Abstract
In this study, the batch technique was used for the sorption of Sr(II) and Cs(I) onto silica loaded with Aspergillus brasiliensis (AB@S). The precipitation technique was used to synthesize the AB@S bio-sorbent, which was then evaluated using several analytical instruments such as XRD, FT-IR, and SEM. The results of this investigation revealed that the sorption process had a short equilibrium time (40 min). The distribution coefficient values have a sequence order Sr(II) ˃ Cs(I). AB@S sorbent has saturation capacity for Sr(II) and Cs(I) 72.2 and 26.1 mg g−1, respectively. The reaction kinetics follow the pseudo-second-order model with capacity values of 5.01 and 3.53 mg g−1 for Sr(II) and Cs(I), respectively. Applicability of Langmuir isotherms has capacity values of 66.3 and 23.38 mg g−1 for Sr(II) and Cs(I), respectively. Thermodynamics data are endothermic and spontaneous. The AB@S is a promising bio-sorbent for the removal of 85Sr and 134Cs from simulated radioactive waste (SRW). The investigation proved that the AB@S is suitable to adsorb Sr(II) and Cs(I) from aqueous solutions and could be considered potential material for the purification of effluent contaminated with these ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The risks caused by radionuclides in water have attracted considerable attention since when radioactive fallout from nuclear facilities and the nuclear industry enters the environment, it can seriously harm human health (Yu et al., 2020). Additionally, the global worry over heavy metal poisoning of water sources is growing. Natural sources (volcanic emissions, deep-sea vents, forest fires, and geysers) and anthropogenic sources (mining and smelting sites, painting and coating businesses, metal-manufacturing factories, and tanneries) are the two main ways that heavy metals enter the environment (Ayele et al., 2021).
One of the biggest issues facing the nuclear industry today is the treatment of wastewater that contains radionuclides. Owing to the production of a sizable amount of radioactive wastewater and its discharge into the nearby soil, subterranean water, and saltwater as a result of the serious catastrophe at Fukushima, Japan, in 2011, this matter was made apparent (Aglan et al., 2019; Yang et al., 2016). Radioactive 137Cs and 90Sr are of particular concern among the potential radioactive contamination in nuclear waste. A powerful beta-gamma emitter, 137Cs has a half-life of 30.17 years, while 90Sr has a half-life of 28.8 years, making it a huge source of radiation and a beta emitter. 137Cs, in particular, is a dominant source of activity in radioactive waste due to their solubility (Liu et al., 2023). Therefore, before their long-term storage, it is necessary to find an effective and affordable method for removing 137Cs and 90Sr from waste streams (Aguila et al., 2016; Emara et al., 2019; Gu et al., 2022; Hao et al., 2023). Radioactive strontium leakage during production and consumption poses a serious risk to both human and environmental health since it may cause bone sarcoma and leukemia (Long et al., 2017).
Additionally, due to their high solubility, Cs+ and Sr2+ are easily discharged into aquatic systems, providing a long-term threat to the biosphere from radiation exposure. Adsorption is regarded to be the most efficient way to remove radioactive elements from radioactive wastewater (Emara et al., 2019; Lee et al., 2016). To remove radionuclides from contaminated wastewater, earlier studies used solvent extraction, ion exchange, and chemical precipitation. However, these techniques are ineffective at low concentrations and frequently too expensive for extensive remediation applications for the removal of Cs(I) and Sr(II) (Long et al., 2017; Yu et al., 2020).
In the past, researchers have used chemical precipitation, ion exchange, and solvent extraction to remove radionuclides from contaminated wastewater. However, for large-scale remedial applications, these techniques are frequently too expensive and ineffective at low concentrations (Abu Taleb et al., 2022; Khani et al., 2012; Lee et al., 2016), while enzymatic processes and inorganic microprecipitation are further divisions of passive adsorption (Yu et al., 2020).
Aspergillus terreus exhibited the highest strontium uptake capacity at 15 °C at an initial strontium ion concentration of 876 mg L−1 (Khani et al., 2012). Several adsorbents for the sequestration of metal ions from wastewater have been described, including alumina (Al2O3), activated carbon, graphene oxide, titanium dioxide (TiO2), kaolin clay, silica, etc. Biomolecules as the biological sources of adsorbents have been given the greatest importance for adsorbent hybridization with other commercial materials for the removal of HMs from wastewater due to their exceptional qualities, which include being inexpensive, chemically stable, non-toxic, and environmentally acceptable (Abu Taleb et al., 2022). With their renewable surface properties, biocompatibility, non-toxicity, non-polluting qualities, mechanical integrity, and economically affordable materials, hybrid bio-adsorbents provide interesting benefits for metal adsorption (Abu Taleb et al., 2022). Fe3O4 nanoparticles, Lindnera jadinii yeast, and raffia fibers undergo hydrothermal carbonization to produce hydrochar biocomposite (Staroń et al., 2023), Also, microorganism-graphene oxide was used as biocomposites for the removal of uranium (VI) from aqueous solutions via immobilized Lysinibacillus sp. on graphene oxide (GO) (Zhao et al., 2019).
To the best of our knowledge, no studies have been published in the open literature on the removal of Sr(II) and Cs(I) by silica loaded with Aspergillus brasiliensis (AB@S) bio-sorbent which was prepared by precipitation technique. The novelty of this work was the hybridization between the fungal biomass and nano-silica to form a composite with improved chemical characteristics and improve the sorption behavior toward heavy metals and radioactive waste.
This work aims to study the sorption of Sr(II) and Cs(I) from aqueous solutions using AB@S bio-sorbent prepared by precipitation technique. Different parameters like the influence of pH, ionic strength, shaking time, metal ion concentrations, and temperatures were studied to determine the optimum sorption conditions for the uptake of Sr(II) and Cs(I) from liquid solutions.
2 Experimental
2.1 Materials and Instruments
SrCl2, CsCl, and NaOH were from Alpha Chemika, India. HCl and HNO3 were provided from Merck, Germany. The chemicals used in this study were all analytical grades and did not undergo any additional purification. For all of the studies, double-distilled water (DDW) was used. AB@S bio-sorbent was analyzed with X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FT-IR) using Brucker XRD diffractometer D2 Phaser II, Germany, and Alpha II Bruker, Germany, respectively. A scanning electron microscopy (SEM) model Philips XL 30 was utilized to determine the surface morphology of AB@S bio-sorbent.
2.2 Fungal Media Composition
Fungi were grown using potato dextrose agar (PDA) media. It includes dextrose as a carbohydrate source that promotes growth and the Infusion of potatoes that gives most fungi a nutritional base for growth (Andriamafana et al., 2018).
