Abstract
The aim of the study was the synthesis of polymer biocomposites based on acrylates with the addition of various amounts of chitosan as an eco-filler. The composites (BPA.DA + NVP) were prepared using the photopolymerization technique. The obtained materials were subjected to physicochemical tests, determining, among others, their hardness and thermal properties by the DSC and TG/DTG methods. The ATR/FT-IR analysis and optical profilometer as well as the values of the solid surface tension calculated from the contact angle of model liquids and van Oss et al. approach to the solid-liquid interface tension were performed to confirm the chemical structure of the obtained biocomposites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently the application of natural fibers in new environmentally friendly materials has become an important issue [1, 2]. As a result, natural fibers such as wood [3], chitosan [4], sisal [5], banana fiber [6], bagasse [7], etc. are gaining more and more interest as reinforcement for both thermoplastic and thermosetting polymer composites. This makes them a promising alternative to replacing synthetic fiber. Many researchers have shown a great interest in the production of materials that could replace synthetic ones. That is why in recent years there has been an increasing demand for the commercial use of natural fiber-based composites in various industrial sectors. Natural fibers are sustainable materials that are easily available in nature and have advantages like low cost, biodegradability, renewability, lightweight, and high specific properties [8, 9]. Among the various renewable polymers, chitosan is one of the most important commercial materials, provided it has significant properties that recommend it for many different applications. Chitosan is also known for its excellent mechanical and thermal properties. For example, Husseinsyah et al. [10] examined the effect of the chemical modification of chitosan by acrylic acid on the mechanical and thermal properties of chitosan-filled polypropylene (PP) composites with varying filler loadings. They showed that the addition of chitosan improved the thermal stability of the PP/chitosan composite as compared to that of neat PP. Therefore, chitosan is a promising product as a bio-filler in the composites that will replace various materials such as construction materials, furniture, and many plastic products in a variety of future industrial applications [11].
Chitosan is the second most plentiful natural fiber after cellulose. It is obtained by extraction from the shells of crustaceans such as crabs, prawns, and lobsters [12]. Chitosan is a crystalline polymer and it possesses several valuable physicochemical properties, such as biocompatibility, biodegradability, non-toxicity, hydrophilicity, ease of modification, a great affinity for metals, dyes, and proteins, the ability to create polycations in the acidic environment, film-forming ability, etc. [13,14,15,16]. These features make it suitable for use in medicine and pharmacy, in various industries, in environmental protection, water purification processes, different types of separation processes, and so on [16,17,18]. The nature of chitosan allows the incorporation of synthetic materials into its polymeric structure [9, 19,20,21]. For example, polyacrylates possess good mechanical properties and moreover, chitosan can react with synthetic polymers, improving their final properties [22]. Over the last few years, numerous studies have been successfully carried out on the grafting of glycidyl methacrylate (GMA) onto chitosan for use in naturally synthesized hydrogels [23, 24]. Torabi et al. [24] reported an environmentally-friendly process for the preparation of acrylic/chitosan films with antibacterial activity and nontoxic properties using water-based acrylic resin. It was found that the obtained films enhanced antibacterial activity against Escherichia coli and Staphylococcus aureus, and the cytotoxicity analysis showed a reasonably non-toxic behavior of the composite films. In another work, Iordacheet al. [25] prepared magnetic-chitosan grafted (alkyl acrylate) composite particles with the purpose of increasing the surface area, mechanical stability, sorbent separation, and re-usability compared to the raw chitosan. They proved the high stability and recovery capacity of the composite particles and they suggested their potential use for the Ni(II) ions removal from wastewater using the magnetically assisted adsorption technology.
