Abstract
In the present study, the bacterial community structure of enrichment cultures degrading benzene under microaerobic conditions was investigated through culturing and 16S rRNA gene Illumina amplicon sequencing. Enrichments were dominated by members of the genus Rhodoferax followed by Pseudomonas and Acidovorax. Additionally, a pale amber-coloured, motile, Gram-stain-negative bacterium, designated B7T was isolated from the microaerobic benzene-degrading enrichment cultures and characterized using a polyphasic approach to determine its taxonomic position. The 16S rRNA gene and whole genome-based phylogenetic analyses revealed that strain B7T formed a lineage within the family Comamonadaceae, clustered as a member of the genus Ideonella and most closely related to Ideonella dechloratans CCUG 30977T. The sole respiratory quinone is ubiquinone-8. The major fatty acids are C16:0 and summed feature 3 (C16:1 ω7c/iso-C15:0 2-OH). The DNA G + C content of the type strain is 68.8 mol%. The orthologous average nucleotide identity (OrthoANI) and in silico DNA–DNA hybridization (dDDH) relatedness values between strain B7T and closest relatives were below the threshold values for species demarcation. The genome of strain B7T, which is approximately 4.5 Mb, contains a phenol degradation gene cluster, encoding a multicomponent phenol hydroxylase (mPH) together with a complete meta-cleavage pathway including a I.2.C-type catechol 2,3-dioxygenase (C23O) gene. As predicted by the genome, the type strain is involved in aromatic hydrocarbon-degradation: benzene, toluene and ethylbenzene are degraded aerobically and also microaerobically as sole source of carbon and energy. Based on phenotypic characteristics and phylogenetic analysis, strain B7T is a member of the genus Ideonella and represents a novel species for which the name Ideonella benzenivorans sp. nov. is proposed. The type strain of the species is strain B7T (= LMG 32,345T = NCAIM B.02664T).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing level of petroleum hydrocarbon pollution significantly damages the ecosystem or even the human health. Simple aromatic hydrocarbons such as benzene, toluene, ethylbenzene, and xylene (BTEX) are the most common contaminants of the groundwater and can therefore easily contaminate drinking water due to their relatively high water solubility. Benzene is an important industrial chemical (Conte et al. 2021) and due to the discharge or disposal of product, benzene can pass into air, water, and soil as well. Accidental leakage of the underground storage tanks release benzene into water and soil where it breaks down more slowly than in air. Exposure to benzene can occur through the lungs, gastrointestinal tract, and across the skin (Abdel-Rahman et al. 1988; da Poça et al. 2021). Benzene can damage the blood and the immune system and can cause cancer such as acute myeloid leukemia and its genotoxic properties are also known (Whysner 2000; Natelson 2007; Guo et al. 2021; da Poça et al. 2021; Wang et al. 2021). Aromatic hydrocarbons decompose most rapidly and completely under aerobic conditions and the bioremediation has proven to be the most successful in solving problems of a widespread contamination. In bioremediation procedures, bacteria are used to eliminate petroleum hydrocarbons as a source of carbon and energy for their metabolic processes. Due to the presence of aerobic microorganisms the concentration of dissolved oxygen in the contaminated soil decreases rapidly partly because the BTEX degraders use mono- and dioxygenases for the hydroxylation and the cleavage of the aromatic ring and these enzymes require molecular oxygen as a co-substrate (Peréz-Pantoja et al. 2019). The availability of dissolved oxygen has a key role in the biodegradation because benzene, para- and ortho-xylene can be persistent contaminants under anaerobic conditions. Several research suggest that microbial communities in oxygen-limited, aromatic hydrocarbon-contaminated subsurface are generally dominated by members of the Betaproteobacteriales, including genera of the Comamonadaceae family (Fahy et al. 2006; Nestler et al. 2007; Martínez-Lavanchy et al. 2015). Enzymes belonging to the subfamily I.2.C of extradiol dioxygenases are adapted to low oxygen concentrations resulting a large diversity of catechol 2,3-dioxygenase (C23O) genes in these environments. These C23O genes are mostly associated with Betaproteobacteriales bacteria (Táncsics et al. 2010, 2012, 2013), and some of them are evidently involved in the microaerobic degradation of BTEXs, e.g. in case of the toluene-degrader Zoogloea oleivorans (Táncsics et al. 2020) or Variovorax paradoxus, which was capable of degrading all the six BTEX-compounds under both aerobic and microaerobic conditions (Benedek et al. 2021). However, still little is known about the role of microorganisms and I.2.C-type C23O genes in microaerobic benzene degradation, since only handful of known cultured bacteria are available yet for the purpose. Consequently, exploration of the bacterial communities of aromatic hydrocarbon contaminated, hypoxic environments, and investigation of bacteria and their functional genes that can participate in biodegradation of benzene and other BTEX components under microaerobic/hypoxic conditions have a current importance.
The genus Ideonella was first proposed by Malmqvist et al. (1994) with the description of Ideonella dechloratans, which was isolated from activated sludge from a municipal wastewater treatment plant. A significant emendation of the genus was carried out by Noar and Buckley (2009) with the description of Ideonella azotifigens isolated from grass rhizosphere soil. To date, five species of the genus Ideonella with validly published names have been reported (https://lpsn.dsmz.de/genus/ideonella). The genus Ideonella is a member of the family Comamonadaceae belonging to the Rubrivivax–Roseateles–Leptothrix–Azohydromonas–Aquincola–Ideonella branch of the order Burkholderiales (Garrity et al. 2005). The family Comamonadaceae includes several genera involved in the biodegradation of petroleum hydrocarbons as dominant community members in contaminated subsurface environments (Benedek et al. 2018; Révész et al. 2020a,b; Banerjee et al. 2021). Furthermore, they showed a possession of diverse C230 genes in such environments (Táncsics et al. 2010, 2012, 2013). Members of the genus Ideonella are characterized as Gram-stain-negative, rod-shaped bacteria, which contain ubiquinone-8 (Q-8) as predominant isoprenoid quinone; C16:0 and summed feature 3 (comprising C16 : 1 ω7c and/or C16 : 1 ω6c) as major fatty acids and the G + C content of the genomic DNA is between 67.4 and 70.4 mol% (Malmqvist et al. 1994; Noar and Buckley 2009; Sheu et al. 2016; Tanasupawat et al. 2016; Chen et al. 2020). In this study, a novel species of the genus Ideonella isolated from a microaerobic benzene-degrading enrichment culture having BTEX-degrading capability has been described with polyphasic characterization. In addition, whole-genome analysis of strain Ideonella designated B7T has been explored providing deeper insights into the genetic background of its BTEX-degrading capability.