2.3 Isolation of Fungal Isolates
The microorganism was isolated from liquid radioactive effluent that was gathered from the Waste Management Facility at the Egyptian Atomic Energy Authority’s Hot Laboratories Center. For the sterile isolating of fungi, 0.5 mL of wastewater was mixed with potato dextrose agar (PDA) media on two Petri dishes. For growing fungus, the plates were incubated at 28 °C for 72 h. The newly obtained colonies were purified for identification (Shaaban et al., 2020). The pure colonies were separated on potato dextrose agar slants and stored in a refrigerator for further use.
2.4 Production of Fungal Biomass
A loopful of the fungal spores from the cultivated plates was taken out and transferred to 500-mL conical flasks containing 150 mL potato dextrose broth for the growth of fungal isolate. These conical flasks were incubated at 28 °C for 3 days in the orbital environment shaker. The obtained cultures were then transferred to 1-L conical flasks containing one-third of their capacity for potato dextrose broth. The cultures were then incubated at 28 °C for 2 days in an orbital shaking incubator, and the mycelia were harvested by filtration on filter paper. Bi-distilled water was used to thoroughly wash the obtained mycelia. The produced fungal biomass was kept in the fridge until use (Abdel-Razek et al., 2009).
2.5 Molecular Identification of the Fungal Isolate
On Czapek’s yeast extract agar (CYA) medium, the fungal isolate was grown and incubated at 28 °C for 5 days (Pitt & Hocking, 2022). At Assiut University’s Molecular Biology Research Unit, DNA was extracted using the Patho-gene-spin DNA/RNA extraction kit (made by Korea’s Intron Biotechnology Company). SolGent Company, Daejeon, South Korea, assisted with the polymerase chain reaction (PCR) and sequencing. Using the universal primers ITS1 (forward) and ITS4 (reverse), which were inserted into the reaction mixture, the ITS sections of the rRNA genes for samples 3 and F8 were amplified. Primers have the following composition: ITS1 (5′-TCCGTAGGTGAA CCTGCGG-3′), and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The purified PCR product was sequenced with the same primers with the incorporation of dideoxynucleotide triphosphates (ddNTPs) in the reaction mixture (White et al., 1990). The Basic Local Alignment Search Tool (BLAST) available on the National Center for Biotechnology Information (NCBI) website was used to examine the obtained sequences. Analysis of sequences and establishment of phylogenetic trees were done using MegAlign (DNA Star) software version 5.0.
2.6 Preparation of AB@S Bio-sorbent
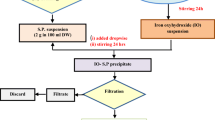
The precipitation method was utilized for the production of the AB@S bio-sorbent. In this method, 10% fungal bio-sorbent (W/V) was dropwise to 10% Na2SiO3 solution (V/V) by various volumetric ratios as represented in Table 1. Concentrated HCl was dropwise to the mixtures with constant stirring at (25 ± 1 °C) until pH was approximately (8–9), the gel was obtained and aged for 1 day, wash the mixture with DDW to remove impurities and reached pH 7, then decantation of filtrate from precipitate, and dried the precipitate at 60 °C. The dried sample (AB@S bio-sorbent) was transformed into the H+ form by immersing in 0.1 M HNO3 for 1 day and drying at 60 °C.
2.7 Sorbent Choice Studies
The optimal sorbent for analytical purposes was determined by evaluating the sorption efficiency (% S.E.) of Cs(I) and Sr(II) onto variously produced samples of AB@S bio-sorbents. This was done by stirring 0.05 g samples with 5 mL of Cs(I) and Sr(II) (50 mg L−1 and V/m = 100 mL g−1) at 25 ± 1 °C for 24 h. Both initial and final concentrations Co and Cf of Cs(I) and Sr(II) used were detected by atomic absorption spectrophotometer. % S.E. can be calculated by using Eq. (1) (Hamed et al., 2016b),
Table 1 exhibits that the % S.E. of Cs(I) and Sr(II) onto prepared composites has order Sr(II) ˃ Cs(I), and S-4 Na2SiO3: fungal (1:1) is the best sorbent for this work depending on their high % S.E. and used for further experiments.
2.8 Point of Zero Charges (pHPzc)
The surface of the sorbent used plays an important role in the sorption processes, so the pHPZC is a very important character of the sorbent. In this article, the pHPZC of AB@S bio-sorbent was determined. Various samples (5 mL) of 0.1 M NaCl were adopted in the pH range (1–12) using 0.1 M HCl and/or NaOH, and then 0.1 g of AB@S bio-sorbent was added to each sample. These different samples were mixed for 24 h at 2 ± 1 °C. The various samples were decanted and the pH of the filtrates was detected (pH final). The change between initial pHi and final pHf (ΔpH = pHf − pHi) was plotted against the pHi. The point of intersection at which ΔpH = zero is called the (pHPzc) (Abass et al. 2022c).
2.9 Chemical Stability
Different solvents like acids, alkali, and organic solvents were used to study the stability of AB@S bio-sorbent to different solvents by shaking 0.3 g of prepared sorbent and 30 mL of tested solvents for 48 h at 25 ± 1 °C. The amount of AB@S bio-sorbent transferred into the solution mixture was detected gravimetrically (Abass et al. 2022c, d; Ibrahim et al., 2021; Kasem et al., 2021).
2.10 Sorption Studies
Many parameters like pH (1–7), shaking time (5–90 min) at different temperatures (25, 45, and 65 °C), and metal ion concentrations (25–800 mg L−1) are checked carefully to get the optimal condition for sorption. Sr(II) and Cs(I) solution and the AB@S bio-sorbent were mixed in a batchwise method. The samples were eliminated from the solution by decantation. All work measurements were carried out by mixing 0.05 g of AB@S bio-sorbent with 5 mL of Sr(II) and Cs(I) (mixed system) of 50 mg L−1 with V/m = 100 mL g−1 in a shaker thermostat (Kotterman D-1362, Germany). The adsorption capacities of Sr(II) and Cs(I) at any time t (qt, mg g−1) and equilibrium (qe, mg g−1) reserved on the AB@S bio-sorbent were determined utilizing the next equations, respectively (Hamed et al., 2019; Metwally et al., 2019):
Co, Ce, and Ct are the initial, equilibrium concentration, and concentration at time t of Sr(II) and Cs(I) (mg L−1), respectively; V is the volume of the solution (L), and m is the mass of AB@S bio-sorbent (g).
2.11 Saturation Capacity
Repeated batch technique of Sr(II) and Cs(I) with AB@S bio-sorbent in a batch factor = 100 mL g−1, at optimum sorption conditions include pH (7), shaking time (40 min), temperatures (25 °C), and metal ion concentrations (100 mg L−1) were performed until no further uptake of Sr(II) and Cs(I) for the detection of saturation capacity, qe (mg g−1), that calculated from Eq. (3). The saturated capacity of AB@S bio-sorbent equals the summation of successive qe for Sr(II) and Cs(I). It could be determined by applying Eq. (4) (Dakroury et al., 2021).
where n is the number of qe.
