Abstract
Graphene-based polymer composites with improved physical properties are of great interest due to their lightweight, conductivity, and durability. They have the potential to partially replace metals and ceramics in several applications which can reduce energy and cost. The obtained properties of graphene-based polymer composites are often linked to the way graphene is dispersed in the polymer matrix. Preparation techniques like solution mixing, melt blending, and in-situ polymerization have been used to obtain graphene-based polymer composites. Dispersing and aligning graphene fillers within the composite is a key factor in enhancing the thermal and electrical conductivity values of the composites due to graphene’s anisotropic properties. The effect of the preparation methods of these composites on their physical-chemical properties is discussed in this review where we presented the advances that were achieved so far in the preparation techniques used showing the highest values ever achieved for electrical and thermal conductivity for these graphene-based polymer composites. Also, we presented the possible applications where graphene-based composites can be utilized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Graphene is a two-dimensional network of carbon atoms that are organized in a honeycomb structure with a specific surface area of 2600 m2/g and very high thermal, mechanical, and electrical properties making it the wonder material of the 21st century [1]. It can also be used as a filler that can dramatically improve the thermal, physical, and mechanical properties of polymers even with low filler loading [2, 3]. The Young's modulus and the mechanical strength of a defect-free single monolayer of graphene can reach up to 1 TPa and 130 GPa, respectively making it 200 times stronger than steel of a similar thickness [4]. The in-plane thermal conductivity for a defect-free single-layer of graphene has a thermal conductivity value of 3000–5150 W/mK at room temperature making it one of the most thermally conductive materials ever known [5]. The electrical conductivity of graphene can reach up to 6000 S/cm making it a very conductive carbon-based filler conducting electricity better than Copper [6]. In addition to that, graphene has other favorable properties like being non-toxic, anti-microbial, and flexible as shown in Fig. 1 [1, 7, 8].
Graphene is a transparent 2D material with incredibly high physical-chemical properties. It has a Young’s Modulus of ~ 1 TPa, electrical conductivity of ~ 6000 S/cm, and in-plane thermal conductivity of ~ 5000 W/mK. Graphene and its derivatives have very promising antimicrobial properties and good flame-retardant performance [9,10,11]
These superior properties of graphene attracted many researchers to use it as a filler in different polymers to improve the electrical, thermal, and mechanical properties of a polymer matrix that is usually thermally and electrically insulative [3, 12, 13]. Graphene has a higher surface-to-volume ratio compared to Carbon nanotube (CNT), which makes it a favorable filler for improving the properties of polymer matrices [12]. Therefore, in the last decade, graphene was added to many polymers including polyvinylidene fluoride (PVDF) [14], poly(ethylene-co-methacrylic acid) (PEMMA) [11], poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) [4], and polyethylene (PE) [15]. For this purpose, researchers used different manufacturing methods to fabricate graphene-based polymer composites with enhanced physical properties using melt blending, solution mixing and molding process, and in-situ polymerization. Researchers were able to achieve graphene-based polymer composites with good thermal and electrical conductivity making these composites semiconductors. These composites will partially replace metals and ceramics because of their lightweight, low cost, and resistance to corrosion allowing researchers to use this material in many applications like electronics, energy storage, e-textiles, and heat sinks [16,17,18,19,20].
Different parameters affect the physical-chemical properties of the resulting graphene-based polymer composites including (1) the type of graphene used and its intrinsic properties which are related to the purity, size, and aspect ratio of the graphene filler [4, 10], (2) the level of wrinkling and defects in graphene and the polymer composite [21], (3) the interfacial interaction between the polymer and graphene, and (4) the network structure of graphene in the matrix [12]. All these parameters have a direct effect on the properties of obtained graphene-based polymer composites affecting the thermal conductivity, the electrical conductivity, and the mechanical properties of these composites.
Graphene can form a conductive network providing conductive channels within the insulative polymer matrix. However, the dispersion of graphene within the polymer matrix is usually a challenge as poor dispersion may occur due to the strong Van der Waals force of attraction between graphene fillers during the preparation process [3]. The poor dispersion of graphene in various polymers and their tendency for agglomeration negatively impact the physical properties of the resulting composites [10]. Nevertheless, with the development of different fabrication techniques for graphene, and the techniques used to improve the physical properties of obtained composites, these challenges are being gradually overcome. In this review paper, the preparation approaches of graphene-based polymer composites are introduced, described, and compared. The parameters that are involved in choosing the preparation process that is suitable for a specific graphene filler and polymer types are also discussed showing the advances in the electrical and thermal conductivity values that were achieved so far in the last decade. To the best of our knowledge, a limited number of previous review papers studied thoroughly the effect of the preparation methods on the properties of obtained composites with a pure focus on graphene-based polymer composites [22,23,24,25].