Materials and methods
Experimental design of enrichment culturing
To reach the goals of the present study, microaerobic benzene-degrading enrichment cultures were set up. All three replicates of the enrichment cultures were inoculated with petroleum hydrocarbon contaminated groundwater of the “Siklos” site of Hungary, (45° 51 × 25.8˝ N 18° 17 × 32.3˝ E) (Táncsics et al. 2012). This area is one of the best characterized BTEX (benzene, toluene, ethylbenzene, and xylenes) contaminated environments of the country, located in Southwest-Hungary. The site has been thoroughly studied and described by Táncsics et al. earlier (2012, 2013). In the course of experiment 100-mL serum bottles were used, sealed with butyl-rubber septa and aluminium crimp seals. To assemble the enrichment cultures, 45 mL of a mineral salt medium (MS) (Fahy et al. 2006) supplemented with vitamins (1 mg thiamine l− 1, 15 µg biotin l− 1 and 20 µg vitamin B12 l− 1) was used for each bottle. The enrichment medium was sparged with N2/CO2 (80:20, v/v) for 10 min and then sterile air (0.2 μm pore size filtered) was injected through the septa to set the 0.5 mg L− 1 concentration of dissolved oxygen. Microaerobic condition was monitored non-invasively by Fibox 3 trace v3 fibre optic oxygen meter with PSt3 sensor spots (PreSens). In addition, each enrichment culture contained 5 mL groundwater sample and benzene as sole carbon and energy source at a concentration of 1 mM. To maintain the microaerobic conditions the oxygen was continuously replenished in the enrichments. After 1 week incubation in shaking thermostat (28 °C, 150 rpm) 5 mL of each enrichment was inoculated into fresh MS medium. The transfer was performed for five consecutive weeks. During the 5th week of the enrichment process, the concentration of benzene was detected for every 24 h by headspace analysis on an ISQ Single Quadrupole gas chromatography-mass spectrometer (GC–MS) (Thermo Fischer Scientific) via an SLB-5 ms fused silica capillary column (Supelco Analytical). The oven temperature was set to 40 °C for 3 min, then ramped at a rate of 20 ºC min − 1 to 190 °C, and held for 1 min. The mass spectrometer was operated at 250 °C in full scan mode.
16S rRNA gene amplicon sequencing and data handling
After the 5th transfer, 45 mL of the enrichments was centrifuged at 2360 g at 4 °C for 10 min using a Rotanta 460 R centrifuge (Hettich) to harvest the bacterial biomass. Subsequently, DNeasy UltraClean Microbial Kit (Qiagen) was used to isolate DNA from the pellets according to the instructions of the manufacturer. V3 and V4 variable regions of the 16S rRNA gene were amplified with the universal primer pair SD (S-D-Bact-0341-b-S-17/S-D-Bact-0785-a-A-21) (Klindworth et al. 2013). Amplification was performed according to the 16S metagenomic sequencing library preparation guide of Illumina using KAPA HiFi HotStart Ready Mix (KAPA Biosystems). Paired-end fragment reads were generated on an Illumina MiSeq sequencer using MiSeq Reagent Kit v3 (600-cycle) by SeqOmics Biotechnology Ltd. (Mórahalom, Hungary). The raw sequencing data were analysed according to Banerjee et al. (2022) to cluster sequences into OTUs and calculate the diversity of the samples using rarefaction curves. Read numbers were between 40 000 and 50 000 during the data processing performed by MiSeq SOP of mothur v1.41.1 (Schloss et al. 2009; Kozich et al. 2013). Sequence reads were deposited in NCBI SRA under BioProject ID PRJNA704528.
Strain isolation and Sanger-sequencing of 16S rRNA and I.2.C C23O genes.
To gain bacterial isolates, 5 mL was taken from each of the 5th week enrichment cultures and mixed, then 1 mL of this pooled sample was serially diluted with 0.85% (w/v) sterile saline solution and spread on the surface of R2A plates (DSM medium No. 830) in an amount of 100 µL. After 10 days of incubation at 15 °C, bacterial colonies were picked up. The UltraClean Microbial DNA Kit (Qiagen) was used according to the instructions of the manufacturer to isolate DNA from the pure cultures made by repeated streaking. Isolated strains were identified based on the 16S rRNA gene sequence. The 16S rRNA gene of strains was amplified and sequenced using the following primers: 27F, 338F, 803F and 1492R (Soergel et al. 2012). The 16S rRNA gene sequence of the strains was determined by using BigDye Terminator version 3.1 Cycle Sequencing Kit (ThermoFisher Scientific) according to the manufacturer’s instructions. Sequencing products were separated on a Model 3130 Genetic Analyzer (Applied Biosystems). To select bacterial strains involved in microaerobic benzene degradation, PCR amplifications of the subfamily I.2.C C23O gene were carried out on DNA extracted from strains by using the forward primer XYLE3F, 5´-TGYTGGGAYGARTGGGAYAA-3´, and the reverse primer XYLE3R, 5´-TCASGTRTASACITCSGTRAA-3´ (Táncsics et al. 2013). Sanger sequencing of the 16S rRNA and C23O genes were performed according to Benedek et al. (2018).
Phylogenetic analysis
To determine an approximate phylogenetic affiliation of strain B7T, the nearly complete 16S rRNA gene sequence was compared with other closely related strains available in the EzBiocloud server (https://www.ezbiocloud.net) (Yoon et al. 2017). Phylogenetic and molecular evolutionary analyses were conducted using MEGA X. (Kumar et al. 2018). Phylogenetic trees were constructed by using the neighbor-joining (NJ) (Saitou and Nei 1987), maximum-likelihood (ML) (Felsenstein 1981) and maximum-parsimony (MP) methods. Kimura’s two-parameter calculation model (Kimura 1980) was selected for NJ and ML analyses, respectively. Tree topologies and distances were evaluated by bootstrap analysis based on 1000 replicates.
Whole genome sequencing and genome data analysis
The whole-genome sequencing of strain B7T was performed by SeqOmics Biotechnology Ltd., Mórahalom, Hungary. In vitro fragment libraries were prepared using the NEBNext® Ultra™ II DNA Library Prep Kit for Illumina. Paired-end fragment reads were generated on an Illumina NextSeq sequencer using TG NextSeq® 500/550 High Output Kit v2 (300 cycles). Primary data analysis (base-calling) was carried out with Bbcl2fastq^ software (v2.17.1.14, Illumina). Trimmed sequences were de novo assembled and scaffolding were performed with SPAdes (v3.13.0) (Nurk et al. 2013). The genome assembly was submitted to NCBI Prokaryotic Genomes Annotation Pipeline (PGAP) v4.5 for automatic annotation (Tatusova et al. 2016). An up-to-date bacterial core gene set (UBCG, concatenated alignment of 92 core genes) and pipeline was utilized for phylogenetic tree construction as described by Na et al. (2018). Genome-to-Genome Distance Calculator (GGDC, https://ggdc.dsmz.de/) version 2.1. were used to determine the digital DNA–DNA hybridization values among strain B7T and related species with available genome sequences (Meier-Kolthoff et al. 2013), which were retrieved from the GenBank database (http://www.ncbi.nlm.nih.gov/genbank). The calculations of orthologous average nucleotide identity (OrthoANI) values between strain B7T and its closest relatives were performed by using the OAT software (Lee et al. 2016). The whole-genome-based phylogenetic analysis was carried out by the MiGA pipeline (http://microbial-genomes.org/) (Rodriguez et al. 2018). In order to determine which BTEX-compounds could potentially be degraded by strain B7T, the genome was annotated by using the Genoscope platform MAGE (Vallenet et al. 2006, 2009) and then analysis was performed by combining automated annotation from MAGE and manual curation using information from MetaCyc (Caspi et al. 2014), KEGG (Kanehisa and Goto 2000) and UniProt (Bateman 2019). CLC Genomics Workbench Tool v21 (Qiagen) was also used to identify gene clusters involved in the degradation of aromatic hydrocarbons.