The goal of the present paper was to prepare polymer composites based on bisphenol A diacrylate (BPA.DA), N-vinyl-2-pyrrolidone (NVP), and chitosan (CHI) as an ecological filler. Various amounts of chitosan were used (in the range of 0 to 15%). UV-initiated polymerization was used to produce the biocomposites. 2,2-Dimethoxy-2-phenyl acetophenone (Irgacure 651, IQ) acting as a photoinitiator. The chemical structure of the composites was confirmed by the ATR/FT-IR, optical profilometer, and solid surface tension analysis. The thermal properties were characterized by the differential scanning calorimeter (DSC) and the TG/DTG method.
2 Materials and methods
2.1 Materials
Bisphenol A glycerolate (1 glycerol/phenol) diacrylate (BPA.DA), N-vinyl-2-pyrrolidone (NVP), chitosan (CHI), and 2,2-dimethoxy-2-phenyl acetophenone (Irgacure 651, IQ) were obtained from Sigma-Aldrich. Formamide (> 99.5%) and diiodomethane (> 99%) were bought from Sigma-Aldrich (Germany) and used without further purification.
2.2 Preparation of biocomposites
The BPA.DA + NVP composites with the addition of eco-filler (chitosan, CHI) were prepared using the photopolymerization technique [26,27,28]. Before their synthesis, the main component of composites – bisphenol A glycerolate (1 glycerol/phenol) diacrylate (BPA.DA) was heated in the laboratory dryer to 60 °C. Then its proper amounts were weighed into the glass vessels. Then, the second monomer, N-vinyl-2-pyrrolidone (NVP) was added dropwise to these glass vessels while mixing. The monomers were stirred at room temperature until a homogenous mixture was obtained. The ratio of BPA.DA to NVP was 10:3. In the next stage, the appropriate amount of chitosan (CHI) (5; 10; 15% w/w) was added, and stirring was continued. Finally, the calculated amount of UV initiator (2% w/w) (Irqacure 651) was added to the sample. The contents of the beaker were poured into the glass molds (10 mm×8 mm×2 mm) and polymerized under the UV lamp for 0.5 h. In consequence, the composites in the form of white or yellow plates were prepared as can be seen in Fig. 1. The obtained chitosan composites possess a homogeneous structure. The photopolymerization of the composite without the chitosan content was also performed to compare the properties. Detailed information regarding the reagents, a fragment of polymer unit, and amounts of used reagents are presented in Fig. 2 and also in Table 1.
2.3 Methods
The Fourier transform infrared (FT-IR) spectra were recorded with a Bruker Tensor 27 FTIR spectrometer (Germany) using the attenuated total reflectance (ATR) technique. All spectra were obtained at room temperature after averaging 32 scans between 600 and 4000 cm− 1 with a resolution of 4 cm− 1 in the absorbance mode.
Thermogravimetry (TG/DTG) was performed with the Netzsch STA 449 F1 Jupiter thermal analyzer (Germany) under the following operational conditions: the heating rate of 10 K min− 1, the dynamic atmosphere of helium (flow 20 ml min− 1), the temperature range of 30–800 °C, the sample mass ~ 10 mg, the sensor thermocouple type S TG-DSC. All TG measurements were taken in Al2O3 crucibles. As a reference, an empty Al2O3 crucible was used.
The differential scanning calorimetry (DSC) curves were obtained using the DSC Netzsch 204 calorimeter (Netzsch, Günzbung, Germany). The DSC measurements were taken in the aluminum pans with a pierced lid of the sample weight of ~ 5 to 15 mg in the nitrogen atmosphere (30 mL min− 1). The empty aluminum crucible was applied as a reference. Dynamic scans were obtained at the heating rate of 10 K min− 1 in the temperature range of 20–550 °C. The parameters such as decomposition temperatures (Tonset, Toffset), final decomposition temperature (Td), and enthalpy of decomposition (ΔHd) were determined.
The Shore hardness tests were carried out with the Zwick 7206/H04 durometer (Germany), type D. The readings were taken after 15 seconds at the temperature of 21 °C.
The images of the investigated surfaces being investigated were recorded using the optical profilometer (Contour GT, Veeco). Moreover, the Wyko Vision software was applied for the surface roughness analysis. The apparatus equipped with the optical surface-profiling system (3D) was used for the measurements of surface morphology and its accuracy was from the sub-nanometre up to 10 mm size.