2 Properties of Graphene and Carbon-Based Fillers
Two main kinds of conductive fillers can be added to polymers to improve their physical properties: metallic fillers and Carbon-based fillers. Metallic fillers like Copper or Silver particles are being added to polymers. Also, Carbon-based fillers that include graphene oxide, graphite, and Carbon nanotubes (CNT) with their respective properties are shown in Table 1.
The properties of the conductive filler will affect the physical properties of the obtained polymer composites. For example, the effective thermal/electrical conductivity of the composites obtained is usually approximated by the weighted average of conductivities of the filler and the matrix according to the rule of mixing [4]. However, the purity of the fillers and the interfacial bond between the filler and the matrix are critical. Fillers with high purity will be more conductive and will have better intrinsic properties [21, 33]. Therefore, achieving higher values for electrical and thermal conductivity for obtained composites will be possible while using defect-free graphene fillers that are well dispersed and aligned across the matrix. Another parameter that also affects the properties of these composites is the shape, size, and aspect ratio of the Carbon-based filler [10]. One of the most challenging issues while using Carbon-based fillers is the dispersion of the filler within the polymer matrix. Large amounts of wrinkling of graphene usually cause a low aspect ratio and decrease the effectiveness of the graphene flakes within the composites [34]. Also, fillers with large aspect ratios can help in achieving high electrical and thermal conductivity values, as presented by many researchers [9, 35]. Carbon-based fillers like CNT and graphene tend to agglomerate within the polymer matrix, which may decrease the efficiency of these fillers within the composite. If graphene layers are not well separated from each other, it usually creates irreversible agglomerates or even restacks to form graphite through Van der Waals interactions. The special properties of graphene are only associated with individual sheets, so it is essential to have a uniformly distributed and segregated structure with no aggregation based on the process that is suitable for graphene fillers. Therefore, it is very critical to choose the right preparation process that can suit the shape and the size of the filler within the polymer matrix.
3 Preparation Methods of Graphene-Based Composites
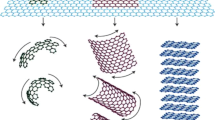
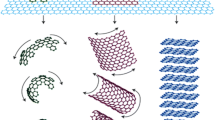
As shown in Fig. 2 most polymers that are thermally and electrically insulating become semiconductors when adding graphene improving both the electrical and thermal conductivity of polymer composites [36].
The improvement in the thermal and electrical conductivity of graphene-based polymer composites in comparison to metals and polymers [36]. BN Boron Nitride, GO Graphene Oxide, GN Graphene Nanosheets
Many techniques were studied to prepare graphene-based polymer composites with improved properties, including melt blending, solvent blending, and in-situ polymerization [37]. The key to obtaining enhanced properties by these preparation methods is the uniform and proper dispersion of the graphene filler in the polymer matrix. These fillers can form conductive networks to provide good conductivity for electrons and heat with low resistance across the polymer matrix. The interface between the polymer and the graphene filler plays a vital role in composites. Heat in composites is transported by phonons which are the main form of thermal conductance in Carbon-based materials, but the bad coupling in vibration modes at the graphene-polymer interface will generate massive thermal resistance causing these phonons to scatter reducing the thermal conductivity of these composites. The excellent bonding between graphene and the polymer can efficiently decrease the phonon scattering and eventually increase the thermal conductivity of the composite [3]. Graphene-based conductive fillers that have a specific shape, size, and aspect ratio can be added to insulating polymers to improve the properties of these composites as shown in Fig. 2. The critical issue in any preparation process is distributing the graphene evenly in the polymer matrix to obtain efficient processability. An even distribution will form multiple conductive channels where phonons and electrons can be transported across the composite [6, 12].