2.12 Distribution Coefficients (Kd) and Selectivity
Distribution coefficients (Kd, mL g−1) and separation factors \(({\mathrm{\alpha }}_{{\text{Cs}}}^{{\text{Sr}}})\) as a function of pH were calculated with the subsequent equations (Ahmed et al., 2017; Metwally et al., 2019):
in which V is the solution volume (mL).
2.13 Simulated Radioactive Waste (SRW) Studies
At Inshas, Egypt, the second Egyptian training research reactor (ETRR-2) was used to obtain 134Cs and 85Sr radioactive tracers. Wrapping definite grams of CsCl and SrCl2 in a thin thickness high-purity aluminum foil then it was held in a thick aluminum irradiation capsule after that the capsule was subjected to a pile of neutrons in the reactor. A definite quantity of the irradiated CsCl and SrCl2 was dissolved in DDW. Both initial (Ai) and final activities (Af) of 134Cs and 85Sr existent in this SRW were detected using a germanium scintillation detector attached to the multi-channel analyzer (Genie 2000, USA). The % removal (%R) can be computed by Eq. (7) (Aglan et al., 2019; Karimi-Maleh et al., 2021),
3 Results and Discussion
3.1 Molecular Identification of the Fungal Isolate
Phylogenetic tree based on ITS sequences of rDNA of the fungal sample isolated in the present study (Aspergillus brasiliensis AUMC16067, arrowed) aligned with closely related strains accessed from the GenBank as represented in Fig. 1 This strain exhibited 99.29–99.30% identity and 98–100% coverage with many strains belonging to the same species, including the parent material A. brasiliensis ATCC MYA4553 with GenBank accession number NR_111414. Fusarium solani represents an outgroup strain. A. = Aspergillus.
3.2 XRD Analysis
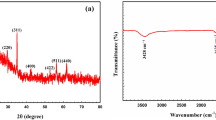
X-ray diffraction (XRD) was used to suggest the crystalline structure analysis of the studied bio-sorbent. Figure 2(a) depicts the XRD patterns of dry AB@S bio-sorbent. This diagram shows that AB@S has a crystalline structure with multiple sharp peaks centered at 27.85°, 31.86°, 45.64°, 56.72°, 66.37°, 75.38°, and 82.97° related to Miller index indications (115, 008, 420, 130, 913, 53–8, and 245), respectively, with JCPDS number 00-210-8040 (Andrews et al., 1988). These data were matched with XRD of piperazine silicate with structure formula (C4H12N2)2+(Si6O13)2− revealing their crystalline nature with the monoclinic system. These data confirm the successful impregnation of fungi inside silicate layers.
3.3 FT-IR Analysis
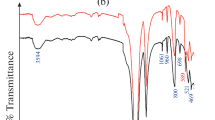
The function groups presented in AB@S bio-sorbent before and after sorption of Sr(II) and Cs(I) were examined using the FT-IR spectrum. Figure 2(b) shows that two bands appeared at 3409 and 3116 cm−1 for a stretching vibration for N–H and O–H, respectively (Al-Yassir & Le Van Mao, 2007; Lee et al., 2012). Two bands appeared at 2926 and 2816 cm−1 and 1450 and 1332 cm−1 due to stretching and bending vibrations for asymmetric and symmetric CH2, respectively (Saufi & Ismail, 2002; Wang et al., 2007). A band at 1830 cm−1 due to C = O stretching (anhydride), and bands at 1727 and 1588 cm−1 could denote medium C = C stretching and a strong N–O stretching, respectively (El-Bondkly & El-Gendy, 2022). Furthermore, the band was detected at 1332 indicating medium O–H bending (phenol). A band at 1223 cm−1 to asymmetric C–N the bending vibration (Saufi & Ismail, 2002). The two bands at 1033 and 660 are due to Si–O–Si (Borai et al., 2015; Li et al., 2012). A band at 967 is due to Si–OH deformation vibration (Abou-Mesalam et al., 2016; Nabi et al., 2011). The band at 865 is due to Si–CH2 (Mirzayev et al., 2021). Band 491–711 cm−1 is due to Si–H (Mirzayev et al., 2021). A fourth later absorption band confirmed the impregnation of Si in a structure of fungi biomass. FT-IR analysis shows that the spectrum either of AB@S bio-sorbent before or after sorption of Sr(II) and Cs(I) is approximately the same with a very slight shift except for the peak observed at 531 cm−1, which confirms the sorption of Sr(II) and Cs(I) onto the surface of AB@S bio-sorbent. Moreover, the intensity of the band at 3116 and 1332 cm−1 was increased which confirms the successful loading of Sr(II) and Cs(I) onto the surface of the AB@S bio-sorbent.
3.4 SEM
SEM picture of AB@S bio-sorbent presented in Fig. 3(a) shows a spherical shape with various diameters. These uneven circles are joined together to make an irregular elongated shape. The presence of impure phases is indicated by the uneven morphology.
3.5 Point of Zero Charges (PZC)
Based on our understanding of PZC, we can hypothesize the ionization of functional groups and their contact with Cs(I) and Sr(II). Plots of pH vs. pHi (Fig. 3(b)) to determine PZC for AB@S bio-sorbent show that pH rises with increasing pHi, then decreases, and PZC was observed at pH = 4.2, which is consistent with the sorption data shown later.
3.6 Chemical Stability
The percent of solubility for AB@S bio-sorbent toward different solvents as shown in Table 2 reflected that AB@S bio-sorbent was very steady in mineral acids, alkaline, and organic solvents. Also, Table 2 shows that AB@S bio-sorbent has high stability to different solvents compared to other sorbents (Abdel-Galil et al., 2020, 2021; Ibrahim et al., 2021).
3.7 Sorption Study
The sorption of Sr(II) and Cs(I) (mixed system) from aqueous solutions by AB@S bio-sorbent was done utilizing batch methods. To maximize their sorption on AB@S bio-sorbent, the main parameters impacting their sorption were examined independently. The findings are discussed in the sections that follow.
3.7.1 Influence of pH
The effect of the solution pH value on the AB@S bio-sorbent sorption efficiency of Sr(II) and Cs(I) (mixed system) is shown in Fig. 4(a). The sorption efficiency rises with increasing pH [from 39.01 to 96.62 for Sr(II) and from 1.3 to 69.5 for Cs(I)] at pH 7. It was also shown that at low pH values, the sorption efficiency of Sr(II) and Cs(I) is low, which is most likely owing to the protonation of the surface active sites and an increase in H3O+ ions in the aqueous solution. As a result of the positively charged surface sites, H3O+ and studied cations competed for the accessible binding surface active site, resulting in decreased Sr(II) and Cs(I) uptake. While the concentration of H3O+ ions decreased, the concentration of OH− ions increased, causing sorbent surface deprotonation; such results indicate that the surface of the AB@S bio-sorbent has a negative charge. As a result, the attraction between the sorbent’s surface and the positive charge of metal ions in the solution was strengthened. These results are similar to the sorption behavior of Sr(II) and Cs(I) onto different sorbent materials (Abass et al., 2022a; Emara et al., 2019; Fang et al., 2017).