The advancing contact angle for water, formamide, and diiodomethane was measured on the surfaces of composites in a thermostated chamber employing the sessile drop method, DSA30 measuring system (Krüss) at 20 °C ± 0.1 °C. In the procedure vapor of a given liquid, whose contact angle was measured, was used for saturation of the apparatus chamber by placing a cell filled with this liquid a few hours before making the measurements. Its contact angle was measured for at least 20 drops and good reproducibility was found. The standard deviation was less than 1.2° for each set in most cases.
3 Results and discussion
3.1 ATR/FT-IR analysis
Figure 3 shows the ATR/FT-IR spectra of the composites based on the BPA.DA + NVP polymer with different addition of chitosan (CHI) as an eco-filler. As can be seen in Fig. 3, all the obtained spectra have similar absorption bands. The most characteristic signal is the broad band in the range 3380–3399 cm− 1 derived from the stretching vibrations of a hydroxyl group (-OH) present in both the BPA.DA and CHI molecules. In the modified composites with CHI, this signal becomes clearer. The signals located at 2870–2961 cm− 1 originate from the stretching vibrations of the C–H in –CH2– and –CH3 groups. Another specific signal for acrylates is also the band from the stretching vibrations of the carbonyl group –C = O. It is present in all the shown spectra in the range 1664–1729 cm− 1. The peak at 1505–1508 cm− 1 is associated with the stretching vibrations of –C = C in the benzene rings and the aromatic skeletal vibrations. The peaks at approximately 1428–1435 cm− 1 originate from the C–H deformation in the –CH2– and –CH3 groups. In the spectra, there are also observed the stretching vibrations of the ester group C–O–C results in a doublet at 1170–1174 and 1245–1258 cm− 1. The bands at 1029–1035 cm− 1 are derived from the stretching vibrations of C-O bonds in the alkyl-aryl ethers. In turn, the signals at 806–823 cm− 1 can be assigned to the C-H vibrations from the benzene ring. All things considered, the increasing content of chitosan does not change drastically the course of the ATR/FT-IR curves of the polymeric composites, however, it increases the intensity of the signals, especially of those derived from the –OH and C–O–C groups.
3.2 Thermal behavior of composites
3.2.1 DSC analysis
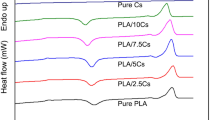
The DSC analysis was carried out in the temperature range from − 30 to 250 °C, after the polymerization process for all prepared samples. The curves are presented in Fig. 4. The endothermic peak occurring in the temperature range of 50–100 °C with the maximum at about 70 °C has probably associated with the evaporation of residual amounts of monomers and water. On the DSC curves, no exothermic effects below 200 oC (Fig. 4) characteristic for the crosslinking reaction are present. This can be interpreted as 100% double bond conversion. Only in the case of BPA.DA-NVP parent composition’s small effect is visible these were related to the further post-polymerization reactions between the used monomers (BPA.DA and NVP). The addition of chitosan filler improves the crosslinking effect in the compositions. It is probably connected with the chemical structure of chitosan and a possible reaction between amine groups and monomers.
3.2.2 TG/DTG analysis
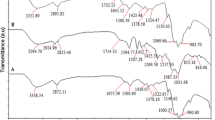
To determine the thermal stability of BPA.DA + NVP and its composites with CHI, the thermogravimetric analysis (TG/DTG) was prepared in the helium atmosphere in the temperature range of 30–800 °C. The curves obtained from the TG and DTG measurements are presented in Figs. 5 and 6. The obtained data are also listed in Table 2. The initial degradation temperatures ranged from 115 to about 200 °C for all the studied materials due to the evaporation of residue monomers and water.
