Abstract
The sorption of Zr(IV) and Y(III) was examined using an iron-tin silicate (FeSnSi) composite prepared by the co-precipitation technique. The analytical tools that characterize prepared composite are FT-IR, SEM, EDX, XRD, and XRF. The effects of temperature, pH, ion concentrations, and shaking time are all considered in the sorption studies conducted on Zr(IV) and Y(III). The sorption of studied metal ions depends on pH, and the pseudo-2nd-order model governs the kinetics of reactions. Negative Gibbs energy values confirmed the excellent feasibility and spontaneity of the sorption process. Positive enthalpy values indicate that this process was endothermic. Positive entropy values demonstrated that the disorder between the solid and liquid phases was enhanced during adsorption. Freundlich and Langmuir models are used to study isotherms. The results of the binary system verify that Zr(IV) may be separated from the Zr-Y system at various pHs. According to the findings, the produced composite may effectively remove Zr(IV) and Y(III) from aqueous solutions. It may also be viable for purifying wastewater contaminated with these metal ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare earth elements (REEs) comprise 15 lanthanides and two pseudo-lanthanides (scandium and yttrium). These elements can be further classified into light rare earth elements (LREEs) and heavy rare earth elements (HREEs). The LREEs are La, Ce, Pr, Nd, Pm, Eu, Gd, and Sm. The list of HREEs is Dy, Y, Tb, Ho, Er, Tm, Yb, and Lu. REEs, are sometimes known as the "industrial vitamin," because they play critical roles in the development of worldwide sustainable energy technologies [1, 2]. The combination of five crucial REEs (Dy, Eu, Nd, Tb, and Y) collectively accounts for as much as 63% of the overall weight of all REEs in the final product, with a purity of 99.4%. Yttrium is extensively utilized in several industries for its exceptional physicochemical qualities, serving as catalysts, sensors, fiber optics, and flattened screen displays [3, 4]. Moreover, yttrium is commonly utilized in the field of medicine for purposes such as tumor therapy, food irradiation methods, and sewage treatment procedures. One of the most frequent radioisotopes found in liquid radioactive waste due to the fission reaction is 90Sr. It undergoes decay with a half-life of 28 years by emitting electrons (beta minus decay) and transforms into 90Y. Some recent investigations have found that long-term yttrium exposure in humans can result in carcinoma of the lungs, and damaged liver function [5,6,7,8]. Recovering yttrium from wastewater not only reduces the ecological and health hazards linked to yttrium-contaminated aquatic ecosystems but also contributes to fulfilling the increasing worldwide need for yttrium [9].
Zirconium is employed in nuclear power plant technology as a cladding material in the form of zircalloys for uranium fuel and other components within the reactor. The deterioration of reactor vessels due to aging results in erosion, which in turn leads to the contamination of coolant water with radioactive zirconium species, hence creating risks to the ecosystem [10]. Zirconium and its derivatives have extensive applications in several industries such as the chemical industry, steel and cast production, satellite surface agents, lamp filaments, welding fluxes, vacuum tubes, water-resistant textiles, dye pigments, ceramics, glass, and abrasives. Therefore, it is crucial to purify the coolant water in the reactor by removing radioactive zirconium and other generated radioactive waste before it can be safely discharge in the environment [10, 11].
Wastewater contaminated with hazardous metal ions has been treated using a variety of techniques, such as chemical precipitation, membrane separation, electrodeposition, ion exchange, solvent extraction, and adsorption [12, 13]. The most economical of these techniques are traditional chemical precipitation, however, it is ineffective for diluted solutions. Reverse osmosis and ion exchange are generally efficient, however, they have relatively significant operational and maintenance expenses. One of the few viable choices for this application is adsorption, due to its cost-effective and simple handling. Furthermore, one of the most important features of the adsorption technique is the possibility of separating one or several similar elements from the mixed solution and quantitatively determining [14]. Silica gels are a beneficial material used as a pre-concentration surface for a variety of compounds, including metals from aquatic systems. Matrix that can host a wide range of components can be created by chemically modifying silica gels with organic or inorganic molecules or both, these matrices have vast technological applications. Wastewater treatment is reliably enhanced by these cutting-edge materials, which are both environmentally safe and highly effective [14].
Many studies have been carried out for the sorption of Y(III) and Zr(IV) using different sorbent materials [15,16,17,18,19,20], but FeSnSi composite has not been used previously for the removal of Y(III) and Zr(IV). In this work, FeSnSi composite was prepared as novel material and used for the sorption of Y(III) and Zr(IV) from aqueous solutions. This sorbent material is prepared by incorporating iron chloride and stannous chloride into the silica matrix. The synthesized sorbent was characterized using different analytical tools. This composite was tested under various batch experimental circumstances to see if it could be used to solid-phase extract Zr(IV) and Y(III) from their respective aqueous solutions.