Aromatic hydrocarbon degradation analyses
To explore the ability of the strain B7T to degrade BTEX components, monoaromatic hydrocarbons were added individually or in a 1:1 mixture of all BTEX components to 50 ml of MS medium supplemented with vitamins at a total concentration of 6 mg L− 1. The experiment was carried out in triplicates of 100-ml crimp top serum bottles under aerobic (7–8 mg L− 1 dissolved O2) and microaerobic (0.5 mg L− 1 dissolved O2) conditions. Bottles were inoculated with 100 µl suspensions of B7T cells (OD600 0.5) no older than 24 h. Then the bottles were incubated in a rotary shaker at 28 °C, 150 rpm Triplicates of abiotic controls were also used. Concentrations of individual or mixed BTEX compounds were detected for every 24 h by GC-MS according to the parameters mentioned above.
Phenotypical and biochemical characteristics
Native preparation of B7T was studied by Gram staining (Claus 1992) to determine the cell size, shape and arrangement. The cell morphology and flagellation type were investigated by transmission electron microscopy (H-7100; Hitachi) by applying the shadow-casting technique described by Ohad et al. (1963). Physiological and biochemical tests were performed according to the protocols of Barrow and Feltham (2004) and Smibert and Krieg (1994). The influence of temperature, salinity and pH on growth was investigated by using R2A broth based on its absorbance (600 nm). The growth test was examined between 4 and 45 °C, pH 4.0–11.0 and 0.1–7% (w/v) of NaCl. The pH of the broth was adjusted after autoclaving with sterile, 20% KOH or HCl. API tests (API ZYM, 20 NE, 50 CH test kits; bioMérieux, France) were performed according to the manufacturers’ instructions to determine the enzyme activities and other biochemical characteristics of B7T. Growth under anaerobic conditions was investigated in the presence of 0.15% (w/v) KNO3 as described earlier by Farkas et al. (2015). The whole-cell fatty acids, respiratory quinones and polar lipids were analysed by the Identification Service of DSMZ (Braunschweig, Germany). Sufficient cells of comparable physiological age could be harvested from the third streak quadrant of the R2A agar plates after cultivation at 28 °C for 48 h. Analysis of fatty acid methyl esters was performed based on the standard protocol of the Sherlock Microbial Identification System version 6.1 (MIDI; MIDI Inc., USA) and identified using the Calculation Method TSBA40 database. Identification of the summed features was performed by GC-MS analysis. The polar lipids and respiratory quinones were extracted based on the protocol modified after Bligh and Dyer (1959). The detection of the total lipid material and functional groups were performed according to described by Tindall et al. (2007). The respiratory quinones were analysed by using reversed-phase HPLC (LDC Analytical; Thermo Separation Products) using methanol:heptane 9 : 1 (v/v) as eluant.
Results and discussion
The composition of microaerobic benzene-degrading microbial community revealed by 16 S rRNA gene amplicon sequencing
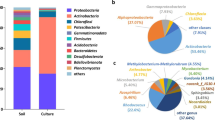
By using the BTEX-contaminated groundwater inoculum, microaerobic enrichments were set up in triplicates with benzene as a sole source of carbon and energy. Based on the measurements carried out in the 5th week of enrichment, the benzene was almost depleted by the third day of incubation from each microaerobic benzene-degrading enrichment. This result suggested that highly efficient microaerobic benzene-degrading communities evolved in the enrichments. Rarefaction curves were created for each sample (Fig. S1) and showed high sequencing coverage in all samples, suggesting that the sequencing depth was adequate. All of the samples showed OTU-based saturation around 55–65 OTUs. In two of the parallel enrichments the bacterial communities were overwhelmingly dominated by members of the genus Rhodoferax (BEN_MA1: 62.8%; BEN_MA3: 58.1%) followed by Acidovorax (BEN_MA1: 11.8%; BEN_MA3: 28.8%) and Pseudomonas (BEN_MA1: 15.6%; BEN_MA3: 5.6%). In case of enrichment BEN_MA2, members of the genus Acidovorax (79.4%) were the most dominant community members, while the abundance of Rhodoferax was only marginal here for some yet unknown reason (Fig. 1). Besides the most dominant genera, members of Sediminibacterium, Xanthobacter and Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium were also detectable with significant abundance (> 1%) as well in at least one of the enrichment communities. Little is known about the ecological role of the genus Rhodoferax in hydrocarbon contaminated subsurface, as the genus contains only eight validly described species (https://lpsn.dsmz.de/genus/rhodoferax). Some previous studies have shown the presence of genus Rhodoferax-related bacteria in ecosystems contaminated with aromatic hydrocarbons, especially occurrence of species closely related to Rhodoferax ferrireducens and R. antarticus were common (Alfreider and Vogt 2007; Aburto and Peimbert 2011), and the role of Rhodoferax sp. in sulfolane degradation has also been reported (Kasanke et al. 2019). The presence of the genus Rhodoferax has also been previously described in the BTEX-contaminated area of Siklós (Táncsics et al. 2012, 2013; Farkas et al. 2017). The genus Pseudomonas contains model organisms for the studies of aerobic BTEX degradation, especially aerobic toluene degrading capability of certain Pseudomonas strains is deeply investigated. Aburto et al. (2011) reported the presence of all three genera (Rhodoferax, Acidovorax and Pseudomonas) in different samples of the SIReN site (UK) and suggested that these bacteria were potential benzene and toluene degraders even under microaerobic conditions. Acidovorax genus related bacteria are frequently observed in petroleum hydrocarbon–contaminated environments (Singleton et al. 2018; Révész et al. 2020a,b), e.g., bacteria most closely related to Acidovorax defluvii and A. delafieldii were detected in BTEX-contaminated environments (Alfreider and Vogt 2007; Révész et al. 2020a, b). According to previous studies, members of the genera Acidovorax and Pseudomonas occurred in benzene-, toluene-, and xylene-degrading enrichments as well (Révész et al. 2020a; Banerjee et al. 2022). The genus Sediminibacterium and Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium have been also found to be present in petroleum-contaminated environments (Lindstrom et al. 2003; Yessica et al. 2013; Poi et al. 2018; Banerjee et al. 2022; Chen et al. 2022). Members of the genus Xanthobacter are often used for biodegradation purposes, as they can play role in the degradation of chlorinated hydrocarbons, alkenes, cyclohexane and phenol as well (van Ginkel and de Bont 1986; Janssen et al. 1989;; Trower et al. 1985; Nagamani et al. 2009).