3.7.2 Distribution Coefficients (Kd) and Separation Factors
The variation in Kd values for Sr(II) and Cs(I) onto AB@S bio-sorbent as a function of pH is shown in Fig. 4(b). The Kd values of studied cations increase with increasing pH values. At pH less than 3, the increase in Kd values for Sr(II) and Cs(I) is small, which is due to the presence of extra protons in the solution competing with the investigated metal ions and preferring occupying the binding sites available in AB@S (Metwally et al., 2019; Moloukhia, 2010). The Kd values increase with increasing pH above 3 due to reduced proton competition, with maximum absorption attained at pH 7 with a minor change above this value, and all experimental work was performed at this pH. Kd and \({\mathrm{\alpha }}_{{\text{Cs}}}^{{\text{Sr}}}\) in different pHs (1–7) were calculated and are shown in Table 3, indicating that Kd has the following affinity sequence at different pHs, Sr(II) > > Cs(I), indicating that sorption of Sr(II) and Cs(I) was achieved in the case of ionic radii [Sr(II) has ionic radii 0.126 nm and Cs(I) has ionic radii 0.165 nm]. Sr(II) with lower ir easily enter the sorbent cavities resulting in higher uptake (Abou-Mesalam et al., 2020; Moloukhia, 2010). Separation factors indicated that Sr(II) has a very high \({\mathrm{\alpha }}_{{\text{Cs}}}^{{\text{Sr}}}\) at all pHs reflecting applicability to separate Sr(II) from Cs(I). Also, the data in Table 3 prove that AB@S has Kd values for Sr(II) and Cs(I) higher than ZrSnP sorbent (Abass et al. 2022d) and lower than BNC (Emara et al., 2019). For the cations investigated, nonlinear relationships between log Kd and pH were detected, as illustrated in Fig. 4(c). This relationship indicates the non-ideality of the exchange reaction, which means the ion exchange is dominant but not the only mechanism. Another mechanism such as surface adsorption was shared with the ion exchange to control the sorption process (Abou-Mesalam et al., 2018).
3.7.3 Effect of Ionic Strength
Figure 4(d) shows the plots between the sorption efficiency of Sr(II) and Cs(I) onto AB@S bio-sorbent and the ionic strength of NaCl (0.01–0.5 M). The experiment was performed at [Co = 50 mg.g−1, V/m = 100 mL.g−1, shaking time 40 min, and pH = 7]. Figure 5c shows a noticeable reduction in the sorption efficiency of Sr(II) and Cs(I) with increasing molar concentration of NaCl from 0.01 to 0.5 M. The sorption efficiency decreased from 99.68 to 76.35% for Sr(II) and decreased from 70.23 to 33.56% for Cs(I) with increasing NaCl concentration up to 0.5 M. This reduction could be referred to as the presence of Na(I) retarded the movement of Sr(II) and Cs(I) from bulk solution toward the surface of the AB@S bio-sorbent. Ion exchange or outer-sphere surface complexation is primarily responsible for the strong ionic strength-dependent sorption (Ghaly et al., 2023; Sheha et al., 2020).
3.7.4 Influence of Shaking Time
The effect of contact time on sorption efficiency of Sr(II) and Cs(I) onto AB@S bio-sorbent was studied at different temperatures (25, 45, and 65 °C), Co = 50 mg L−1, V/m = 100 mL g−1, agitating time (5–90 min), and pH = 7. The obtained results are represented in Fig. 5 and show that the sorption efficiency of Sr(II) and Cs(I) onto AB@S bio-sorbent enhances with time attained to equilibrium at = 40 min. The rate of Sr(II) and Cs(I) uptake onto AB@S bio-sorbent rapidly rises with time from 5 to 20 min and slowly rises from 25 to 40 min, and 40 min was used as equilibrium time for further experimental. Also, the results represented in Fig. 5 prove the sorption efficiency of Sr(II) and Cs(I) onto AB@S bio-sorbent increases with increasing temperature reflecting the endothermic nature of the process.
3.8 Saturation Capacity
The saturation capacity of Sr(II) and Cs(I) onto AB@S bio-sorbent was determined at a fixed temperature and the data are tabulated in Table 4. These results show that AB@S bio-sorbent had a high saturation capacity when compared to other composites (Abass et al., 2021; Abass et al., 2022b, d; Bakhotmah et al., 2023; Metwally et al., 2017; Șenilă et al., 2023), and also demonstrate the AB@S bio-sorbent has high selectivity for Sr(II). Table 4 compares the capacity of Sr(II) and Cs(I) onto AB@S bio-sorbent with other composites produced in the literature. It can be found that AB@S bio-sorbent has a better saturation capacity than different composites. It shows that AB@S bio-sorbent can be considered a talented bio-sorbent for the capture of Sr(II) and Cs(I) from liquid waste solutions.
3.9 Kinetic Investigation
In this paper, pseudo-first-order and pseudo-second-order methods are used to analyze and interpret data from the sorption of Sr(II) and Cs(I) onto AB@S bio-sorbent at different reaction temperatures. The pseudo-first-order and pseudo-second-order can be computed from linear equations (El-Naggar et al., 2016)
in which, Kf (min−1) and Ks (g mg−1 min−1) are the rate constants of two kinetic models. Plotting log (qe-qt) and time for the former linear equation and t/qt against t for the later linear equation as shown in Fig. 6. Pseudo-first-order plot exhibits straight lines with low linearity whereas the pseudo-second-order plot shows straight lines have very good linearity. The constants computed from these plots and R2 are tabulated in Table 5. The obtained data reflect that pseudo-second-order is the main mechanism, as R2 for Sr(II) and Cs(I) is high and closer to unity and is higher compared to the results obtained from the pseudo-first-order kinetic model. This means that Sr(II) and Cs(I) uptake is governed by a chemisorption process (Abdel-Galil et al., 2016; Borai et al., 2019). Also, the data presented in Table 5 show that the calculated amount sorbed (qe, mg/g) for Sr(II) and Cs(I) onto AB@S bio-sorbent improved by rising reaction temperature, proving the endothermic nature of the process with a slight change for Sr(II) and noticeable change of the Cs(II).