3.1 Solvent-Based Blending Method
The solvent-based blending method is mainly based on a solvent system where the polymer is soluble, and the graphene filler is added to the solution and mixed. In general, solution blending methods can be categorized into multiple techniques including Casting/molding, electro-spinning, and coating which are all solvent-based methods that can be used to prepare graphene-based polymer composites. The solution blending process is a widely used method for the preparation of graphene-based polymer composites since it is relatively direct, quick, and scalable. Tarhini et al. used this method to prepare their graphene-based polymer composites as shown in Fig. 3a-b [4, 11]. The molding process involves dissolving the polymer and suspending the filler (graphene as a Carbon-based filler) in a solvent as shown in Fig. 3b under continuous mixing. Both solutions were mixed to form composites with various graphene weight %. The mixed solution was poured into a silicon mold and dried to evaporate the solvent to obtain graphene-based polymer composites. Therefore, the solvent-based blending method that includes the molding technique is usually a process with three parts that include first dispersing the graphene and the polymer in a suitable solvent, then mixing the graphene suspension with the polymer solution, and finally pouring the mixture into a mold to dry the composites and evaporate the solvent. During this process, proper dispersion of the graphene filler is needed in the polymer matrix, and this can be achieved by vigorous stirring and ultra-sonication to make sure that graphene fillers are not aggregated in the mixture. After solvent evaporation, the polymer chains reassemble, and this will hold the graphene filler together in the composite creating multiple conductive channels. The advantage of this preparation method is that using a solution-based method allows researchers to obtain a lower viscosity solution allowing uniform dispersing and mixing of the graphene filler across the polymer matrix. One disadvantage of this process is that finding a compatible solvent for the polymer and the filler can be challenging. Also, one of the main concerns in solvent-based blending is the cost of a large amount of solvent used during this process. The evaporated solvent should be recovered and reused to safeguard the environment and reduce the cost of preparation. The precipitated composite can then be extracted, dried, and then processed using other techniques. This process has been widely reported in the literature before [9, 11, 38]. It was used for graphene and graphene derivatives like Graphene oxide (GO). Also, it was applied to various polymers like polyacrylamide, poly (methyl methacrylate) (PMMA), polycarbonate, and polyimides [4, 11]. This technique offers a simple route to disperse Carbon-based fillers in different polymers as shown in Figs. 2 and 3.
(a) A schematic of a solvent-based blending process (b) A solution blending process where the polymer (PVDF-HFP) and graphene flakes (GNF) were dispersed in a solvent DMF (Dimethylformamide) to prepare graphene-based polymer composites [4] (c) Schematic of melt blending process (d) Schematic of in-situ polymerization process of graphene-based polymer composites
Different researchers tried to use solvent-based blending methods to fabricate Carbon-based composites with enhanced properties. Yu et al. used this solution blending to prepare graphene-based PVDF composites and they improved the in-plane thermal conductivity of the polymer reaching a value of 0.45 W/mK only by adding 0.5 wt% of the graphene filler [39]. Cao et al. used solution blending and used different Carbon-based fillers: two-dimensional graphene Sheets, one-dimensional Carbon Nanotubes (CNT), and zero-dimensional fullerene with PVDF under the same loading [38]. The thermal conductivity of graphene/PVDF reached a value of 2.06 W/mK at a filler loading content of 20 wt%, which is an increase of about ten times higher than that of neat PVDF. Coating and laminating substrates with a protective layer of polymer containing graphene to enhance their in-plane thermal and electrical conductivities were also done by other researchers [40]. Malekpour et al. prepared some highly conductive graphene laminates on polyethylene terephthalate (PET) substrates using a slit coating machine [41]. They succeeded in coating PET films with a graphene layer improving the thermal conductivity of the composite by 600 times that of the original film. Figure 4a-b presents cross-sectional SEM images of an uncompressed sample and a compressed sample of graphene laminate on PET with the corresponding thermal conductivity value as a function of graphene flake size. Compressed samples with larger average graphene flake size showed higher in-plane thermal conductivity than uncompressed samples with lower average graphene flake size which show the importance of large average graphene flake size and compressing graphene-based composites in improving the in-plane thermal conductivity of the samples obtained. Other coating techniques, such as the air-brush technique, are also used and being widely studied to obtain some conductive composites that can be used for many applications [3, 42]. Delfaure et al. used this technique for the fabrication of electrodes and supercapacitor cells [43]. They reported an air-brush technique with specific parameters that they used to produce highly regular mats with finely tuned thicknesses and graphene weight [43]. The airbrush technique is easy and scalable, but the main disadvantage is that it is hard to control the orientation and the dispersion of the graphene filler across the substrate using this technique.