The composites containing chitosan in their structure show a different course of the decomposition process compared to the BPA.DA + NVP composite. All synthesized materials have two decomposition peaks. In the case of BPA.DA + NVP, the first peak at 398 °C is due to the major degradation of the polymer chain, while the second peak at 604 °C is due to the degradation of the aromatic parts present in BPA.DA + NVP. Analyzing the obtained data, one can conclude that the largest thermal resistance was found in the case of BPA.DA + NVP + 15% CHI. In turn, polymeric materials with the addition of chitosan possess a very similar degradation profile. The first peak on the DTG plot observed for only the chitosan-containing materials in the temperature range of 290–298 °C (Tmax) can be associated with the degradation of aliphatic fragments and oxygen functionalities from chitosan. These materials decomposed in one main step in the range 320–470 °C with the maximum temperature at 411–415 °C. The final decomposition temperatures (Tf) are from 498 to 695 °C. The residual ash (RM) at 800 °C was in the range from 1.67 to 12.24% of the initial mass for all synthesized polymers and it grew with the increasing chitosan content in the composites. The characteristic features of the thermal behavior of the chitosan composites are given in Table 2.
3.3 Shore hardness
The measurements of hardness were based on the vertical immersion of the indenter into the composite surface. The obtained values are expressed in the D scale in Table 3.
The hardness of the studied materials was in the range of 84.5–86 units. After the addition of chitosan, hardness did not change significantly. The highest value was obtained for the sample with the maximum amount of eco-filler (15 wt %). Generally, one can conclude that the addition of chitosan does not deteriorate the hardness of the resulting composite (as is the case in many applications of biopolymers) and can be efficiently used as a bio-filler.
3.4 Surface properties
There were determined the surface roughness parameters as average roughness (Ra), root-mean-squared roughness (Rq), and peak-to-valley difference (Rt) (Table 4) based on the optical profilometer images (Fig. 7).
As follows from Table 4 the values of the Ra and Rq increased from 9.9 to 294.3 nm and from 11.9 to 709.2 nm, respectively after the addition of 15% of CHI compared with the chitosan-free surface [29]. Moreover, the increase of Rt values was significant with the CHI concentration to increase. These parameters are reflected in the vales of components and parameters and surface tension,\({ \gamma }_{S}\), of the studied composites (Table 5) calculated using van Oss et al. method [30,31,32] and the contact angle (\(\theta\)) of model liquids, water (W), formamide (F) and diiodomethane (D) on the composite surfaces presented on Fig. 8. From this Figure it results that all studied surfaces have a weakly hydrophobic character which is reflected in the values of the water contact angle in the range of 90o >\(\theta\)> (56–65 o) [33], i.e. \({\theta }_{\text{W}}\) = 75o and \({\theta }_{\text{W}}\) = 90o for BPA.DA-NVP and BPA.DA+NVP+15% CHI, respectively. The increase of chitosan content caused not only the increase of the contact angle of water but also the values of \(\theta\) for formamide and diiodomethane on the surface of the studied composites. The obtained \({\theta }_{\text{W}}\), \({\theta }_{\text{F}}\) and\({\theta }_{\text{D}}\) as well as the components and parameters of the surface tension of model liquids proposed by Zdziennicka et al. [34] were applied for the calculation of the values index of \({\gamma }_{S}\), the Lifshitz-van der Waals component as well as electron-acceptor and electron-donor parameters of the acid−base component of the solid surface tension. The water, formamide, and diiodomethane surface tension values were 72.8, 58.0 and 50.8 mN/m, respectively.
As follows from Table 5 the components and parameters of the surface tension of composites, similar to the values of the contact angle (Fig. 8), are dependent on the CHI percentage. On the other hand, the values of \({\gamma }_{S}^{+}\) are small and change significantly for the solids with 5, 10 and 15% of CHI. However, after the chitosan addition the values of \({\gamma }_{S}^{LW}\) and \({\gamma }_{S}\) decrease largely. Interestingly values of \({\gamma }_{S}\) are similar to those obtained by the other researches [35, 36] and that for CHI \({ C}_{p}\)= 15% is similar to that of polyethylene [37].