2 Experimental
2.1 Materials and instruments
The main reagents used were YCl3, HNO3, and HCl (Merck, Germany). FeCl3.6H2O (Loba Chemie, India), SnCl2.2H2O, ZrOCl2, and Na2SiO3 (Alpha Chemika, India), as well as NaOH and NH3 (El-Nasr Co, Egypt). The compounds and components used in this article are of analytical quality, meaning they do not require any additional purification. Double distilled water (DDW) was used for all experiments involving the preparation of composites and solutions. We used an Alpha II Bruker from Germany for Fourier-transform infrared spectroscopy (FT-IR) and a Brucker XRD diffractometer D2 Phaser II from Germany for X-ray diffraction (XRD) analysis of the FeSnSi composite. We used a Philips sequential X-ray spectrometer-2400 to determine the FeSnSi composite's elemental composition. The Super-Q quantitative application program dictated SiO2, Fe2O3, and SnO2 percentages. The morphology and elemental analysis of the FeSnSi composite were examined using scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) analysis model (ZEISS—EVO 15, UK).
2.2 Preparation
2.2.1 Preparation of reagents
Using 600 mL of DDW and 60 g of Na2SiO3 powder, a 10% (w/v) solution was made by mixing them for 60 min. After dissolving 4.866 g of FeCl3 powder in 150 mL of DDW for 1 h, a 0.2 M solution of FeCl3 was obtained. 17.79 g of SnCl2.2H2O powder was dissolved in 300 mL of 4 M HCl for 2 h to produce a SnCl2.2H2O solution (0.2 M).
2.2.2 Preparation of iron tin silicate (FeSnSi) composite
The SnSi and FeSnSi composites were prepared using a co-precipitation technique. In this method, FeCl3 solution (0.2 M) and SnCl2.2H2O solution (0.2 M) were dropwise to Na2SiO3 solution by various volumetric ratios at constant stirring as shown in Table 1. The reaction mixture was diluted to one litter and let to settle for one day after total addition, which produced a brownish-red tint. Subsequently, NH4OH (10% v/v) was added dropwise to the combined solution until a precipitate formed at pH 7.2. Precipitate washed several times to remove free chloride ions. Drying the precipitate at 55 ± 1 ◦C for 24 h followed by grinding and sieving it for different mesh sizes was followed by storage at 25 ± 1 ◦C.
2.3 The percent removal studies
To select the optimal sample for percent removal (% R), SnSi and FeSnSi composites were made using various volumetric ratios of Zr(IV) and Y(III) (50 mg L−1) and agitated with 0.05 g solid, 5 mL of Zr(IV) and Y(III) (V/m = 100 mL g−1), at 30 ± 1˚C for 24 h. At this point, the agitator is turned off, then solid and solution are immediately separated. UV–visible recording spectrophotometer (UV-160A) made and supplied by Shimadzu Kyoto, Japan, was utilized to measure the initial and final concentrations (Ci and Cf) of Y(III) and Zr(IV). Using (Eq. (1)), one may get the % R [21, 22]:
The data shown in Table 1 demonstrates the % R of Zr(IV) and Y(III) onto SnSi and FeSnSi composites, which were prepared using various volumetric ratios. The results indicate that Zr(IV) removal is more than Y(III) removal, and sample 3 (S-3) exhibits the highest efficiency. Consequently, it was selected for this study based on its higher percentage of elimination.
2.4 pH titration
Topp and Pepper's method was used to study the pH titration of FeSnSi composite utilizing the NaOH-NaCl system [23]. Each NaOH-NaCl system was supplemented with 0.1 g of solid powder and subjected to continuous shaking in 10 mL volumes. The pH of the solution was measured using a laboratory pH meter at regular one-day intervals until it achieved equilibrium.
2.5 Chemical stability
The chemical stability of S-3 was assessed by subjecting 0.2 g of solid and 20 mL of various solvents, including mineral acids, and alkaline solution to agitation for approximately 3 days at a temperature of 25 ± 1 °C. The quantity of S-3 discharged into the solution was measured by gravimetric analysis [21, 22].
2.6 Sorption investigations
The ideal conditions for sorption are determined by carefully examining many parameters, including pH levels ranging from 0.5 to 5, batch factor V/m values ranging from 50 to 1000 mL g−1, reaction temperatures of 298, 308, 318, 328, and 338 K, metal ion concentrations ranging from 25 to 600 mg L−1, and agitation times ranging from 2 to 180 min. The sorbent and sorbate solution were brought into contact using a batch process. After sorption occurred, the samples were removed from the solution using filtration. The equilibrium measurements were conducted by agitating 0.05 g of S-3 with 5 mL of Zr(IV) and Y(III) solutions, which had an initial concentration of 50 mg L−1, using an agitator thermostat (Kottermann D-1362, Germany) at a volume-to-mass ratio of 100 mL g−1. In this inquiry, all the recorded experimental data was obtained by averaging the results of two identical tests conducted in duplicate. The equilibrium adsorption capacity (qe), mg g−1 of Zr(IV) and Y(III) on S-3 were obtained using the relevant formula [24,25,26]:
where Ce is the equilibrium concentration of Zr(IV) and Y(III), mg L−1. V and m represent the volume of the effluent solution in liters and the amount of S-3 per gram, respectively.