SEM images for the cross-section of the (a) uncompressed composite and (b) compressed graphene layer on Polyethylene terephthalate (PET) (c) In-plane thermal conductivity of polymer composites as a function of graphene average flake size. [41] (adapted from [41], copyright 2014, with permission from American Chemical Society)
3.2 Melt Blending
Melt blending is a preparation process that is usually used with thermoplastic polymers. During this process, the polymer is first melted and then combined with a specific amount of Carbon-based filler using high-shear mixers at a temperature above the melting temperature of the polymer or the glass transition point as shown in the schematic of Fig. 3c. Hence, it does not require a solvent to combine both the graphene filler and the polymer. It is usually the preferred method for industrial applications because it can be used simply for large-scale production systems because of its low cost and simplicity. It is also known to be environmentally friendly because it does not need any solvent during the process. Therefore, this process avoids the use of solvents that may harm the environment later. However, melt blending is not as effective as a solution blending method, in terms of the ability to achieve good dispersion of the graphene filler within the polymer matrix [44]. Also, the use of high shear forces can sometimes break the graphene flakes and reduce their effectiveness in the matrix. Melt blending was used a lot with CNT, and it was reported for various polymer types, including polyesters, polyolefins, polystyrene, polyamides, and polyurethane. However, melt blending is generally less effective compared to solvent blending with dispersing CNT in polymers, and it is only limited to lower concentrations of the filler because of the high viscosity of the composites at higher nanotube loadings [26]. Similarly, to CNT, melt blending is less effective in dispersing graphene sheets compared to solvent blending due to the higher viscosity of the composite at increased sheet loading. Besides, this process can be applied to both polar and non-polar polymers. However, it is more practical with thermos-plastic polymers, especially in large-scale production systems [45]. Melt blending or mixing can also be categorized into multiple categories: Extrusion, hot-pressing, and compaction/injection molding. These are different manufacturing techniques that are based on melting and mixing the polymer with Carbon-based fillers without the presence of any solvent. The main parameters that are considered in these manufacturing processes are temperature, pressure, and time [46]. Usually, a higher temperature is used than the melting temperature of the polymer coupled with shear forces to incorporate the graphene filler with the polymer matrix. Also, high pressure is applied to shape melted blends in a specific mold, as for screw mixers that are used to mix both the polymer with the graphene filler. Compaction molding is a process that was used by Jung et al. due to the anisotropic behavior of the graphene flakes, un-aligned graphene composites had inferior properties compared to aligned-graphene composites [47]. Jung et al. tried to have a proper orientation of graphene layers along the matrix direction using a melt compression process using an L-shaped tube increasing the thermal conductivity to 10 W/mK at 25 vol % of graphene flakes in PVDF [47]. Also, the electrical conductivity of the composites obtained increased to reach 30 S/m for 25 vol % of graphene flakes. Therefore, they were able to achieve high in-plane thermal conductivity values for the composites obtained using the compaction molding technique since they directed most of the graphene flakes in one direction, improving the alignment of these flakes across the composite. Also, injection molding is a standard method that is used where specific molds are used to inject the melted blend of the polymer with the graphene filler in defined shapes at high pressure to achieve the intended design. Complex structures with excellent finish can be obtained by injection molding. Therefore, it is commonly used in the automotive industry, where many structures are created using this technique. Graphene 3D printing is becoming very popular in many industries, especially for nano-scale industries like biomedical applications, and it is based on the extrusion method. A suspended conductive graphene-based solution with a specific viscosity is used within extrusion-based 3D printers to print nano-electric circuits that can be used for many applications. Inkjet-printed thin films of liquid-exfoliated graphene were reported by various researchers lately [13]. Araby et al. reported that printing graphene flakes to form a film with different thicknesses is possible while improving the electrical conductivity of the film up to 3000 S/m. Another method that is based on melt blending is hot pressing. In the hot-pressing technique, a thin film for a graphene-based composite can be obtained when the film is placed between two heated flat plates at high pressure to obtain a thin film with improved physical properties. This process is commonly used with thermoplastic polymers. Many researchers checked the effect of hot pressing on graphene-based composites, and they found that this technique decreases porosities within the composite and lowers the distance between the graphene flakes by heat pressing, thus improving the physical properties in terms of thermal and electrical conductivity of the composites obtained [48].