3.10 Mass Transport Kinetics
3.10.1 Intra-particle Diffusion Model
The adsorption mechanism of Sr(II) and Cs(I) onto AB@S bio-sorbent was examined by the intra-particle diffusion model (Dakroury et al., 2021; Karaca et al., 2013).
in which C (intercept) and Kid (slope) are the intra-particle diffusion rate constant (mg min−0.5 g−1). This model was established through three steps (Dakroury et al., 2021; Karaca et al., 2013). The first stage includes the diffusion of Sr(II) and Cs(I) from the solution to the surface of the AB@S bio-sorbent (5–20 min). The second stage (25–35 min) describes the gradual sorption on the surface of the AB@S bio-sorbent, which may be the rate-limiting step. The third stage (40–90 min) is the equilibrium stage. The relation between qt and t0.5 for the adsorption of Sr(II) and Cs(I) onto AB@S bio-sorbent at different reaction temperatures is shown in Fig. 7, and the three steps of the sorption mechanism are noticed in this figure. The thickness of the surface is the intercept which gave information about the contribution of the surface sorption in the rate-determining step. The higher the intercept, the greater its contribution. The parameters computed from these linear plots are shown in Table 6. The sorption mechanism of Sr(II) and Cs(I) onto AB@S bio-sorbent at different reaction temperatures was rapid at the initial mixing time and then became fixed with the increasing mixing time.
3.10.2 Film Diffusion Model
The adsorption rate during the initial adsorption period only can be controlled by film diffusion model controls. When the solute molecules are transmitted from a liquid phase up to a solid phase, the boundary layer plays the most significant role in sorption. The film diffusion model may be calculated using Eq. (11) as defined by El-Deen et al. (2017):
F and Kfd are the fractional attainments of equilibrium (F = qt/qe) and the film diffusion rate constant. A linear plot of log (1 – F) and t with zero intercepts should suggest that the kinetics of the sorption process are controlled by diffusion through the liquid film surrounding the solid AB@S bio-sorbent. The relation between log (1 – F) and t for the adsorption of Sr(II) and Cs(I) onto AB@S bio-sorbent at different reaction temperatures is shown in Fig. 7. The Kfd for the film diffusion is computed from the slope. This constant and the value of the correlation coefficient (R2) are shown in Table 6. From Fig. 7 and Table 6, the plots give a straight line but do not pass through the origin, also the low values of R2 (0.941, 0.965, and 0.928 and 0.805, 0.809, and 0.781) for Sr(II) and Cs(I) at different reaction temperatures, respectively. These data prove that the external diffusion model will have only limited applicability in the adsorption of Sr(II) and Cs(I) onto AB@S bio-sorbent at different reaction temperatures.
3.11 Sorption Isotherms
3.11.1 Influence of Ion Concentrations
Figure 8(a) displays the plot between the % uptake of Sr(II) and Cs(I) onto AB@S bio-sorbent and the start concentrations of Sr(II) and Cs(I). The experiment was done at conditions [Co = 25–800 mg L−1, V/m = 100 mL g−1, contact time 40 min, and pH = 7]. Figure 8(a) exhibits a great decrease in the % uptake of Sr(II) and Cs(I) with rising concentrations of Sr(II) and Cs(I) and the highest uptake was attained at start concentrations of 25 mg L−1.
3.11.2 Langmuir Isotherm
The theoretical monolayer capacity (Qmax, mg g−1) was estimated by the Langmuir isotherm equation that occurred by complete monolayer adsorption on the adsorbent surface (Hamed, 2014). The Langmuir isotherm was displayed by the following formula (Abass et al., 2021; Hamed et al., 2016a):
where b is the sorption equilibrium constant linked to the sorption energy. The plots Ce/qe and Ce give a linear relation as presented in Fig. 8(b), with high values of R2 close to unity (0.995 and 0.992 for Sr(II) and Cs(I), respectively) as represented in Table 7 reflecting the applicability of the Langmuir isotherm, and the Qmax values are 66.31 and 23.38 mg g−1 for Sr(II) and Cs(I), respectively.
3.11.3 Freundlich Isotherm
The Freundlich isotherm is an empirical equation that depends on multilayer sorption. The Freundlich isotherm is given as (Abass et al., 2021)
KF and n−1 are the Freundlich constants calculated from the intercept and slope of the linear plot of log qe and log Ce as represented in Fig. 8(c) related to the relative sorption capacity and heterogeneity factor respectively. The values of KF, n−1, and R2 are presented in Table 7, and the data reveal that R2 = 0.957 and 0.941 for Sr(II) and Cs(I), respectively. The values of R2 of the Freundlich model were lower than the Langmuir model. The values of Freundlich exponent n−1 > 1 prove the favorable sorption (Abdel-Galil et al., 2016). However, based on the R2 values, the Freundlich model was less applicable than the Langmuir model.
3.12 Thermodynamic Studies
The linear relation between ln Kd of Sr(II) and Cs(I) onto AB@S bio-sorbent and 1000/T are shown in Fig. 9(a) based on Van’t Hoff relation (Abass et al. 2022d):
where ∆S˚, ∆H˚, R, and T are the entropy change of adsorption, enthalpy change of adsorption, gas constant, and absolute temperature, respectively. As represented in Fig. 9(a), Kd of Sr(II) and Cs(I) increased with increasing reaction temperature from 298 to 338 K. This enhancement of the adsorption process is related to the acceleration of previously slow adsorption steps and the formation of new active sites on the surface of AB@S bio-sorbent (Abdel-Galil et al., 2016). From both slopes and intercepts of the straight lines exhibited in Fig. 9(a), ∆H˚ and ∆S˚ were computed and revealed that the change in ∆H˚ during the adsorption process was 22.7 and 4.51 kJ mol−1 for Sr(II) and Cs(I), respectively. Temperature increase showed a positive influence on Sr(II) and Cs(I) sorption in the endothermic adsorption process. With the rising temperature, the amount adsorbed increased. The entropy change, ΔS˚, was 159.91 and 60.48 J mol−1 K−1 for Sr(II) and Cs(I), respectively; these data revealed that the adsorption process was random. A positive entropy could be regarded as an increase in the randomness of the adsorption system as a result of the adsorbent’s high affinity (Şenol et al., 2022). The free energy change of specific adsorption (∆G˚) was calculated using Eq. (15):
Free energy change was − 24.95, − 28.15, and − 31.35 kJ mol−1 for Sr(II), while − 13.51, − 14.72, and − 15.93 kJ mol−1 for Cs(I) at 298, 318, and 338 K, respectively. The negative values of ∆G˚ reveal that the adsorption process is spontaneous (El-Naggar et al., 2010). The larger availability of ΔG˚ at higher temperatures was related to increased mobility of Sr(II) and Cs(I) onto the AB@S bio-sorbent surface, and increased electrostatic interaction among studied cations and different active groups on the AB@S bio-sorbent surface.