2.7 Study of the rate of a chemical reaction
To elucidate the adsorption process, we will discuss the nonlinear form of the pseudo-1st-order equation (PFO) (Eq. (3)) and the pseudo-2nd-order equation (PSO) (Eq. (4)). The PFO model is a model of a system consisting of a solid and a liquid, which is based on the adsorption capacity of the adsorbent [27]. The PSO model relies on the quantity of active sites present on the adsorbent surface and the adsorption capacity of the solid phase [28].
qt and qe represent the quantity of substance adsorbed at time t and the quantity of substance adsorbed at equilibrium, mg g−1, respectively. t represents the time in min, while K1 and K2 represent the rate constants of the PFO and PSO models, respectively. The rate constant of the PFO model is measured in minutes to the power of a negative one, while the rate constant of the PSO model is measured in grams per milligram per minute.
2.8 Impact of temperature
Thermodynamic calculations can be used to ascertain the spontaneity of the adsorption process. Moreover, by utilizing thermodynamic characteristics at various reaction temperatures (298, 308, 318, 328, and 338 K), we can demonstrate the impact of temperature on the sorption of Zr(IV) and Y(III) onto S-3. The thermodynamic parameters were determined by studying the percentage of Zr(IV) and Y(III) sorption onto S-3 at pH 2.5 and 4 for Zr(IV) and Y(III). The equations employed for the determination of enthalpy (∆H˚), entropy (∆S˚), and Gibbs free energy (∆G˚) are as follows: [29,30,31];
Kd represents the distribution coefficient, mL g−1.
2.9 Modelling of isotherms
The concentration data acquired for the determination of the isotherms of Zr(IV) and Y(III) loaded onto FeSnSi were examined using a non-linear version of the Langmuir Eqs. (9&10) and the Freundlich Eq. (11). Sorption isotherms were conducted at pH 4 and 2.5 for Y(III) and Zr(IV), respectively, with initial concentrations ranging from 25 to 600 mg L−1. According to the Langmuir model, the adsorbent surface has active centers of adsorption that are evenly distributed [32]. The Freundlich model elucidates the hyperbolic adsorption behavior and offers insights into the heterogeneity of the adsorbent surface [33].
qm, RL, and KL represent the maximum sorption capacity, mg g−1, the dimensionless constant of separation factor, and the parameters for the Langmuir isotherm, respectively. KF and 1/n represent the Freundlich constant and the degree of adsorbent surface heterogeneity, respectively.
2.10 Binary system
This study focuses on a binary system consisting of Zr and Y. A solution containing 5 mL of Zr-Y combination with an initial concentration of 50 mg L−1 is used. The ratio of solution volume to mass is 100 mL g−1. The contact time is set at 90 min, and the initial pH ranges from 1 to 3. The separation factors \(({\alpha }_{Y}^{Zr})\) were determined by evaluating the equation as a function of pH [24, 34]:
3 Results and Discussion
3.1 X-ray diffraction analysis
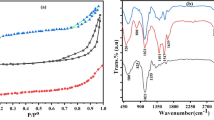
The semi-crystalline nature of FeSnSi was observed from X-ray diffraction (XRD) as shown in Fig. 1a. The broad peak centered at 2θ from 9 ̊ to 26 ̊ is a known typical characteristic of amorphous silica [35, 36]. Sharp peaks at 2θ ≈ 32°and 45.4 ̊ characteristics to Fe2O3 and SnO2 respectively [37,38,39].
3.2 FT-IR analysis
The FT-IR spectrum of the FeSnSi composite in Fig. 1b shows two bands at 3301 and 1635 cm−1. These bands correspond to the stretching and bending vibrations of the OH frequencies of water molecules within the composite structure, respectively [40]. The presence of a band at 1246 cm−1 indicates that there is a connection between Sn–O and an OH group, specifically in the form of a bending vibration. This band serves as evidence that Sn has been incorporated within a layer of FeSnSi, as demonstrated by the subsequent XRF results [41]. The band at 1049 is due to the metal–oxygen bond (Si–O-Si) [42]. The band at 788 cm−1 possibly results from Si–O bonding and is classified as a silicate group (SiO3) [43, 44]. Bands at 562 and 470 cm−1 are attributed to metal oxide bonds (Fe–O and Sn–O), respectively [45].