Isolates
Based on different colony morphology and growth pattern altogether 13 strains were isolated from the pooled sample of the enrichments, which represented eight different genera (Table S1): Brucella, Pseudomonas, Rhizobium, Rhodococcus, Ideonella, Xanthobacter, Pseudoxanthomonas and Pinisolibacter. Most of the isolates belonged to the Hyphomicrobiales (Hyphomicrobiales -synonym: Rhizobiales), many of which are known to occur in ecosystems contaminated with petroleum hydrocarbons and some of them are potential hydrocarbon degraders (Rochman et al. 2017). Several members of the genus Rhodococcus, Pseudoxanthomonas and Pseudomonas are also known to degrade a wide range of xenobiotics (Táncsics et al. 2008; Wasi et al. 2013; Rochman et al. 2017; Lu et al. 2019; Révész et al. 2020b). Some members of these genera involved in petroleum hydrocarbon degradation, e.g., Rhodococcus aetherivorans was isolated from a mixture of petroleum compounds (Auffret et al. 2009) and the ability of members of the genus Pseudoxanthomonas to metabolize aromatic hydrocarbons and other pollutants are also studied (Patel et al. 2012; Kim 2008). The 16S rRNA gene sequences of the isolates were deposited at GenBank under the accession numbers OM570587-OM570577, MZ041034 and MZ047316 subsequently. The lack of Acidovorax and Rhodoferax isolates is striking, however it is a well known phenomenon that culture dependent, and independent microbial ecological approaches tend to yield results with minimal overlap.
Amid the isolates, only the Ideonella strain designated as B7 possessed subfamily I.2.C-type C23O gene. The closest relatives of this C23O gene were found in other species of the genus Ideonella, for instance in Ideonella dechloratans CCUG 30977T and in Ideonella sp. strain B508-1 with a low sequence similarity (98.41% and 98.09%) suggesting that occurrence of the C23O gene is not unique among the members of the genus. Genus Ideonella was also detectable by 16 S rRNA gene amplicon sequencing in the enrichment communities, although with low abundance (< 1%). These results suggest that the strain Ideonella sp. B7 was a relevant member of the microaerobic benzene-degrading enrichment cultures involved actively in BTEX degradation. Consequently, strain B7 was chosen for further investigation to explore its role in microaerobic benzene-degradation and to clarify its phylogenetic position.
Phylogenetic analysis of strain B7T
The 16 S rRNA gene seqence of strain B7T was 1413 bp long and it was deposited at GenBank database under the accession number MZ041034. Phylogenetic analysis based on the 16 S rRNA gene sequence showed that strain B7T formed a lineage within the family Comamonadaceae and clustered as a member of the genus Ideonella (Fig. 2). Comparative 16 S rRNA gene sequence analysis revealed that strain B7T was most closely related to Ideonella dechloratans CCUG 30977T (99.15%) followed by I. livida TBM-1T (98.51%), I. azotifigens 1a22T (98.28%), I. paludis KBP-31T (98.08%) and I. sakaiensis 201-F6T (98.02%). Strain B7T clustered with I. dechloratans, and I. livida in all of the three (NJ, ML and MP) phylogenetic trees. Furthermore, it formed a separate lineage within this cluster in ML and MP trees, which fact supported the identification of strain B7T as a possible novel member of the genus Ideonella (Figs. 2, S2, S3).
Maximum-likelihood phylogenetic tree with Kimura’s two-parameter calculation model based on 16S rRNA gene sequences highlighting the position of Ideonella benzenivorans strain B7T relative to other closely related species. Filled circles indicate that the corresponding nodes were also recovered using the maximum-likelihood (ML), neighbour-joining (NG) and maximum-parsimony (MP) tree-making algorithms. Sequences were aligned using CLUSTAL W with default parameters and phylogenies were carried out by the software MEGA X. Bootstrap values (> 50%) based on 1000 bootstrap replicates are shown at branch nodes. Pseudorhodoferax aquiterrae BCRC:80210T was used as an out-group. The respective GenBank accession numbers for 16S rRNA genes are indicated in parentheses. Bar, 0.01 substitutions per nucleotide site
Genomic analysis of strain B7T
Whole genome sequence of strain B7T has been deposited at GenBank under the accession number JAGWCB000000000. The high-quality draft genome of strain B7T had a total length of 4,490, 200 bps. The genome sequence was assembled into 21 contigs and has an average coverage value of 1823. The DNA G + C content of strain B7T is 68.8%, which value falls within the range of Ideonella species. The MiGA pipeline indicated high completeness value for the whole-genome sequence (100%), while contamination was low (0.9%). The number of predicted proteins was 4044. The dDDH values were much lower than the species threshold of 70% recommended for species demarcation (Chun et al. 2018). dDDH relatedness between strain B7T and Ideonella dechloratans, Ideonella azotifigens and Ideonella livida were 30.2%, 22.7% and 22.0%, respectively. In case of the other Ideonella species, the dDDH value was below 22%. Moreover, OrthoANI values were also below the treshold values (95–96%) recommended for the species level delineation (Chun et al. 2018) (Fig. 3). These results indicated that strain B7T should be assigned to a novel species of the genus Ideonella. Furthermore, a genome-based phylogenetic tree was also reconstructed with the UBCGs software (Fig. 4). The phylogenetic tree based on the coding sequences of 92 protein clusters showed that strain B7T formed a distinct phyletic lineage of the genus Ideonella.
The catechol 2,3-dioxygenase (C23O) gene was found in a phenol degradation gene cluster, encoding a multicomponent phenol hydroxylase (mPH) together with a complete meta-cleavage pathway (Fig. 5), providing aromatic hydrocarbon-degrading ability to strain B7T. The operon is regulated by a σ54-interacting transcriptional regulator and it contains Rieske-type ferredoxin that mediates electron transfer from the reductase to the oxygenase. Benzene, toluene and ethylbenzene degradation pathways were annotated by using the Genoscope platform MAGE, predicting which compounds are degradable by B7T. For the strain B7T, the target C23O gene (about 800 bp) was also detected successfully with Sanger-sequencing. The I.2.C catechol-2,3-dioxygenase gene sequence of strain B7T obtained by Sanger-method was compared with the C23O gene sequence extracted from the genome assembly and showed 100% similarity.
Physical map of the phenol degradation gene cluster encoding the multicomponent phenol hydroxylase (mPH) components, the catechol 2,3-dioxygenase and other lower meta-cleavage pathway enzymes in the genome of strain B7T. The map was performed using CLC Genomics Workbench Tool version 21. Arrows represent predicted ORFs, the direction of the arrow represents the direction of transcription. The colours denote different functional groups of genes involved in aromatic hydrocarbon degradation
The presence of a subfamily I.2.C-type C23O gene in the genome implied the possibility that strain B7T is capable of degrading monoaromatic componds under microaerobic conditions. Analyses of the genome of strain B7T revealed the presence of genes encoding cbb3-type cytochrome c oxidase (between locus tags KGA65_1305 and KGA65_13040), further hinting on this possibility. It is well known that this oxidase has high affinity for oxygen and is expressed under microaerobic conditions (van der Oost et al. 1994). Besides, genes encoding cytochrome o ubiquinol oxidase were also detected in the genome (locus tags KGA65_11435–KGA65_11455), assuming that under ample supply of oxygen this oxidase accommodates most of the electron flow (Dinamarca et al. 2002).