3.3 In-situ polymerization
In-situ polymerization is used in preparing graphene-based polymer composites where graphene derivatives are incorporated during the polymerization of the monomer to improve the dispersion of the derivative between two phases as shown in Fig. 3d. In this process, a monomer solution and suspensions of graphene-based materials are mixed under suitable reaction conditions and in the presence of catalysts to obtain a graphene-based polymer composite providing a strong interaction between the matrix and the filler. Epoxy is usually a typical polymer that is suitable for in-situ polymerization. A graphene suspension can be mixed with epoxy resins and subjected to high-shear mixing. While stirring and heating the mixture to remove the solvent, the epoxy curing agent can be added to finalize the polymerization process [49]. This technique can be used on graphene and graphene derivatives like Graphene oxide (GO) and Reduced Graphene Oxide (rGO). In-situ polymerization is significant for the preparation of insoluble and thermally unstable polymers because such matrices cannot be dissolved in solvents or fused. Therefore, they cannot be processed by solution or melt blending [25]. Furthermore, in-situ polymerization methods enable covalent bonding between functionalized Carbon-based filler and the polymer matrix using various chemical reactions. In-situ polymerization has also shown great potential in manufacturing polyolefin/graphene nanocomposites, mainly graphene-based polypropylene, Linear low-density polyethylene, and ultra-high-molecular-weight polyethylene composites [25]. Song et al. prepared graphene/epoxy composites using in-situ polymerization and succeeded in enhancing the thermal conductivity of the composites by 66.5% [50]. Also, in-situ polymerization was used by Ma et al. on Carbon-based filler with polyurethane achieving a high thermal conductivity of 0.3 W/mK for a filler weight % of 0.6% [51]. Wang et al. dramatically improved the mechanical and thermal properties of epoxy by adding graphene platelets using in-situ polymerization. This technique was used by Kim et al. where they dispersed thermally reduced graphene within thermoplastic polyurethane using all three techniques (solution mixing, melt blending, and in situ polymerization) and studied the distribution obtained by TEM images [52]. They found that in-situ polymerization produced the best dispersion for the graphene filler in the matrix. Despite its several advantages including presenting an effective method that allows Carbon-based fillers to be dispersed uniformly in the matrix, thereby providing a strong interaction between the matrix and the filler, the in-situ polymerization has some shortcomings. It is not very popular for the preparation of specific sets of materials because it requires low-viscosity monomers and other starting materials. Furthermore, the major disadvantage of this method is the increase of viscosity along with the preparation progress of the polymerization process which hinders the manipulation and limits the load fraction of the graphene filler in the polymer matrix [53]. By employing the methods above (solution blending, melt blending, in-situ polymerization), graphene and graphene derivatives are mostly dispersed in the polymer matrix. The advantages and shortcomings of each preparation method are summarized in Table 2. Multiple techniques can be used to enhance the dispersion and alignment of graphene filler in the composites including hot pressing and ultrasonication. These techniques have a direct effect on the thermal, electrical, and mechanical properties of the obtained composites.
4 Electrical Conductivity of Graphene-Based Composites
The electrical conductivity is dictated by the flow of free electrons in the lattice. In electrically conductive metals, free valance electrons can be excited quickly due to their band structure in crystalline structures. Whereas in insulators large excitation energy is needed due to the large bandgap [37]. Most polymers are insulators with electrical conductivity values ranging between 10-14 to 10-17 S/cm [54]. Due to graphene’s large aspect ratio, graphene is considered one of the most efficient fillers for improving electrical conductivity as simulation and experimental results showed that fillers with large aspect ratios led to better electrical conductivity [13]. Table 3 summarizes the recent values obtained for the electrical conductivity of different graphene-based polymer composites that were prepared using different preparation methods.