3.3 XRF analysis
The elemental composition of the FeSnSi composite can be determined using XRF analysis and is presented in Table 2. These results confirm that the % of metals present in the FeSnSi composite is 85.86, 3.92, and 6.15 for SiO2, Fe2O3, and SnO2, respectively. As well as some traces such as Na2O, SO3, and MgO. The data has verified that the main components found in the FeSnSi composite are SiO2, Fe2O3, and SnO2.
3.4 Morphology analysis
The SEM and EDX analysis can be used to identify the morphological properties and surface elemental composition of the FeSnSi composite, as depicted in Fig. 2. Figure 2a illustrates the surface morphology of the FeSnSi composite indicating that the irregular surface and presence of different pores with different diameters confirmed the presence of different components. Multi-elemental EDX mapping images for FeSnSi composite signified the presence of O, Si, Sn, and Fe constituents as shown in Fig. 2b. EDX confirmed the presence of all atoms, with oxygen being the most prevalent ion at a mass percent of 50.6%. Silica, tin, and iron were also detected, accounting for 40%, 5%, and 3.9% respectively. Moreover, the maps corresponding to these peaks as shown in Fig. 2c revealed that there are bright spots with green, yellow, blue, and red colors with heterogeneous distribution for oxygen, silica, tin, and iron respectively in the view of the cross-section.
3.5 pH titration
Figure 3 illustrates the pH titration of the FeSnSi composite. The X-axis represents the amount of 0.1 M NaOH in millimoles per gram of FeSnSi composite, while the Y-axis shows the pH of the liquid that comes out. The pH titration of the FeSnSi composite has a single inflection point, indicating that the composite is monofunctionally. This chemical reaction has similarities to cerium(IV) molybdate [46] and SnSiMo [47].
3.6 Chemical stability
According to the results of the solubility test conducted on various solvents (Table 3), the FeSnSi composite exhibited remarkable stability when exposed to common mineral acids and alkalis. The chemical stability of the FeSnSi composite is comparatively high among alternative sorbents, as demonstrated in Table 3 [20, 39, 48].
3.7 Metal ions hydrolysis
The primary determinant of the separated elements by the suggested adsorbent is the metal hydrolysis side reaction, which is highly responsive to the pH of the solution. Regarding this, several examinations have been conducted autonomously. Thus, at various pH values ranging from 0.5 to 6, distinct samples (10 mL each) containing 50 mg L−1 of each element (in distilled water) were individually prepared. The samples were filtered following 30 min of agitation. Subsequently, the concentrations of each element at every pH value were determined using spectrophotometry to compute the precipitation percentage, as illustrated in Table 4. Before pH 5, Y(III) precipitated, and Zr(IV) precipitated at pH 3, respectively. To the outcomes of metal hydrolysis, the subsequent experiments were conducted at pH 2.5 and 4 for Zr(IV) and Y(III), respectively, to simulate acidic conditions and prevent the hydrolysis of the metal ions.
3.8 Impact of pH
The removal of Y(III) and Zr(IV) from aqueous solutions by the FeSnSi composite was studied as represented in Fig. 4a. The % R rises with increasing pH (1–4) from 29.7% to 84.8% for Y(III) with the continuous increase at pH 5, (% R = 88.9% but this pH value Y(III) started to precipitate based on metal ion hydrolysis data mentioned in Table 4. The % R increases with increasing pH (0.5–2.5) from 57.6% to 97.3% for Zr(IV) with a slight increase of the % R with increasing of pH above this value due to the precipitation of Zr(IV) as mentioned in Table 4. All experimental procedures involving Y(III) and Zr(IV) were conducted at pH 4 and 2.5, respectively. Additionally, it was noted that the percentage R of Y(III) and Zr(IV) is diminished at low pH levels. This phenomenon can likely be attributed to the protonation of the surface-active sites and the accumulation of H3O+ ions in the aqueous solution. Thus, the competition between H3O+ and both Y(III) and Zr(IV) for the available binding surface active site was caused by the positively charged surface sites, which reduced Y(III) and Zr(IV) uptake. As the initial pH values increased, there was a concurrent decrease in the concentration of H3O+ ions and an increase in the concentration of OH− ions. This phenomenon induces deprotonation at the sorbent surface. These findings indicate that the FeSnSi composite surface generally possesses a negative charge. As a result, an increase in attraction was observed between the positive charge of metal ions in the solution and the surface of the composite.
3.9 Impact of mixing time
At pH 4 and 2.5, the impact of contact time on the percent R of Y(III) and Zr(IV) on the FeSnSi composite was investigated, respectively. As shown in Fig. 4b, the % R of Y(III) and Zr(IV) deposited onto the FeSnSi composite increases with time until it reaches equilibrium at approximately 90 min. The uptake rates of Y(III) and Zr(IV) onto the FeSnSi composite increase very rapidly from 2 to 60 min, then gradually from 60 to 90 min; beyond this time, there is no change in the uptake; therefore, 90 min was designated as the equilibrium time for subsequent experimental investigations.