Aromatic hydrocarbon degradation analyses
As predicted by the genome analysis, it was observed that strain B7T was capable of degrading both aerobically and microaerobically benzene, toluene and ethylbenzene as sole source of carbon and energy (Fig. 6). The aerobic degradation of toluene and ethylbenzene was completed within 48 h of incubation at 28 °C and degradation of same concentration of benzene within around 72 h under similar conditions. However, microaerobic degradation proved to be slightly more efficient. Considerable degradation of toluene and ethylbenzene was observed within 24 h and the total amount of benzene was utilized by strain B7T within approximetaly 48 h. Strain B7T was unable to utilize xylenes as a sole carbon and energy source. In contrast, in those experimental set-ups where the mixture of all BTEX components were present at the same time, the amount of all xylene-isomers were also significantly reduced within 48 h indicating co-metabolism of xylenes in the presence of other monoaromatic hydrocarbons (data not shown).
Aerobic and microaerobic degradation of a benzene, b toluene, c ethylbenzene by Ideonella sp. strain B7T. Abiotic controls were used for each experiment. The concentration of BTEXs were measured by GC-MS analysis for every 24 h. The averages of triplicate experiments ± standard errors of the means, indicated by error bars, are shown
Phenotypical and biochemical characteristics
Ideonella dechloratans CCUG 30898T and I. azotifigens 1a22T DSM 21438T were used as reference strains for the comparative phenotypic analyses. Strain B7T proved to be strictly aerobic, as growth was not observed in anaerobic R2A broth supplemented with KNO3. The cells of strain B7T are rod-shaped, Gram-stain-negative and motile with polar flagellum. The approximate lenght of the cells was 1.8–2.0 μm (Fig. S4). The small and medium size colonies are pale amber, entire round, shiny and peak shaped after 2–3 days of incubation on R2A agar at 28 °C. Strain B7T hydrolysed Tweens 80, urea, esculin and gelatin, showed positive results for oxidase and casease and weakly positive for catalase. Strain B7T grew well but the reference strains were unable to grow at 45 °C. In addition, intense growth was observed after 24 h incubation at 35 °C on R2A plates. In API ZYM tests, B7T showed high diversity of enzymatic activities similar to the reference strains, but based on API 20NE and 50CH tests, strain B7T was unable for the assimilation and fermentation of any carbohydrates of those that were examined. Other differential physiological characteristics of B7T are given in Table 1 with its closest relatives.
The major fatty acids of strain B7T were C16:0 and summed feature 3 (C16:1 ω7c/iso-C15:0 2-OH) and C18:1 ω7c was present in significant quantities, similar with the reference strains. The additional GC-MS analysis revealed that summed feature 3 contained only the fatty acid C16:1 ω7c. The presence of C15:0 and C16:1 2-OH differentiates strain B7T from other phylogenetically related species of the genus Ideonella (Table 2). The sole respiratory quinone was ubiquinone-8 (Q-8) and the major polar lipids were phosphatidylethanolamine (PE), diphosphatidylglycerol (DPG) and phosphatidylglycerol (PG). In addition, an unidentified aminophospholipid (APL) an aminolipid (AL) and a polar lipid (L) were also detected (Fig. S5).
Based on above discussed data, strain B7T represents a novel species within the genus Ideonella for which the name Ideonella benzenivorans sp. nov. is proposed.
Description of Ideonella benzenivorans sp. nov.
Ideonella benzenivorans (ben.ze.ni.vo’rans. N.L. n. benzenum benzene; L. v. vorare to devour; L. part. adj. vorans devouring, digesting; N.L. part. adj. benzenivorans digesting benzene).
The cells (1.8–2.0 μm long and 0.6–0.7 μm wide) are rod-shaped, strictly aerobic, Gram-stain-negative and motile with a polar flagellum. Colonies on R2A are small and medium size, pale amber, circular, shiny and peak shaped after 2–3 days at 28 °C. Colonies grow at 15–45 °C (optimum, 28–37 °C) and pH 4.0–10.0 (optimum pH, 6.0–8.0) and can tolerate maximum 2% NaCl (w/v) (optimum 0.3% w/v). Oxidase and casease are positive and catalase is weakly positive. The type strain is able to reduce nitrate to nitrite or ammonium and can perform hydrolysis of Tween 80, urea, esculin and gelatin. Glucose and other carbohydrates are not metabolised. The type strain shows the following enzyme activities: positive for esterase (C4), esterase lipase (C8), leucine arylamidase, valine arylamidase, α-chymotrypsin, napthol-AS-BI-phosphohydrolase, α-glucosidase, weakly positive for alkaline phosphatase, lipase (C14), trypsin, N-acetyl-β-glucosaminidase. The sole respiratory quinone is Q-8. The major polar lipids are phosphatidylethanolamine, diphosphatidylglycerol and phosphatidylglycerol. The major fatty acids are C16:0 and C16:1 ω7c. The DNA G + C content of the type strain is 68.8%. It is able to degrade aerobically and microaerobically benzene, toluene ethylbenzene as sole source of carbon and energy, and the xylene isomers are degraded co-metabolically in the presence of the other BTEX components.
The type strain B7T (= LMG 32345T = NCAIM B.02664T) was isolated from a benzene-degrading enrichment culture inoculated with groundwater sample of the „Siklós” BTEX contaminated site (Hungary). The GenBank accession numbers for the 16 S rRNA gene sequence and the whole genome sequence of strain B7T are MZ041034 and JAGWCB000000000, respectively.
Data availability
Correspondence and requests for materials should be addressed to A.T. The whole genome data of Ideonella benzenivorans B7T could be accessed under accession number JAGWCB000000000 in the NCBI database (www.ncbi.nlm.nih.gov). The 16 S rRNA gene amplicon sequence reads were deposited in NCBI SRA under BioProject ID PRJNA704528. The 16 S rRNA gene sequences of the isolates were deposited at NCBI GenBank under accession numbers OM570587-OM570577, MZ041034 and MZ047316.