The enhancement of electrical conductivity depends on the preparation method used to fabricate graphene-based polymer composites. The polymers used are usually insulators, and the corresponding electrical conductivity of the graphene-based polymer composites corresponds to a significant improvement in the electrical conductivity making these composites comparable to conductive metals. Moreover, this conductivity is also attributed to good interfacial interaction between the graphene filler and the polymer matrix, the high aspect ratio of the graphene filler, and the preferential orientation of graphene fillers along the film direction which improves the electrical pathway where electrons can pass across the composite [9, 60, 61]. The improvement of the electrical conductivity of the graphene-based polymer composite usually follows a percolation model where the filler volume fraction should surpass a threshold value for the polymer composite to be conductive as shown in Fig. 5 [13, 62].
(a) The electrical conductivity of the composite plotted follows a percolation model [13]. (b) A plot of the electrical conductivity as a function of the filler loading for graphene and graphite filler in PET [63] (adapted from [63], copyright 2010, with permission from Elsevier). (c) Example plot of percolation threshold of CNT and graphene in High-density polyethylene (adapted from [64], copyright 2011, with permission from Elsevier)
For a random distribution of Graphene in the polymer matrix, a conductive network of graphene after a specific loading of the filler is called the percolation threshold. Then after adding more filler, the electrical conductivity of the graphene-based polymer composite increased rapidly and the graph of the electrical conductivity takes an S shape with three main sections of the graph: The insulating region, the percolation region where tunneling between graphene happens within the composite, and the conductive region as shown in Fig. 5 [4, 13]. In the insulative region, there is no conductive path, then the electrons cannot flow, and the composite remains insulative due to the insulative matrix. However, when the graphene fillers can form multiple conductive channels across the composite, electrons can flow, and the composite becomes conductive. Agglomerations, defects, and the size of the graphene filler affect the percolation threshold as seen by both simulations and experiments [6, 10, 12, 59]. Graphene fillers with large aspect ratios lead to reduced percolation thresholds and higher electrical conductivity [10, 13].
5 Thermal Conductivity of Graphene-Based Polymer Composites
Most polymers have very low thermal conductivities in the range of 0.1 - 0.5 W/mK. However, metals have very high thermal conductivity values that can reach up to 385 W/mK for copper, for example. Polymers are made from repeated structural unit chains like propylene, vinyl chloride, ethylene, and styrene. In polymers, heat will reach first the units in the structure that are the closest to the heat source then it will slowly propagate to the neighbor units. Heat is modeled as vibrational waves that at low frequencies (less than 100 kHz) are referred to as phonons [65]. Phonons can travel linearly through a continuous path of chemically bonded atoms in solids. Therefore it is expected that phonons would be affected and scattered by the random curvature along a polymer chain axis [65]. This scattering across the polymer makes heat propagate slower which reduces the value of the thermal conductivity for the polymer matrix [48]. So it is reported that phonons in amorphous polymers typically propagate for less than 10 nm since they cannot propagate far away due to the random curvature and structure of the polymer chains which is different than crystalline structures like in metals [66]. In crystalline structures, heat dissipates as a wave across the whole lattice structure. Due to this reason, the thermal conductivity values for metals are much higher than for polymers. Researchers expected a dramatic enhancement in the thermal conductivity of graphene-based polymer composites after adding high conductive graphene fillers with thermal conductivity of ~3000-5000 W/mK into polymers that have low thermal conductivity value (typically in the range of ~0.2 W/mK). Following the rule of mixing the expected value of the obtained thermal conductivity of the polymer composites should be in the range of ~300 W/mK for 10 wt.% of graphene [38, 47]. However, that was not the case, and the values were instead in the range of 0.3-10 W/mK. This was due to many parameters that affect the properties of graphene-based composites including the graphene shape and properties, the interface between the graphene and the polymer, and the polymer properties. After adding graphene to the polymer matrix, many interfaces are produced, which lead to phonon scattering and the presence of high interfacial resistance called Kapitza resistance as shown in Fig. 6 [67].