3.10 Impact of batch factor (V/m)
Impact of V/m on % R of Y(III) and Zr(IV) onto the FeSnSi composite (Fig. 4c), was done at pH = 2.5 for Zr(IV) and 4 for Y(III). From this Figure, it is found that retention of Y(III) and Zr(IV) onto the FeSnSi composite highly reduced with rising the V/m ratio from (50–1000 mL g−1) and V/m ratio (100 mL g−1) was applied as a V/m to subsequent experimental procedures.
3.11 Kinetic study
To analyze the kinetics of adsorption, the experimental data were fitted with PFO and PSO model equations. Y(III) and Zr(IV) were adsorbed onto the FeSnSi composite in two distinct phases (Fig. 5). A phase of rapid adsorption transpired for a duration of 60 min during the initial stage. In contrast to the rapid and quantitatively dominant nature of the first stage, the second was markedly sluggish and quantitatively insignificant. Several active centers were readily accessible on the FeSnSi surface during the early stages of adsorption. The equilibrium state was attained once these centers were occupied; so, the second stage doesn't increase at a noticeable rate. Upon comparing the R2 values of the PFO and PSO models (Table 5), it was determined that the PSO provided a more accurate representation of the kinetics. Moreover, the experimental and theoretically determined qe values were comparable, indicating that the PSO model was consistent. The obtained results indicate that the adsorption process adhered to the rate kinetics of PSO.
3.12 Studies on thermodynamics
The effect of reaction temperature on the % R of Y(III) and Zr(IV) onto FeSnSi composite was done at initial concentration of 50 mg L−1, shaking time = 90 min, pH 4 and 2.5 for Y(III) and Zr(IV), respectively. The result is shown in Fig. 6a. This Figure shows how an increase in the % R of Y(III) and Zr(IV) with increasing reaction temperature reflects the endothermic nature of the sorption process. The thermodynamic behavior of the adsorption process was examined at temperatures of 298, 308, 318, 328, and 338 K (Fig. 6b). For Y(III) and Zr(IV) adsorption onto the FeSnSi composite, the change in ∆H˚ during the adsorption process was 16.8 and 42.8 kJ mol−1, respectively. In the endothermic adsorption process, Y(III) and Zr(IV) sorption exhibited a favorable response to temperature increases. Furthermore, ΔS˚ was a 108.3 and 209.7 J mol−1 K−1 for Y(III) and Zr(IV), respectively. This result demonstrated the random nature of the adsorption process. Because of the high affinity of the adsorbent, a positive entropy could be interpreted as an increase in the randomness of the adsorption system [49]. At 298, 308, 318, 328, and 338 K, respectively, the free energy change for Y(III) was -15.4, -16.5, -17.6, -18.7, and -19.8 kJ mol−1, while for Zr(IV), it was -19.7, -21.8, -23.9, -26.0, and -28.1 kJ mol−1. Negative values of ΔG˚ indicate that the adsorption process is spontaneous. In addition, the increase in temperature results in greater mobility of Y(III) and Zr(IV) on the surface of the FeSnSi composite. This leads to more significant electrostatic attraction between these cations and various active groups on the composite surface, which can be attributed to the higher values of ΔG˚ observed at higher temperatures.
3.13 Sorption isotherms
To find a suitable model for the design process, a variety of isotherm models were employed to examine the equilibrium data. The study utilized the Langmuir and Freundlich isotherm equations to examine the sorption of Zr(IV) and Y(III) onto the FeSnSi composite. Isotherm equations are consistently demonstrated to be applicable by the correlation coefficients (R2). The interaction mechanism of FeSnSi with Y(III) and Zr(IV) at equilibrium was determined using adsorption isotherms. The comparison of R2 values between the Freundlich isotherm and Langmuir isotherm models (Fig. 7, Table 6) indicates that the adsorption process of Y(III) and Zr(IV) conformed to the Langmuir isotherm, offering a better fit with R2 = 0.995 and 0.983 for Y(III) and Zr(IV), respectively. The favorable sorption isotherms of Y(III) and Zr(IV) were found to be reflected in the RL values of (0.092 and 0.028) [50]. The maximum sorption capacity was 34.8 and 41.3 mg g−1, for Y(III) and Zr(IV), respectively. These findings verified that the Langmuir model fits the Y(III) and Zr(IV) adsorption onto the FeSnSi composite better.
3.14 Comparison of maximum sorption capacity with different composites
The maximum sorption capacity of FeSnSi composite for the sorption of Y(III) and Zr(IV) was compared with other composites reported in the literature. As represented in Table 7, the maximum sorption capacity of FeSnSi is higher than the previously reported values which suggested that this is a promising composites to remove Y(III) and Zr(IV) from aqueous solutions [15,16,17,18,19].