References
Aburto A, Peimbert M (2011) Degradation of a benzene–toluene mixture by hydrocarbon-adapted bacterial communities. Ann Microbiol 61:553–562. https://doi.org/10.1007/s13213-010-0173-6
Alfreider A, Vogt C (2007) Bacterial diversity and aerobic biodegradation potential in a BTEX-contaminated aquifer. Water Air Soil Pollut 183:415–426. https://doi.org/10.1007/s11270-007-9390-4
Auffret M, Labbé D, Thouand G, Greer CW, Fayolle-Guichard F (2009) Degradation of a mixture of hydrocarbons, gasoline, and diesel oil additives by Rhodococcus aetherivorans and Rhodococcus wratislaviensis. Appl Environ Microbiol 75:7774–7782. https://doi.org/10.1128/AEM.01117-09
Banerjee S, Táncsics A, Tóth E, Révész F, Bóka K, Kriszt B (2021) Hydrogenophaga aromaticivorans sp. nov., isolated from a para-xylene-degrading enrichment culture, capable of degrading benzene, meta-and para-xylene. Int J Syst Evol Microbiol 71:004743. https://doi.org/10.1099/ijsem.0.004743
Banerjee S, Bedics A, Harkai P, Kriszt B, Alpula N, Táncsics A (2022) Evaluating the aerobic xylenedegrading potential of the intrinsic microbial community of a legacy BTEXcontaminated aquifer by enrichment culturing coupled with multiomics analysis: uncovering the role of Hydrogenophaga strains in xylene degradation. Environ Sci Pollut Res 29: 28431–28445. https://doi.org/10.1007/s11356-021-18300-w
Barrow GI, Feltham RKA (2004) Cowan and steel’s manual for the identification of medical bacteria, 3rd edn. Cambridge University Press, Cambridge
Benedek T, Szentgyörgyi F, Szabó I, Kriszt B, Révész F, Radó J, Maróti G, Táncsics A (2018) Aerobic and oxygen-limited enrichment of BTEX-degrading biofilm bacteria: dominance of Malikia versus Acidovorax species. Environ Sci Pollut Res 25:32178–32195. https://doi.org/10.1007/s11356-018-3096-6
Benedek T, Szentgyörgyi F, Gergócs V, Menashe O, Gonzalez PAF, Probst AJ et al. (2021) Potential of Variovorax paradoxus isolate BFB1_13 for bioremediation of BTEX contaminated sites. AMB Express, 11:1–17. https://doi.org/10.1371/journal.pone.0189276
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Caspi R, Billington R, Ferrer L et al (2016) The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res 42:D459–D471. https://doi.org/10.1093/nar/gkv1164
Chen WM, Chen LC, Sheu DS, Tsai JM, Sheu SY (2020) Ideonella livida sp. nov., isolated from a freshwater lake. Int J Syst Evol Microbiol 70:4942–4950. https://doi.org/10.1099/ijsem.0.004363
Chen S, Yang C, Zhu G, Zhang H, Yan N, Zhang Y, Rittmann BE (2022) Selective acceleration of 2-hydroxyl pyridine mono-oxygenation using specially acclimated biomass. J Environ Manage 301:113887. https://doi.org/10.1016/j.jenvman.2021.113887
Chun J, Oren A, Ventosa A et al (2018) Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol 68:461–466. https://doi.org/10.1099/ijsem.0.002516.
Claus D (1992) A standardized Gram staining procedure. World J Microbiol Biotechnol 8:451–452. https://doi.org/10.1007/BF01198764
Conte S, Lagacé F, Netchiporouk E, Sasseville D, Litvinov IV (2021) Benzene, a Known Human Carcinogen, Detected in Suncare Products. J Cutan Med Surg 25:650–651. https://doi.org/10.1177/12034754211034507
da Poça KS, Giardini I, Silva PVB et al (2021) Gasoline-station workers in Brazil: Benzene exposure; Genotoxic and immunotoxic effects. Mutat Res Genet Toxicol Environ Mutagen 865:503322. https://doi.org/10.1016/j.mrgentox.2021.503322
Dinamarca MA, Ruiz-Manzano A, Rojo F (2002) Inactivation of cytochrome o obiquinol oxidase relieves catabolic repression of the Pseudomonas putida GPo1 alkane degradation pathway. J Bacteriol 184:3758–3793. https://doi.org/10.1128/JB.184.14.3785-3793.2002
Fahy A, McGenity TJ, Timmis KN, Ball AS (2006) Heterogeneous aerobic benzene-degrading communities in oxygen-depleted groundwaters. FEMS Microbiol Ecol 58:260–270. https://doi.org/10.1111/j.1574-6941.2006.00162.x
Farkas M, Táncsics A, Kriszt B, Benedek T, Tóth EM, Kéki Z, Veres PG, Szoboszlay S (2015) Zoogloea oleivorans sp. nov., a floc-forming, petroleum hydrocarbon-degrading bacterium isolated from biofilm. Int J Syst Evol Microbiol 65:274–279. https://doi.org/10.1099/ijs.0.068486-0
Farkas M, Szoboszlay S, Benedek T, Révész F, Veres PG, KRiszt B, Táncsics A (2017) Enrichment of dissimilatory Fe (III)-reducing bacteria from groundwater of the Siklós BTEX-contaminated site (Hungary). Folia Microbiol 62:63–71. https://doi.org/10.1007/s12223-016-0473-8.
Felsenstein J (1981) Evolutionary trees from DNA sequences: A maximum likelihood approach. J Mol Evol 17:368–376. https://doi.org/10.1007/BF01734359
Guo H, Ahn S, Zhang L (2021) Benzene-associated immunosuppression and chronic inflammation in humans: a systematic review. Occup Environ Med 78:377–384. https://doi.org/10.1136/oemed-2020-106517
Janssen DB, Pries F, van der Ploeg J, Kazemier B, Terpstra P, Witholt B (1989) Cloning of 1, 2-dichloroethane degradation genes of Xanthobacter autotrophicus GJ10 and expression and sequencing of the dhlA gene. J Bacteriol 171:6791–6799. https://doi.org/10.1128/jb.171.12.6791-6799.1989
Kaden R, Spröer C, Beyer D, Krolla-Sidenstein P (2014) Rhodoferax saidenbachensis sp. nov., a psychrotolerant, very slowly growing bacterium within the family Comamonadaceae, proposal of appropriate taxonomic position of Albidiferax ferrireducens strain T118T in the genus Rhodoferax and emended description of the genus Rhodoferax. Int J Syst Evol Microbiol 64:1186–1193. https://doi.org/10.1099/ijs.0.054031-0
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30. https://doi.org/10.1093/nar/28.1.27
Kasanke CP, Collins RE, Leigh MB (2019) Identification and characterization of a dominant sulfolane-degrading Rhodoferax sp. via stable isotope probing combined with metagenomics. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-40000-2
Kim JM, Le NT, Chung BS, Park JH, Bae J-W, MAdsen EL, Jeon CO (2008) Influence of soil components on the biodegradation of benzene, toluene, ethylbenzene, and o-, m-, and p-xylenes by the newly isolated bacterium Pseudoxanthomonas spadix BD-a59. Appl Environ Microbiol 74:7313–7320. https://doi.org/10.1128/AEM.01695-08
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1. https://doi.org/10.1093/nar/gks808
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. https://doi.org/10.1128/AEM.01043-13
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547. https://doi.org/10.1093/molbev/msy096
Lee I, Kim OY, Park S-C, Chun J (2016) OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol 66:1100–1103. https://doi.org/10.1099/ijsem.0.000760.