The thermal conductivity in solids can be separated into two main components:
where k is the thermal conductivity of the composite, \({k}_{e}\) is the thermal conductivity that is caused by energized electrical motion, and \({k}_{p}\) is due to energized collisions (phonons) motion. For conductive polymers, the thermal conductivity that happened because of electron motion can be estimated using the Wiedermann-Franz law:
where the thermal conductivity is caused by the motion of electrons in the composite \({k}_{e}\) can be obtained by multiplying the Lorentz constant (\({L}_{0}=2.44 \times {10}^{-8}\Omega W{K}^{-2}\)) to the electrical conductivity of the graphene-based composite (\({\sigma }_{e})\) and temperature (T). And since most graphene-based polymer composites are semiconductors with an electrical conductivity reaching a range of 2000 S/m, then the thermal conductivity that is contributed by the motion of electrons in graphene-based composites can reach a range of 0.01 W/mK which is very low compared to the total thermal conductivity of the composite. Thus, we can estimate in graphene-based composites that phonons only contributed to the thermal conductivity of these polymer composites. And the focus while studying the thermal conductivity of these composites can be the scattering mechanisms of these phonons in graphene-based polymer composites. Different scattering mechanisms may happen across graphene-based polymer composites from boundary scattering to impurity scattering and defect scattering. The recommended step for preparing a composite with high thermal conductivity would be using a very pure and conductive graphene filler in the polymer matrix. Also, it is advised to use a preparation method that would increase the number of graphene pathways through the proper orientation of graphene filler. This will reduce the resistance between graphene and the graphene-polymer interface, thus reducing the Kapitza resistance [69]. Table 4 summarizes the recent values achieved so far for in-plane thermal conductivity by different research groups recently. Researchers use different techniques like hot-pressing and milling to properly disperse and align graphene flakes in a specific direction across the polymer composite trying to improve the thermal conductivity value of the composite.
These high thermal conductivity values were attributed to the increasing thermal transport capacity in these graphene-based polymer composites due to the alignment of graphene layers across the composite film. The proper orientation of the graphene filler within the composite would provide a path of lower thermal resistance for phonon travel which explains the high thermal conductivity values for some composites that were prepared with specific preparation techniques that provided proper dispersion and alignment of graphene filler in a specific direction. Other factors like the purity of the graphene filler, the crystalline morphology of the polymer matrix, and the hydrophobic interaction between graphene and the polymer are also some factors that can reduce the thermal resistance between the graphene filler and the polymer matrix reducing the boundary phonon scattering across the polymer composites and thus improving the in-plane thermal conductivity values [4].
6 Applications of Graphene-based Polymer Composites
In the last decade, polymer materials with high thermal and electrical conductivity values were required in many emerging industries. Graphene-based polymer composites can be used in many applications such as wearable/stretchable electronic devices, E-textile, batteries, and sensors [3, 28, 72,73,74]. They are lightweight, durable, and conductive materials and they can be metallic components that are typically rigid, heavy, rigid, and prone to corrosion with a high thermal expansion coefficient.
6.1 Energy Storage
Many advances are happening in energy storage systems like batteries and capacitors in the last few years. Due to the high charging/discharging rates of batteries in some cases, the heat generated by these batteries can be larger than the heat dissipated. This mismatch can cause the overheating of the battery causing the battery to be very inefficient with time and maybe catch fire in other cases. Therefore, some researchers tried to use new materials like graphene to improve the performance of batteries in many applications. Graphene fillers were used in lithium-ion batteries to improve their sustainability and performance [75]. Many researchers like Song et al. have developed a new cathode by combining graphene with other polymers like (poly(anthraquinonyl sulfide) and polyimide) in lithium batteries to improve the performance of the battery. Graphene and its derivatives were combined with other polymers in supercapacitors showing a very high electrochemical capacitance (210 F/g) at a discharge rate of 0.3 A/g [76]. Graphene can reduce the charging time and extend the battery’s while having excellent electrical conductivity and a large surface area which improves the efficiency of batteries. The electrochemical analysis was presented in Fig. 7 for different graphene-based polymer composite films to study their efficiency and performance as a material used in batteries showing their electrochemical impedance under typical aqueous conditions. the cyclic voltammetry curves for graphene-based composites showed a typical rectangular behavior depicting an excellent super capacitive behavior. Also, the charge-storage capacity of the composites increased for higher graphene wt% and the impedance values dramatically decreased after adding graphene filler to PVDF-HFP polymer as shown in Fig. 7 [77].