3.15 Binary systems
Figure 8 illustrates the effect of competition on Zr(IV) uptake and separation onto FeSnSi composite at several pHs from the Zr-Y binary system. In the binary system, it is discovered that Zr(IV) has high Kd values at various pHs. Table 8 contains the Kd and \({\alpha }_{Y}^{Zr}\) values at various pHs for the binary system. The Zr-Y binary system data showed that Kd had the following sequence: Zr(IV) > Y(III) at all pHs. This sequence supports the idea that Y(III) and Zr(IV) adsorption was accomplished by ionic radii (I.R.) [Y(III) has an I.R. of 0.093 nm, whereas Zr(IV) has an I.R. of 0.08 nm]. Zr(IV) will more readily penetrate the sorbent cavities, increasing sorption and raising Kd [51]. Also, the data reflect that Zr(IV) is high \({\alpha }_{Y}^{Zr}\) at different pHs. These findings demonstrate that at various pHs, Zr(IV) can be readily sorbed from the Zr-Y system onto FeSnSi composite.
4 Conclusion
In this work, the batch sorption of Zr(IV) and Y(III) from an aqueous solution using a FeSnSi composite prepared by the co-precipitation method. The sorption of Zr(IV) and Y(III) demonstrates that the FeSnSi composite obeys the PSO kinetic model and has an equilibrium time of 90 min. The thermodynamic functions reflected that the sorption process is endothermic and spontaneous. The applicability of the Langmuir model with the maximum sorption capacity was 34.8 and 41.3 mg g−1 for Y(III) and Zr(IV), respectively. According to binary system data, Zr(IV) can be separated from Zr-Y onto FeSnSi composite at different pHs. The previously mentioned data demonstrates that the FeSnSi composite is a material with great potential for the sorption and extraction of Zr(IV) and Y(III) from aqueous solutions.
Data availability
No datasets were generated or analysed during the current study.
References
Iftekhar, S., Srivastava, V., Casas, A., et al.: Synthesis of novel GA-g-PAM/SiO2 nanocomposite for the recovery of rare earth elements (REE) ions from aqueous solution. J. Clean. Prod. 170, 251–259 (2018)
Hu, Y., Florek, J., Larivière, D., et al.: Recent advances in the separation of rare earth elements using mesoporous hybrid materials. Chem. Rec. 18, 1261–1276 (2018)
Ryu, S., Fonseka, C., Naidu, G., et al.: Recovery of rare earth elements (Lu, Y) by adsorption using functionalized SBA-15 and MIL-101 (Cr). Chemosphere 281, 130869 (2021)
Huang, Z., Fan, M., Tiand, H.: Coal and coal byproducts: A large and developable unconventional resource for critical materials–Rare earth elements. J. Rare Earths 36, 337–338 (2018)
Dutta, T., Kim, K.-H., Uchimiya, M., et al.: Global demand for rare earth resources and strategies for green mining. Environ. Res. 150, 182–190 (2016)
Rim, K.T., Koo, K.H., Park, J.S.: Toxicological evaluations of rare earths and their health impacts to workers: a literature review. Saf. Health Work 4, 12–26 (2013)
Shin, S.-H., Kim, H.-O., Rim, K.-T.: Worker safety in the rare earth elements recycling process from the review of toxicity and issues. Saf. Health Work 10, 409–419 (2019)
Pagano, G., Guida, M., Tommasi, F., et al.: Health effects and toxicity mechanisms of rare earth elements—Knowledge gaps and research prospects. Ecotoxicol. Environ. Saf. 115, 40–48 (2015)
Shahnaz, T., Priyan, V.V., Jayakumar, A., et al.: Magnetic nanocellulose from Cyperus rotundas grass in the absorptive removal of rare earth element cerium(III): Toxicity studies and interpretation. Chemosphere 287, 131912 (2022)
Hamed, M.M., Rizk, H.E., Ahmed, I.M.: Adsorption behavior of zirconium and molybdenum from nitric acid medium using low-cost adsorbent. J. Mol. Liq. 249, 361–370 (2018)
Atia, A.A., Donia, A.M., Awed, H.A.: Synthesis of magnetic chelating resins functionalized with tetraethylenepentamine for adsorption of molybdate anions from aqueous solutions. J. Hazard. Mater. 155, 100–108 (2008)
Mahrous, S.S., Abass, M.R., Mansy, M.S.: Bentonite phosphate modified with nickel: Preparation, characterization, and application in the removal of 137Cs and 152+154Eu. Appl. Radiat. Isot. 190, 110445 (2022)