4 Conclusions
As a result of UV photopolymerization a new composites based on bisphenol A glycerolate (1 glycerol/phenol) diacrylate (BPA.DA) and N-vinyl-2-pyrrolidone (NVP) with the addition of ecological filler such as chitosan (CHI) were successfully obtained. The spectroscopic analysis (ATR/FT-IR) proved the effective synthesis of chitosan-containing biocomposites, which was confirms by presence of characteristic bands of appropriate functional groups.
Based on the thermogravimetric data and the values of the components and parameters of the composites surface tension determined using the van Oss et al. approach, it can be stated that the addition of CHI to the acrylate increased its thermal resistance (especially visible in the case of BPA.DA + NVP composites containing 15% of chitosan) and hydrophobicity.
Data Availability
All data are available from the corresponding author upon reasonable request.
References
Rahman, M.R., Huque, M.M., Islam, M.N., Hasan, M.: Mechanical properties of polypropylene composites reinforced with chemically treated abaca, Compos. Part A Appl. Sci. Manuf. 40, 511–517 (2009). https://doi.org/10.1016/j.compositesa.2009.01.013
Tanjung, F.A., Husseinsyah, S., Hussin, K.: Chitosan-filled polypropylene composites: The effect of filler loading and organosolv lignin on mechanical, morphological and thermal properties. Fibers Polym. 15, 800–808 (2014). https://doi.org/10.1007/s12221-014-0800-0
Bledzki, A.K., Mamun, A.A., Volk, J.: Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part. A Appl. Sci. Manuf. 41, 480–488 (2010). https://doi.org/10.1016/j.compositesa.2009.12.004
Hsu, S.-T., Chen, L.-C., Leu, M.-H., Hsiao, W.-F., Lee, W.-Y., Pan, T.-C.: Experimental improvement of Preparation of Acrylic Acid-Modified Middle Deacetylated Chitosan and its application in absorbing paraquat. Polym. Eng. Sci. 53, 468–473 (2013). https://doi.org/10.1002/pen
Li, Y., Hu, C., Yu, Y.: Interfacial studies of sisal fiber reinforced high density polyethylene (HDPE) composites, Compos. Part A Appl. Sci. Manuf. 39, 570–578 (2008). https://doi.org/10.1016/j.compositesa.2007.07.005
Zaman, H.U., Khan, M.A., Khan, R.A.: Banana fiber-reinforced polypropylene composites: A study of the physico-mechanical properties. Fibers Polym. 14, 121–126 (2013). https://doi.org/10.1007/s12221-013-0121-8
Zheng, Y.T., Cao, D.R., Wang, D.S., Chen, J.J.: Study on the interface modification of bagasse fibre and the mechanical properties of its composite with PVC, Compos. Part A Appl. Sci. Manuf. 38, 20–25 (2007). https://doi.org/10.1016/j.compositesa.2006.01.023
Pegoretti, A., Cercená, R., Siengchin, S., Gowda Thyavihalli Girijappa, Y., Mavinkere Rangappa, S., Parameswaranpillai, J.: Natural fibers as sustainable and renewable resource for development of Eco-Friendly Composites: A Comprehensive Review, Front. Mater. | Www Frontiersin Org. 6, 226 (2019). https://doi.org/10.3389/fmats.2019.00226
Silva-Castro, I., Martín-Ramos, P., Matei, P.M., Fernandes-Correa, M., Hernández-Navarro, S., Martín-Gil, J.: Eco-friendly nanocomposites of chitosan with natural extracts, antimicrobial agents, and nanometals. Handb. Compos. from Renew. Mater. 35–60 (2017). https://doi.org/10.1002/9781119441632.ch150