Lindstrom K, Jussila MM, Hintsa H, Kaksonen A, Mokelke L, Mäkeläinen K, Pitkäjärvi J, Suominen L (2003) Potential of the Galega-Rhizobium galegae system for bioremediation of oil-contaminated soil. Food Technol Biotechnol 41:11–16.
Lu Z, Sun W, Li C, Ao X, Yang C, Li S (2019) Bioremoval of non-steroidal anti-inflammatory drugs by Pseudoxanthomonas sp. DIN-3 isolated from biological activated carbon process. Water Res 161:459–472. https://doi.org/10.1016/j.watres.2019.05.065
Malmqvist A, Welander T, Moore E, Ternstrom A, Molin G, Stenstrom I (1994) Ideonella dechloratans, gen. nov., sp. nov., a new bacterium capable of growing anaerobically with chlorate as an electron acceptor. Syst Appl Microbiol 17:58–64. https://doi.org/10.1016/S0723-2020(11)80032-9
Martínez-Lavanchy PM, Chen Z, Lünsmann V, Marin-Cevada V, Vilchez-Vargas R, Pieper DH, Reiche N, Kappelmeyer U, Imparato V, Junca H, Nijenhuis I, Müller JA, Kuschk P, Heipieper HJ (2015) Microbial toluene removal in hypoxic model constructed wetlands occurs predominantly via the ring monooxygenation pathway. Appl Environ Microbiol 81: 6241–6252 https://doi.org/10.1128/AEM.01822-15
Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M (2013) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform 14:60. https://doi.org/10.1186/1471-2105-14-60
Na SI, Kim YO, Yoon SH, Ha SM, Baek I, Chun J (2018) UBCG: up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J Microbiol 56:281–285. https://doi.org/10.1007/s12275-018-8014-6
Nagamani A, Soligalla R, Lowry M (2009) Isolation and characterization of phenol degrading Xanthobacter flavus. Afr J Biotechnol 8:5449–5453.
Natelson EA (2007) Benzene-induced acute myeloid leukemia: A clinician’s perspective. Am J Hematol 82: 826–830. https://doi.org/10.1002/ajh.20934
Nestler H, Kiesel B, Kaschabek SR, Mau M, Schlömann M, Balcke GU (2007) Biodegradation of chlorobenzene under hypoxic and mixed hypoxic-denitrifying conditions. Biodegradation 18:755–767. https://doi.org/10.1007/s10532-007-9104-z
Noar JD, Buckley DH (2009) Ideonella azotifigens sp. nov., an aerobic diazotroph of the Betaproteobacteria isolated from grass rhizosphere soil, and emended description of the genus Ideonella. Int J Syst Evol Microbiol 59:1941–1946. https://doi.org/10.1099/ijs.0.003368-0
Nurk S, Bankevich A, Antipov D et al (2013) Assembling Genomes and Mini-metagenomes from Highly Chimeric Reads. In Deng M, Jiang R, Sun F, Zhang X (eds) Research in Computational Molecular Biology. RECOMB 2013. Lecture Notes in Computer Science, vol 7821. Springer, Berlin, Heidelberg. pp. 158–170 https://doi.org/10.1007/978-3-642-37195-0_13
Ohad I, Danon D, Hestrin S (1963) The use of shadow-casting technique for measurement of the width of elongated particles. J Cell Biol 17:321–326. https://doi.org/10.1083/jcb.17.2.321
Patel V, Cheturvedula S, Madamwar D (2012) Phenanthrene degradation by Pseudoxanthomonas sp. DMVP2 isolated from hydrocarbon contaminated sediment of Amlakhadi canal, Gujarat, India. J Hazard Mater 201:43–51. https://doi.org/10.1016/j.jhazmat.2011.11.002
Poi G, Shahsavari E, Aburto-Medina A, Mok PC, Ball AS (2018) Large scale treatment of total petroleum-hydrocarbon contaminated groundwater using bioaugmentation. J Environ Manage 214:157–163. https://doi.org/10.1016/j.jenvman.2018.02.079
Révész F, Farkas M, Kriszt B, Szoboszlay S, Benedek T, Táncsics A (2020a) Effect of oxygen limitation on the enrichment of bacteria degrading either benzene or toluene and the identification of Malikia spinosa (Comamonadaceae) as prominent aerobic benzene-, toluene-, and ethylbenzene-degrading bacterium: enrichment, isolation and whole-genome analysis. Environ Sci Pollut Res 27:31130–31142. https://doi.org/10.1007/s11356-020-09277-z
Révész F, Figueroa-Gonzalez PA, Probst AJ, Kriszt B, Banerjee S, Szoboszlay S, Maróti G, Táncsics A (2020b) Microaerobic conditions caused the overwhelming dominance of Acinetobacter spp. and the marginalization of Rhodococcus spp. in diesel fuel/crude oil mixture-amended enrichment cultures. Arch Microbiol 202:329–342. https://doi.org/10.1007/s00203-019-01749-2
Rochman FF, Sheremet A, Tamas I et al (2017) Benzene and naphthalene degrading bacterial communities in an oil sands tailings pond. Front Microbiol 8:1845. https://doi.org/10.3389/fmicb.2017.01845
Rodriguez-R LM, Gunturu S, Harvey WT et al (2018) The Microbial Genomes Atlas (MiGA) webserver: taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res 46:282–288. https://doi.org/10.1093/nar/gky467
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Sheu SY, Chen ZH, Young CC, Chen WM (2016) Ideonella paludis sp. nov., isolated from a marsh. Int J Syst Evol 66:1052–1057. https://doi.org/10.1099/ijsem.0.000832
Singleton DR, Lee J, Dickey AN, Stroud A, Scholl EH, Wright FA, Aitken MD (2018) Polyphasic characterization of four soil-derived phenanthrene-degrading Acidovorax strains and proposal of Acidovorax carolinensis sp. nov. Syst Appl Microbiol 41:460–472. https://doi.org/10.1016/j.syapm.2018.06.001
Soergel DA, Dey N, Knight R, Brenner SE (2012) Selection of primers for optimal taxonomic classification of environmental 16S rRNA gene sequences. ISME J 6:1440–1444. https://doi.org/10.1038/ismej.2011.208
Tanasupawat S, Takehana T, Yoshida S, Hiraga K, Oda K (2016) Ideonella sakaiensis sp. nov., isolated from a microbial consortium that degrades poly (ethylene terephthalate). Int J Syst Evol 66:2813–2818. https://doi.org/10.1099/ijsem.0.001058
Táncsics A, Szoboszlay S, Kriszt B, Kukolya J, Baka E, Márialigeti K, Révész S (2008) Applicability of the functional gene catechol 1, 2-dioxygenase as a biomarker in the detection of BTEX‐degrading Rhodococcus species. J Appl Microbiol 105:1026–1033. https://doi.org/10.1111/j.1365-2672.2008.03832.x
Táncsics A, Szabó I, Baka E, Szoboszlay S, Kukolya J, Kriszt B, Márialigeti K (2010) Investigation of catechol 2,3-dioxygenase and 16S rRNA gene diversity in hypoxic, petroleum hydrocarbon contaminated groundwater. Syst Appl Microbiol 33:398–406. https://doi.org/10.1016/j.syapm.2010.08.005