3.13 Simulated Radioactive Waste
The simulated radioactive waste (SRW) results are summarized in Fig. 9(b). This figure reflects the % removal of 85Sr and 134Cs from SRW solution increase from 62.6 to 95.8% for 85Sr and from 33.45 to 67.8% for 134Cs, by increasing AB@S weight from 0.05 to 0.15 g and has an affinity sequence 85Sr ˃ 134Cs. This behavior is similar to the sorption of 85Sr and 134Cs onto ZrSnP sorbent (Abass et al. 2022d). These results confirmed that AB@S biosorbent is a promising material for the removal of 85Sr and 134Cs from SRW.
4 Conclusion
In this work, silica loaded with Aspergillus brasiliensis (AB@S) bio-sorbent was synthesized, characterized, and used for batch sorption of Sr(II) and Cs(I) ions from aqueous solutions. AB@S was prepared by the precipitation technique. The distribution coefficients as a function of pH have selectivity Sr(II) > Cs(I). The kinetics of the sorption process obey pseudo-second-order kinetics. The capacity has the values 72.2 and 26.1 mg g−1 for Sr(II) and Cs(I), respectively. The isotherm data apply to Langmuir with Qmax values 66.31 and 23.38 mg g−1 for Sr(II) and Cs(I), respectively. Negative Gibbs energy values demonstrated the excellent feasibility and spontaneity of the sorption process. Positive enthalpy values indicated that the sorption process was endothermic. Positive entropy values demonstrated that during adsorption, the disorder between the solid and liquid phases enhanced. Finally, AB@S bio-sorbent can work as an effective sorbent for the sorption of 85Sr and 134Cs from simulated radioactive waste.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Abass, M. R., El-Masry, E. H., & Ibrahim, A. B. (2021). Preparation, characterization, and applications of polyacrylonitrile/ball clay nanocomposite synthesized by gamma radiation. Environmental Geochemistry and Health, 43(8), 3169–3188. https://doi.org/10.1007/s10653-021-00813-5
Abass, M. R., Breky, M. M. E., & Maree, R. M. (2022a). Removal of 137Cs and 90Sr from simulated low-level radioactive waste using tin(IV) vanadate sorbent and its potential hazardous parameters. Applied Radiation and Isotopes, 189, 110417.
Abass, M. R., Eid, M. A., & Ibrahim, A. B. (2022b). Silico antimonate nanocomposite material: I-preparation, characterization and its application for separation of strontium and rubidium from aqueous solutions. International Journal of Environmental Analytical Chemistry, 1–16. https://doi.org/10.1080/03067319.2022.2071611
Abass, M. R., El-Masry, E. H., & El-Kenany, W. M. (2022c). Gamma irradiation-induced preparation of polyacrylonitrile acrylamide nano-silica for removal of some hazardous metals. Journal of Inorganic and Organometallic Polymers and Materials, 32, 536–546. https://doi.org/10.1007/s10904-021-02156-1
Abass, M. R., Maree, R. M., & Sami, N. M. (2022d). Adsorptive features of cesium and strontium ions on zirconium tin(IV) phosphate nanocomposite from aqueous solutions. International Journal of Environmental Analytical Chemistry, 1–20. https://doi.org/10.1080/03067319.2021.2016728
Abdel-Galil, E. A., Ibrahim, A. B., & Abou-Mesalam, M. M. (2016). Sorption behavior of some lanthanides on polyacrylamide stannic molybdophosphate as organic-inorganic composite. International Journal of Industrial Chemistry, 7(3), 231–240.
Abdel-Galil, E. A., Eid, M. A., & Hassan, R. S. (2020). Preparation of nanosized stannic silicomolybdate for chromatographic separation of Y(III) from Zr(IV). Particulate Science and Technology, 38(1), 113–120.
Abdel-Galil, E. A., Ibrahim, A. B., & El-Kenany, W. M. (2021). Facile fabrication of a novel silico vanadate ion exchanger: Evaluation of its sorption behavior towards europium and terbium ions. Desalination and Water Treatment, 226, 303–318. https://doi.org/10.5004/dwt.2021.27261
Abdel-Razek, A. S., Abdel-Ghany, T. M., Mahmoud, S. A., El-Sheikh, H. H., & Mahmoud, M. S. (2009). The use of free and immobilized Cunninghamella elegans for removing cobalt ions from aqueous waste solutions. World Journal of Microbiology and Biotechnology, 25, 2137–2145.
Abou-Mesalam, M. M., Abass, M. R., Abdel-Wahab, M. A., Zakaria, E. S., Hassan, A. M., & Khalil, H. F. (2016). Complex doping of d-block elements cobalt, nickel, and cadmium in magneso-silicate composite and its use in the treatment of aqueous waste. Desalination and Water Treatment, 57(53), 25757–25764. https://doi.org/10.1080/19443994.2016.1156031
Abou-Mesalam, M. M., Abass, M. R., Abdel-Wahab, M. A., Zakaria, E. S., & Hassan, A. M. (2018). Polymeric composite materials based on silicate: II. sorption and distribution studies of some hazardous metals on irradiated doped polyacrylamide acrylic acid. Desalination and Water Treatment, 109, 176–187. https://doi.org/10.5004/dwt.2018.22084
Abou-Mesalam, M. M., Abass, M. R., Ibrahim, A. B., & Zakaria, E. S. (2020). Polymeric composite materials based on silicate. III-Capacity and sorption behavior of some hazardous metals on irradiated doped polyacrylamide acrylonitrile. Desalination and Water Treatment, 193, 402–413. https://doi.org/10.5004/dwt.2020.25816
Abu Taleb, M., Halawani, R., Neamtallah, A., Kumar, R., & Barakat, M. (2022). Hybrid bioadsorbents for heavy metal decontamination from wastewater: A review. International Journal of Materials Technology and Innovation, 5–19.
Aglan, R. F., Hamed, M. M., & Saleh, H. M. (2019). Selective and sensitive determination of Cd(II) ions in various samples using a novel modified carbon paste electrode. Journal of Analytical Science and Technology, 10(7), 1–11.
Aguila, B., Banerjee, D., Nie, Z., Shin, Y., Ma, S., & Thallapally, P. K. (2016). Selective removal of cesium and strontium using porous frameworks from high level nuclear waste. Chemical Communications, 52(35), 5940–5942.
Ahmed, I. M., Aglan, R. F., & Hamed, M. M. (2017). Removal of Arsenazo-III and Thorin from radioactive waste solutions by adsorption onto low-cost adsorbent. Journal of Radioanalytical and Nuclear Chemistry, 314(3), 2253–2262.