Husseinsyah, S., Amri, F., Husin, K., Ismail, H.: Mechanical and thermal properties of chitosan-filled polypropylene composites: The effect of acrylic acid. J. Vinyl Addit. Technol. 17, 125–131 (2011). https://doi.org/10.1002/vnl.20268
Das Lala, S., Deoghare, A.B., Chatterjee, S.: Effect of reinforcements on polymer matrix bio-composites - an overview. Sci. Eng. Compos. Mater. 25, 1039–1058 (2018). https://doi.org/10.1515/secm-2017-0281
Martínez-Camacho, A.P., Cortez-Rocha, M.O., Ezquerra-Brauer, J.M., Graciano-Verdugo, A.Z., Rodriguez-Félix, F., Castillo-Ortega, M.M., Yépiz-Gómez, M.S.: Plascencia-Jatomea, Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 82, 305–315 (2010). https://doi.org/10.1016/j.carbpol.2010.04.069
Wan, Y., Xiao, B., Dalai, S., Cao, X., Wu, Q.: Development of polycaprolactone/chitosan blend porous scaffolds. J. Mater. Sci. Mater. Med. 20, 719–724 (2009). https://doi.org/10.1007/s10856-008-3622-z
Struszczyk, M.H.: Chitin and Chitosan. Part I. Properties and production. Polimery. 47, 316–325 (2002)
Peter, M.G.: Chitin and Chitosan from Animal Sources, Part 6: Polysaccharides, in: Biopolymers, p. 481. Wiley, Weinheimt (2005)
Mucha, M.: Chitozan – wszechstronny Polimer ze źródeł Odnawialnych. Wydawnictwo Naukowo-Techniczne, Warsaw (2010)
Rinaudo, M.: Chitin and chitosan: Properties and applications. Prog Polym. Sci. 31, 603–632 (2006). https://doi.org/10.1016/j.progpolymsci.2006.06.001
Kim, S.-K.: Chitin, Chitosan, Oligosaccharides and Their Derivatives. CRC Press, Boca Raton (2011). https://doi.org/10.1271/nogeikagaku1924.66.1360
Lima, P.S., Trocolli, R., Wellen, R.M.R., Rojo, L., Lopez-Manchado, M.A., Fook, M.V.L., Silva, S.M.L.: HDPE/chitosan composites modified with PE-g-MA. Thermal, morphological and antibacterial analysis, polymers (Basel). 11 1559–1579. (2019). https://doi.org/10.3390/polym11101559
Amri, F., Husseinsyah, S., Hussin, K.: Mechanical, morphological and thermal properties of chitosan filled polypropylene composites: The effect of binary modifying agents. Compos. Part. A Appl. Sci. Manuf. 46, 89–95 (2013). https://doi.org/10.1016/j.compositesa.2012.10.014
Liu, D., Yuan, J., Li, J., Zhang, G.: Preparation of Chitosan Poly(methacrylate) composites for Adsorption of Bromocresol Green. ACS Omega. 4, 12680–12686 (2019). https://doi.org/10.1021/acsomega.9b01576
Lee, J.W., Kim, S.Y., Kim, S.S., Lee, Y.M., Lee, K.H., Kim, S.J.: Synthesis and characteristics of interpenetrating polymer network hydrogel composed of chitosan and poly(acrylic acid). J. Appl. Polym. Sci. 73, 113–120 (1999). https://doi.org/10.1002/app.11008
Elizalde-Peña, E.A., Flores-Ramirez, N., Luna-Barcenas, G., Vásquez-García, S.R., Arámbula-Villa, G., García-Gaitán, B., Rutiaga-Quiñones, J.G., González-Hernández, J.: Synthesis and characterization of chitosan-g-glycidyl methacrylate with methyl methacrylate. Eur. Polym. J. 43, 3963–3969 (2007). https://doi.org/10.1016/j.eurpolymj.2007.06.004
Torabi, S., Mahdavian, A.R., Sanei, M., Abdollahi, A.: Chitosan and functionalized acrylic nanoparticles as the precursor of new generation of bio-based antibacterial films. Mater. Sci. Eng. C. 59, 1–9 (2016). https://doi.org/10.1016/j.msec.2015.09.096