Táncsics A, Szoboszlay S, Szabó I, Farkas M, Kovács B, Kukolya J, Mayer Z, Kriszt B (2012) Quantification of subfamily I.2.C catechol 2,3-dioxygenase mRNA transcripts in groundwater samples of an oxygen-limited BTEX-contaminated site. Environ Sci Technol 46:232–240. https://doi.org/10.1021/es201842h
Táncsics A, Farkas M, Szoboszlay S, Szabó I, Kukolya J, Vajna B, Kovács B, Benedek T, Kriszt B (2013) One-year monitoring of meta-cleavage dioxygenase gene expression and microbial community dynamics reveals the relevance of subfamily I.2.C extradiol dioxygenases in hypoxic, BTEX-contaminated groundwater. Syst Appl Microbiol 36:339–350. https://doi.org/10.1016/j.syapm.2013.03.008
Táncsics A, Farkas M, Horváth B, Maróti G, Bradford LM, Lueders T, Kriszt B (2020) Genome analysis provides insights into microaerobic toluene-degradation pathway of Zoogloea oleivorans BucT. Arch. Microbiol 202: 421–426. https://doi.org/10.1007/s00203-019-01743-8
Tatusova T, DiCuccio M, Badretdin A et al (2016) NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44:6614–6624. https://doi.org/10.1093/nar/gkw569
The UniProt Consortium (2019) UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515. https://doi.org/10.1093/nar/gky1049
Trower MK, Buckland RM, Higgins R, Griffin M (1985) Isolation and characterization of a cyclohexane-metabolizing Xanthobacter sp. Appl Environ Microbiol 49:1282–1289. https://doi.org/10.1007/BF00470879
Vallenet D, Labarre L, Rouy Z et al (2006) MaGe: A microbial genome annotation system supported by synteny results. Nucleic Acids Res 34:53–65. https://doi.org/10.1093/nar/gkj406
van der Oost J, de Boer AP, de Gier JW, Zumft WG, Stouthamer AH, van Spanning RJ (1994) The heme-copper oxidase family consists of three distinct types of terminal oxidases and is related to nitric oxide reductase. FEMS Microbiol Lett 121:1–9. https://doi.org/10.1111/j.1574-6968.1994.tb07067.x
van Ginkel CG, ve Bont JAM (1986) Isolation and characterization of alkene-utilizing Xanthobacter spp. Arch Microbiol 145:403–407. https://doi.org/10.1007/BF00470879
Wang J, Guo X, Chen Y, Zhang W, Ren J, Gao A (2021) Association between benzene exposure, serum levels of cytokines and hematological measures in Chinese workers: A cross-sectional study. Ecotoxicol Environ Saf 207:111562. https://doi.org/10.1016/j.ecoenv.2020.111562
Wasi S, Tabrez S, Ahmad M (2013) Use of Pseudomonas spp. for the bioremediation of environmental pollutants: a review. Environ Monit Assess 185:8147–8155. https://doi.org/10.1007/s10661-013-3163-x
Whysner J (2000) Benzene-induced genotoxicity. J Toxicol Environ Health Part A 61:347–351. https://doi.org/10.1080/00984100050166343
Yessica GP, Alejandro A, Ronald F-C et al (2013) Tolerance, growth and degradation of phenanthrene and benzo [a] pyrene by Rhizobium tropici CIAT 899 in liquid culture medium. Appl Soil Ecol 63:105–111. https://doi.org/10.1016/j.apsoil.2012.09.010
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Abdel-Rahman MS, Turkall RM (1988) Determination of exposure of oral and dermal benzene from contaminated soils. In: Kostecki PT, Calabrese EJ (eds) Petroleum contaminated soils: remediation techniques, environmental fate, and risk assessment, 1st edn. CRC Press. pp 301–311.
Garrity GM, Bell JA, Lilburn T, Order I. Burkholderiales ord. nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM (eds) Bergey’s manual of systematic bacteriology, 2nd edn, vol. 2 (The Proteobacteria), part C (The Alpha-, Beta-, Delta-, and Epsilonproteobacteria), Springer, New York, pp. 575.–763
Pérez-Pantoja D, González B, Pieper DH (2019) Aerobic degradation of aromatic hydrocarbons. In: Rojo F (eds) Handbook of hydrocarbon and lipid microbiology. Cham, Switzerland: Springer Nature Switzerland AG. pp. 157–200. https://doi.org/10.1007/978-3-319-50418-6
Smibert RM, Krieg NR (1994) Phenotypic characterization. In : Gerhardt RGE, Murray WA, Krieg NR (eds) Methods for general and molecular bacteriology, American Society for Microbiology, Washington, DC. pp. 603–711
Tindall BJ, Sikorski J, Smibert RM, Kreig NR (2007) Phenotypic characterization and the principles of comparative systematics. In: Reddy CAT et al (eds) Methods for general and molecular microbiology, ASM Press. pp. 330–393 https://doi.org/10.1128/9781555817497.ch15
Vallenet D, Engelen S, Mornico D et al (2009) MicroScope: A platform for microbial genome annotation and comparative genomics. Database 2009:bap021. https://doi.org/10.1093/database/bap021
Funding
Open access funding provided by Hungarian University of Agriculture and Life Sciences. This research was supported by the grant ÚNKP-21-3 New National Excellence Programme of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. The financial support of the Ministry of Innovation and Technology within the framework of the Thematic Excellence Programme 2020, National Challenges Subprogramme (TKP2020-NKA-16) is also acknowledged. András Táncsics was supported by the “OTKA” Young Researcher Excellence Programme (FK 134439). Sinchan Banerjee was supported through the Tempus Public Foundation (Stipendium Hungaricum Scholarship Programme) by the Ministry of Human Capacities of Hungary.
Author information
Authors and Affiliations
Contributions
A.B. made the experiments, analysed the data, wrote the main manuscript text and prepared figures and tables as well as supplementary materials. B.S. performed GC-MS measurements. P.H. organized and implemented the sampling campaign of the contaminated site. B. Kovács constructed the phylogenomic tree with the UBCGs software. K.B. prepared the transmission electron microscopy figure. E.T. performed the biochemical tests. B. Kriszt provided access to the contaminated site. A.T. designed and supervised the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bedics, A., Táncsics, A., Tóth, E. et al. Microaerobic enrichment of benzene-degrading bacteria and description of Ideonella benzenivorans sp. nov., capable of degrading benzene, toluene and ethylbenzene under microaerobic conditions. Antonie van Leeuwenhoek 115, 1113–1128 (2022). https://doi.org/10.1007/s10482-022-01759-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-022-01759-z