Al-Yassir, N., & Le Van Mao, R. (2007). Catalysts for the thermo-catalytic cracking (TCC) process: Interactions between the yttria in yttria-doped alumina aerogel and the mono-oxide MoO3, CeO2, and bi-oxide MoO3–CeO2 species. Applied Catalysis a: General, 332(2), 273–288.
Andrews, S. J., Papiz, M. Z., McMeeking, R., Blake, A. J., Lowe, B. M., Franklin, K. R., et al. (1988). Piperazine silicate (EU 19): The structure of a very small crystal determined with synchrotron radiation. Acta Crystallographica Section b: Structural Science, 44(1), 73–77.
Andriamafana, H. H., Mong, Y., Andriambeloson, O., Ravonizafy, C., Raherimandimby, M., & Rasolomampianina, R. (2018). Isolation and identification of heavy metals and antibiotics resistant strains from Antananarivo Dumpsite, Madagascar. International Journal of Microbiology and Biotechnology, 3(3), 71–78.
Ayele, A., Haile, S., Alemu, D., & Kamaraj, M. (2021). Comparative utilization of dead and live fungal biomass for the removal of heavy metal: A concise review. The Scientific World Journal, 1–10.
Bakhotmah, D. A., Hussein, M. A., El-Said, W., Ismael, M. H., & Elshehy, E. (2023). Efficient removal of cesium and strontium from an aqueous solution using a zirconosilicate/vanadium oxide nanocomposite. Journal of Dispersion Science and Technology, 44(10), 1842–1852.
Borai, E. H., Hamed, M. G., El-Kamash, A. M., Siyam, T., & El-Sayed, G. O. (2015). Synthesis, characterization and application of a modified acrylamide–styrene sulfonate resin and a composite for sorption of some rare earth elements. New Journal of Chemistry, 39(9), 7409–7420.
Borai, E. H., Attallah, M. F., Elgazzar, A. H., & El-Tabl, A. S. (2019). Isotherm and kinetic sorption of some lanthanides and iron from aqueous solution by aluminum silicotitante exchanger. Particulate Science and Technology, 37(4), 414–426.
Dakroury, G. A., El-Shazly, E. A. A., & Hassan, H. S. (2021). Preparation and characterization of ZnO/chitosan nanocomposite for Cs(I) and Sr(II) sorption from aqueous solutions. Journal of Radioanalytical and Nuclear Chemistry, 330(1), 159–174.
El-Bondkly, A. M. A., & El-Gendy, M. M. A. A. (2022). Bioremoval of some heavy metals from aqueous solutions by two different indigenous fungi Aspergillus sp. AHM69 and Penicillium sp. AHM96 isolated from petroleum refining wastewater. Heliyon, 8(7), e09854.
El-Deen, S. E. A. S., Moussa, S. I., Mekawy, Z. A., Shehata, M. K. K., Sadeek, S. A., & Someda, H. H. (2017). Evaluation of CNTs/MnO2 composite for adsorption of 60Co(II), 65Zn(II) and Cd(II) ions from aqueous solutions. Radiochimica Acta, 105(1), 43–55.
El-Naggar, I. M., Mowafy, E. A., Abdel-Galil, E. A., & El-Shahat, M. F. (2010). Synthesis, characterization and ion-exchange properties of a novel ‘organic–inorganic’ hybrid cation-exchanger: Polyacrylamide Sn(IV) molybdophosphate. Global Journal of Physical Chemistry, 1(1), 91–106.
El-Naggar, I. M., Sheneshen, E. S., & Abdel-Galil, E. A. (2016). Diffusion mechanism of Co2+, Cu2+, Cd2+, Cs+, and Pb2+ ions in the particles of polyaniline silicotitanate. Particulate Science and Technology, 34(3), 373–379.
Emara, A. M., El-Sweify, F. H., Abo-Zahra, S. F., Hashim, A. I., & Siyam, T. E. (2019). Removal of Cs-137 and Sr-90 from reactor actual liquid waste samples using a new synthesized bionanocomposite-based carboxymethylcellulose. Radiochimica Acta, 107(8), 695–711.
Fang, X.-H., Fang, F., Lu, C.-H., & Zheng, L. (2017). Removal of Cs+, Sr2+, and Co2+ ions from the mixture of organics and suspended solids aqueous solutions by zeolites. Nuclear Engineering and Technology, 49(3), 556–561.
Ghaly, M., Abass, M. R., & Mekawy, Z. A. (2023). Performance of molybdenum vanadate loaded on bentonite for retention of cesium-134 from aqueous solutions. Environmental Science and Pollution Research, 30(21), 60432–60446. https://doi.org/10.1007/s11356-023-26607-z
Gu, H., Liu, X., Wang, S., Chen, Z., Yang, H., Hu, B., et al. (2022). COF-based composites: Extraordinary removal performance for heavy metals and radionuclides from aqueous solutions. Reviews of Environmental Contamination and Toxicology, 260(1), 23.
Hamed, M. M. (2014). Sorbent extraction behavior of a nonionic surfactant, Triton X-100, onto commercial charcoal from low level-radioactive waste. Journal of Radioanalytical and Nuclear Chemistry, 302(1), 303–313.
Hamed, M. M., Holiel, M., & Ismail, Z. H. (2016a). Removal of 134Cs and 152+154Eu from liquid radioactive waste using Dowex HCR-S/S. Radiochimica Acta, 104(6), 399–413.
Hamed, M. M., Rizk, S. E., & Nayl, A. A. (2016b). Adsorption kinetics and modeling of gadolinium and cobalt ions sorption by an ion-exchange resin. Particulate Science and Technology, 34(6), 716–724.
Hamed, M. M., Hassan, R. S., & Metwally, S. S. (2019). Retardation behavior of alum industrial waste for cationic and anionic radionuclides. Process Safety and Environmental Protection, 124, 31–38.
Hao, M., Liu, Y., Wu, W., Wang, S., Yang, X., Chen, Z., et al. (2023). Advanced porous adsorbents for radionuclides elimination. Energy Chem, 100101.
Ibrahim, A. B., Abass, M. R., EL-Masry, E. H., & Abou-Mesalam, M. M. (2021). Gamma radiation-induced polymerization of polyacrylic acid-dolomite composite and applications for removal of cesium, cobalt, and zirconium from aqueous solutions. Applied Radiation and Isotopes, 178, 109956. https://doi.org/10.1016/j.apradiso.2021.109956
Karaca, S., Gürses, A., Açışlı, Ö., Hassani, A., Kıranşan, M., & Yıkılmaz, K. (2013). Modeling of adsorption isotherms and kinetics of Remazol Red RB adsorption from aqueous solution by modified clay. Desalination and Water Treatment, 51(13–15), 2726–2739.