Iordache, M.L., Dodi, G., Hritcu, D., Draganescu, D., Chiscan, O., Popa, M.I.: Magnetic chitosan grafted (alkyl acrylate) composite particles: Synthesis, characterization and evaluation as adsorbents. Arab. J. Chem. 11, 1032–1043 (2018). https://doi.org/10.1016/j.arabjc.2015.12.010
Podkościelna, B.: The influence of oxidation number of sulfur on the polymerization and thermo-mechanical properties of dimethacrylate copolymers. J. Therm. Anal. Calorim. 111, 1553–1560 (2013). https://doi.org/10.1007/s10973-012-2483-3
Podkościelna, B.: New photoluminescent copolymers of naphthalene-2,7-diol dimethacrylate and N-vinyl-2-pyrrolidone: Synthesis, characterisation and properties. J. Therm. Anal. Calorim. 116, 785–793 (2014). https://doi.org/10.1007/s10973-013-3602-5
Podkościelna, B.: The highly crosslinked dimethacrylic/divinylbenzene copolymers: Characterization and thermal studies. J. Therm. Anal. Calorim. 104, 725–730 (2011). https://doi.org/10.1007/s10973-010-1184-z
Szymczyk, K., Podkościelna, B.: Synthesis and wettability of cellulose based composites by aqueous solutions of nonionic surfactant. Colloids Surf. Physicochem Eng Asp. 623 (2021). https://doi.org/10.1016/j.colsurfa.2021.126709
van Oss, C.J.: Interfacial Forces in Aqueous Media. Marcel Dekker, New York (1994)
van Oss, C.J., Chaudhury, M.K., Good, R.J.: Monopolar surfaces. Adv. Colloid Interface Sci. 28, 35–64 (1987). https://doi.org/10.1016/0001-8686(87)80008-8
van Oss, C.J., Good, R.J., Chaudhury, M.K.: Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir. 4, 884–891 (1988). https://doi.org/10.1021/la00082a018
Drelich, J., Chibowski, E., Meng, D.D., Terpilowski, K.: Hydrophilic and uperhydrophilic surfaces and materials. Soft Matter. 7, 9804–9828 (2011). https://doi.org/10.1039/c1sm05849e
Zdziennicka, A., Krawczyk, J., Szymczyk, K., Jańczuk, B.: Components and parameters of liquids and some polymers surface tension at different temperature. Colloids Surf. Physicochem Eng Asp. 529, 864–875 (2017). https://doi.org/10.1016/j.colsurfa.2017.07.002
Cunha, A.G., Fernandes, S.C.M., Freire, C.S.R., Silvestre, A.J.D., Neto, C.P., Gandini, A.: What is the real value of chitosan’s surface energy? Biomacromolecules. 9, 610–614 (2008). https://doi.org/10.1021/bm701199g
Çaykara, T., Alaslan, A., Eroǧlu, M.S., Güven, O.: Surface energetics of poly(N-vinyl-2-pyrrolidone)/chitosan blend films. Appl. Surf. Sci. 252, 7430–7435 (2006). https://doi.org/10.1016/j.apsusc.2005.08.092
Szymczyk, K., Zdziennicka, A., Jańczuk, B.: Effect of polysorbates on solids wettability and their adsorption properties. Colloids and Interfaces. (2018). 2https://doi.org/10.3390/colloids2030026
Acknowledgements
The authors would like to thank COST Action CA18220 - European network of FURan based chemicals and materials FOR a Sustainable development (FUR4Sustain) for making it possible to exchange experience with the other scientists.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
K.F., B.P., and K.S. wrote the main manuscript text and figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fila, K., Podkościelna, B. & Szymczyk, K. The application of chitosan as an eco-filler of polymeric composites. Adsorption 30, 157–165 (2024). https://doi.org/10.1007/s10450-023-00403-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00403-0