Mansy, M.S., Eid, M.A., Breky, M.M.E., et al.: Sorption behavior of 137Cs, 152+154Eu and 131Ba from aqueous solutions using inorganic sorbent loaded on talc. J. Radioanal. Nucl. Chem. 332, 2971–2987 (2023)
Oladoja, N.A., Ololade, I.A., Alimi, O.A., et al.: Iron incorporated rice husk silica as a sorbent for hexavalent chromium attenuation in aqueous system. Chem. Eng. Res. Des. 91, 2691–2702 (2013)
Cheira, M.F., Kouraim, M.N., Zidan, I.H., et al.: Adsorption of U(VI) from sulfate solution using montmorillonite/polyamide and nano-titanium oxide/polyamide nanocomposites. J. Environ. Chem. Eng. 8, 104427 (2020)
Bai, R., Yang, F., Meng, L., et al.: Polyethylenimine functionalized and scaffolded graphene aerogel and the application in the highly selective separation of thorium from rare earth. Mater. Des. 197, 109195 (2021)
Faghihian, H., Kabiri-Tadi, M.: Removal of zirconium from aqueous solution by modified clinoptilolite. J. Hazard. Mater. 178, 66–73 (2010)
Mekawy, Z.A., El Shazly, E.A.A., Mahmoud, M.R.: Utilization of bentonite as a low-cost adsorbent for removal of 95Zr(IV), 181Hf(IV) and 95Nb(V) radionuclides from aqueous solutions. J. Radioanal. Nucl. Chem. 331, 3935–3948 (2022)
Zolfonoun, E., Monji, A.B., Taghizadeh, M., et al.: Selective and direct sorption of zirconium from acidic leach liquor of zircon concentrate by rice bran. Miner. Eng. 23, 755–756 (2010)
Ibrahim, A.B., Abass, M.R., EL-Masry, E.H., et al.: Gamma radiation-induced polymerization of polyacrylic acid-dolomite composite and applications for removal of cesium, cobalt, and zirconium from aqueous solutions. Appl. Radiat. Isot. 178, 109956 (2021)
Abass, M.R., Kandeel, E.M., Abou-Lilah, R.A., et al.: Effective biosorption of cesium and strontium ions from aqueous solutions using silica loaded with aspergillus brasiliensis. Water Air Soil Pollut. 235, 61 (2024)
Abass, M.R., Abou-Lilah, R.A., Abou-Mesalam, M.M.: Selective separation of cobalt ions from some fission products using synthesized inorganic sorbent. J. Inorg. Organomet. Polym. Mater. 1–14 (2024)
Gupta, V.K., Sharma, G., Pathania, D., et al.: Nanocomposite pectin Zr(IV) selenotungstophosphate for adsorptional/photocatalytic remediation of methylene blue and malachite green dyes from aqueous system. J. Ind. Eng. Chem. 21, 957–964 (2015)
Metwally, S.S., Hassan, H.S., Samy, N.M.: Impact of environmental conditions on the sorption behavior of 60Co and 152+154Eu radionuclides onto polyaniline/zirconium aluminate composite. J. Mol. Liq. 287, 110941 (2019)
Abass, M.R., Ibrahim, A.B., El-Masry, E.H., et al.: Optical properties enhancement for polyacrylonitrile-ball clay nanocomposite by heavy metals saturation technique. J. Radioanal. Nucl. Chem. 329, 849–855 (2021)
Hamed, M.M., Shahr El-Din, A.M., Abdel-Galil, E.A.: Nanocomposite of polyaniline functionalized Tafla: synthesis, characterization, and application as a novel sorbent for selective removal of Fe(III). J. Radioanal. Nucl. Chem. 322, 663–676 (2019)
Lagergren, S.: Zur theorie der sogenannten adsorption geloster stoffe. K Sven Vetenskapsakademiens Handl. 24, 1–39 (1898)
Ho, Y.S., McKay, G.: The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 34, 735–742 (2000)
Abdel-Galil, E.A., Ibrahim, A.B., Abou-Mesalam, M.M.: Sorption behavior of some lanthanides on polyacrylamide stannic molybdophosphate as organic-inorganic composite. Int J Ind Chem. 7, 231–240 (2016)
Şenol, Z.M., Şimşek, S.: Insights into effective adsorption of lead ions from aqueous solutions by using chitosan-bentonite composite beads. J. Polym. Environ. 30, 3677–3687 (2022)
Dakroury, G.A., El-Shazly, E.A.A., Hassan, H.S.: Preparation and characterization of ZnO/Chitosan nanocomposite for Cs(I) and Sr(II) sorption from aqueous solutions. J. Radioanal. Nucl. Chem. 330, 159–174 (2021)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
Freundlich, H.: Über die adsorption in lösungen. Zeitschrift Phys. Chem. 57, 385–470 (1907)
