights and permissions
About this article
Cite this article
Gotoh, T., Moriya, D. & Nachman, G. Development and reproduction of five Tetranychus species (Acari: Tetranychidae): Do they all have the potential to become major pests?. Exp Appl Acarol 66, 453–479 (2015). https://doi.org/10.1007/s10493-015-9919-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-015-9919-y