Karimi-Maleh, H., Alizadeh, M., Orooji, Y., Karimi, F., Baghayeri, M., Rouhi, J., et al. (2021). Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: A docking/experimental investigation. Industrial & Engineering Chemistry Research, 60(2), 816–823.
Kasem, A. E., Abdel-Galil, E. A., Belacy, N., & Badawy, N. A. (2021). Kinetics and adsorption equilibrium of some radionuclides on polyaniline/SiO2 composite. Radiochimica Acta, 109(2), 85–97.
Khani, M. H., Pahlavanzadeh, H., & Alizadeh, K. (2012). Biosorption of strontium from aqueous solution by fungus Aspergillus terreus. Environmental Science and Pollution Research, 19(6), 2408–2418.
Lee, S., Kim, J., Ku, B.-C., Kim, J., & Joh, H.-I. (2012). Structural evolution of polyacrylonitrile fibers in stabilization and carbonization. Advances in Chemical Engineering and Science, 02(02), 275–282. https://doi.org/10.4236/aces.2012.22032
Lee, K.-Y., Kim, K.-W., Park, M., Kim, J., Oh, M., Lee, E.-H., et al. (2016). Novel application of nanozeolite for radioactive cesium removal from high-salt wastewater. Water Research, 95, 134–141.
Li, D., Wang, M., Yang, C., Wang, J., & Ren, G. (2012). Solid state characterizations and analysis of stability in azelnidipine polymorphs. Chemical and Pharmaceutical Bulletin, 60(8), 995–1002.
Liu, X., Xiao, M., Li, Y., Chen, Z., Yang, H., & Wang, X. (2023). Advanced porous materials and emerging technologies for radionuclides removal from Fukushima radioactive water. Eco-Environment & Health.
Long, J., Li, H., Jiang, D., Luo, D., Chen, Y., Xia, J., & Chen, D. (2017). Biosorption of strontium(II) from aqueous solutions by Bacillus cereus isolated from strontium hyperaccumulator Andropogon gayanus. Process Safety and Environmental Protection, 111, 23–30.
Metwally, S. S., Ahmed, I. M., & Rizk, H. E. (2017). Modification of hydroxyapatite for removal of cesium and strontium ions from aqueous solution. Journal of Alloys and Compounds, 709, 438–444.
Metwally, S. S., Hassan, H. S., & Samy, N. M. (2019). Impact of environmental conditions on the sorption behavior of 60Co and 152+154Eu radionuclides onto polyaniline/zirconium aluminate composite. Journal of Molecular Liquids, 287, 110941.
Mirzayev, M. N., Abdurakhimov, B. A., Demir, E., Donkov, A. A., Popov, E., Tashmetov, M. Y., et al. (2021). Investigation of the formation of defects under fast neutrons and gamma irradiation in 3C–SiC nano powder. Physica b: Condensed Matter, 611(January), 412842. https://doi.org/10.1016/j.physb.2021.412842
Moloukhia, H. (2010). Use of animal charcoal prepared from the bivalve chaelatura (chaelatura) companyoi in treatment of waste solution containing cesium and strontium ions. Journal of Radiation Research and Applied Sciences, 3(2), 343–356.
Nabi, S. A., Shahadat, M., Bushra, R., Shalla, A. H., & Azam, A. (2011). Synthesis and characterization of nano-composite ion-exchanger; its adsorption behavior. Colloids and Surfaces b: Biointerfaces, 87(1), 122–128.
Pitt, J. I., & Hocking, A. D. (2022). Ecology of fungal food spoilage. In Fungi and food spoilage (pp. 3–12). Springer.
Saufi, S. M., & Ismail, A. F. (2002). Development and characterization of polyacrylonitrile (PAN) based carbon hollow fiber membrane. Songklanakarin Journal of Science and Technology, 24, 843–854.
Șenilă, M., Neag, E., Tănăselia, C., & Șenilă, L. (2023). Removal of cesium and strontium ions from aqueous solutions by thermally treated natural zeolite. Materials, 16(8), 2965.
Şenol, Z. M., Kaya, S., Şimşek, S., Katin, K. P., Özer, A., & Marzouki, R. (2022). Synthesis and characterization of chitosan-vermiculite-lignin ternary composite as an adsorbent for effective removal of uranyl ions from aqueous solution: Experimental and theoretical analyses. International Journal of Biological Macromolecules, 209, 1234–1247.
Shaaban, M. T., Abdel-Razek, A. S., Mahmoud, S. A., & Kandeel, E. M. (2020). Comparative studies to remove cobalt ions from hazardous waste solutions by immobilized microbial species using several techniques: Beads and thin film. Desalination and. Water Treatment, 206, 215–228.
Sheha, R. R., Mekawy, Z. A., Someda, H. H., Shehata, M. K. K., & Mahmoud, W. H. (2020). Assessing the sorptive ability of synthesized graphene oxide-metal oxide composite to remove certain lanthanides. CLEAN-Soil, Air, Water, 48(12), 2000348.
Staroń, P., Kuciakowski, J., & Chwastowski, J. (2023). Biocomposite of hydrochar and Lindnera jadinii with magnetic properties for adsorptive removal of cadmium ions. Journal of Environmental Chemical Engineering, 110270.
Wang, Q., Dong, Z., Du, Y., & Kennedy, J. F. (2007). Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohydrate Polymers, 69(2), 336–343.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications, 18(1), 315–322.
Yang, H.-M., Jang, S.-C., Hong, S. B., Lee, K.-W., Roh, C., Huh, Y. S., & Seo, B.-K. (2016). Prussian blue-functionalized magnetic nanoclusters for the removal of radioactive cesium from water. Journal of Alloys and Compounds, 657, 387–393.
Yu, R., Chai, H., Yu, Z., Wu, X., Liu, Y., Shen, L., et al. (2020). Behavior and mechanism of cesium biosorption from aqueous solution by living Synechococcus PCC7002. Microorganisms, 8(4), 491.
Zhao, C., Liu, J., Deng, Y., Tian, Y., Zhang, G., Liao, J., et al. (2019). Uranium(VI) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. Journal of Cleaner Production, 236, 117624.
Acknowledgements
Great thanks to all members of the Nuclear Fuel Technology Department, and Radiation Protection and Safety Department, Egyptian Atomic Energy Authority for supporting this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohamed R. Abass: conceptualization, visualization, experimental work, writing–reviewing, and editing. Riham A. Abou-Lilah: experimental work. Eman M. Kandeel and Mokhtar K. Mohamed: editing and reviewing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abass, M.R., Kandeel, E.M., Abou-Lilah, R.A. et al. Effective Biosorption of Cesium and Strontium Ions from Aqueous Solutions Using Silica Loaded with Aspergillus brasiliensis. Water Air Soil Pollut 235, 61 (2024). https://doi.org/10.1007/s11270-023-06855-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06855-y