Ahmed, I.M., Aglan, R.F., Hamed, M.M.: Removal of Arsenazo-III and Thorin from radioactive waste solutions by adsorption onto low-cost adsorbent. J. Radioanal. Nucl. Chem. 314, 2253–2262 (2017)
Jafari, V., Allahverdi, A.: Synthesis of nanosilica from silica fume using an acid-base precipitation technique and PVA as a nonionic surfactant. J Ultrafine Grained Nanostructured Mater. 47, 105–112 (2014)
Zulfiqar, U., Subhani, T., Wilayat, H.S.: Synthesis of silica nanoparticles from sodium silicate under alkaline conditions. J. Sol-Gel Sci. Technol. 77, 753–758 (2016)
Taufik, A., Saleh, R.: Synthesis of iron (II, III) oxide/zinc oxide/copper (II) oxide (Fe3O4/ZnO/CuO) nanocomposites and their photosonocatalytic property for organic dye removal. J. Colloid Interface Sci. 491, 27–36 (2017)
Ahmad, M., Islam, I.U., Ahmad, M., et al.: Preparation of iron-modified biochar from rice straw and its application for the removal of lead (Pb+2) from lead-contaminated water by adsorption. Chem. Pap. 76, 3789–3808 (2022)
Abdel-Galil, E.A., Eid, M.A., Hassan, R.S.: Preparation of nanosized stannic silicomolybdate for chromatographic separation of Y(III) from Zr(IV). Part. Sci. Technol. 38, 113–120 (2020)
Abass, M.R., Maree, R.M., Sami, N.M.: Adsorptive features of cesium and strontium ions on zirconium tin(IV) phosphate nanocomposite from aqueous solutions. Int. J. Environ. Anal. Chem. 104, 103–122 (2024)
Yakout, S.M., Hassan, H.S.: Adsorption characteristics of sol gel-derived zirconia for cesium ions from aqueous solutions. Molecules 19, 9160–9172 (2014)
Abou-Mesalam, M.M., Abass, M.R., Abdel-Wahab, M.A., et al.: Complex doping of d-block elements cobalt, nickel, and cadmium in magneso-silicate composite and its use in the treatment of aqueous waste. Desalin. Water Treat. 57, 25757–25764 (2016)
Abass, M.R., Ibrahim, A.B., Abou-Mesalam, M.M.: Retention and selectivity behavior of some lanthanides using bentonite dolomite as a natural material. Chem. Pap. 75, 3751–3759 (2021)
Abou-Mesalam, M.M., Abass, M.R., Zakaria, E.S., et al.: Metal doping silicates as inorganic ion exchange materials for environmental remediation. Silicon 14, 7961–7969 (2022)
Taha, K.K., Suleiman, T.M., Musa, M.A.: Performance of Sudanese activated bentonite in bleaching cottonseed oil. J. Bangladesh Chem. Soc. 24, 191–201 (2011)
Nilchi, A., Maalek, B., Khanchi, A., et al.: Cerium(IV) molybdate cation exchanger: synthesis, properties and ion separation capabilities. Radiat. Phys. Chem. 75, 301–308 (2006)
Nabi, S.A., Khan, A.M.: Synthesis, ion exchange properties and analytical applications of stannic silicomolybdate: Effect of temperature on distribution coefficients of metal ions. React. Funct. Polym. 66, 495–508 (2006)
Abdel-Galil, E.A., Ibrahim, A.B., El-Kenany, W.M.: Facile fabrication of a novel silico vanadate ion exchanger: evaluation of its sorption behavior towards europium and terbium ions. Desalin. Water Treat. 226, 303–318 (2021)
Şenol, Z.M., Kaya, S., Şimşek, S., et al.: Synthesis and characterization of chitosan-vermiculite-lignin ternary composite as an adsorbent for effective removal of uranyl ions from aqueous solution: Experimental and theoretical analyses. Int. J. Biol. Macromol. 209, 1234–1247 (2022)
Hamed, M.M., Holiel, M., Ismail, Z.H.: Removal of 134Cs and 152+154Eu from liquid radioactive waste using Dowex HCR-S/S. Radiochim. Acta 104, 399–413 (2016)
Abass, M.R., Ibrahim, A.B., Abou-Mesalam, M.M.: Comparative studies for natural and synthetic composites based on molybdate for gadolinium ions separation. Int. J. Environ. Anal. Chem. 1–20 (2022)
Acknowledgements
Great thanks to all members of the Nuclear Fuel Technology Department, and Environmental Radioactive Pollution Department, Egyptian Atomic Energy Authority for supporting this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahrous, S.S., Abou-Lilah, R.A. & Abass, M.R. Yttrium and zirconium sorption on iron tin functionalized with silica: preparation, characterization, isotherm, and kinetic modelling. Adsorption (2024). https://doi.org/10.1007/s10450-024-00447-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00447-w