(a) cyclic voltammetry for different graphene-based composite films (b) Charge-storage capacity of graphene-based composites with various graphene wt% (c) Electrochemical impedance spectroscopy of composite films across a different frequency range [77]
6.2 E-Textile
Smart fabric or electronic textiles (E-textiles) are a new segment in the wearable cloth category where embedded electronic devices like sensors, batteries, and computing devices are being added to the cloth to improve their functionality. This can be either aesthetic or performance-enhancing and monitoring. Different materials can be used in smart fabrics which can range from traditional materials like polyester, nylon, and cotton, to advanced composites where conductive graphene-based composites can be used. Producing graphene-based polymer composites with high electrical conductivity and flexibility will be of massive interest in this industry as these composites will sustain the properties of the polymer matrix (flexibility and resistance to corrosion) while being very conductive. Also, many researchers tried to incorporate graphene in textiles for human sensing applications as shown in Fig. 8 where the finger, wrist, elbow, and leg movements were recorded using the change in the resistance of graphene/textile composites [17].
6.3 Thermal Interface Materials (TIM)
Thermal management is the process of monitoring and controlling temperatures produced by devices in electrical enclosures and is a very crucial issue for electronic devices. Electronic devices have become smaller with high power density which dictates the presence of an effective heat conduction path that can lower the temperature of the components in the whole device. Therefore, thermal interface materials (TIM) with high thermal conductivity values are used to provide this effective heat conduction path in these devices. And with the increasing power density of electronic devices, there has been a massive demand for the development of a lightweight TIM with very high thermal conductivity for handling the problem of system overheating within different electrical devices [74]. The thermal conductivity for a single layer of the graphene sheet is in the range between 3080–5150 W/mK which makes graphene and its derivatives a very promising filler [78, 79]. So, developing a light polymer composite with low density and high thermal conductivity is very critical in electronic devices. The role of a TIM is to have a considerable drop in the temperature of the whole system when filling the gaps with a TIM. This need for TIM to dissipate heat very quickly when the electronic chips are working can be very exciting since existing commercial TIM has a relatively low thermal conductivity value which is less than 5 W/mK and improving their conductivity by adding graphene filler will gain a lot of attention in the electronics industry. Shahil et al showed that graphene nanocomposite can be used as the thermal interface material, and it performed better than composites with carbon nanotubes or metal nanoparticles due to graphene’s aspect ratio and lower Kapitza resistance at the graphene–matrix interface as shown in Fig. 9 [16].
6.4 Biomedical Applications
Graphene-based polymer composites have many interesting properties such as biocompatibility, and high physical, chemical, optical, and sensing properties that allow researchers to use this material in many biomedical applications [24]. Graphene has been conjugated with chitosan and biocompatible polyethylene glycol (PEG). This composite showed better stability and solubility in physiological solutions and has been studied for in vitro drug delivery, imaging, and in vivo photothermal therapy for tumors [45]. Other graphene-based hybrid materials were tested and used in drug and gene delivery, tissue engineering, bio-sensing, molecular imaging, and bioelectronics [80,81,82]. Also, graphene-based material can be used as transducers for biosensors because of their electrical conductivity, large surface area, and high electron transfer rate [24, 80] (Figs. 8 and 10).
Graphene-based polymer composites have the potential to be used in many biomedical applications (a) Sensing applications for the nervous system (b) Metabolic sensors (c) Bioelectric electrodes (d) Strain sensors (e) Biological receptors (f) Invasive sensor [24]. (adapted from [24], copyright 2022, with permission from Informa UK Limited)
7 Summary and Outlook
In this review, we introduced different preparation methods for fabricating graphene-based polymer composites. These methods are essential in dictating the physical properties of obtained composites. For fabricating graphene-based composites with high thermal, electrical, and mechanical properties, proper dispersion, and alignment of Carbon-based filler are required. Although there are a lot of advantages to using solution mixing and in situ polymerization methods to produce composites with a high degree of dispersion and homogeneity, as shown by many researchers, these processes have other drawbacks, especially the environmental concerns that are needed to be considered while applying these methods. The melt-mixing process is a commonly used method in industrial applications since it is easy to scale up, but the user will not get a similar dispersion as in the methods above. Incorporating Carbon-based filler in polymer composites usually enhanced the thermal, electrical, and mechanical properties. However, these enhancements are subject to many parameters such as the dispersion of graphene filler in the matrix, the graphene properties and filler loading, the interfacial contact between the graphene filler and the matrix, and the preparation method. Usually, the fabrication method of graphene polymer composites affects the dispersion of the filler in the polymer composites and thus results in different influences on the physical properties of the composites obtained. Also, usually, the highly dispersed graphene filler provides a much higher enhancement in the physical properties than the poorly dispersed ones due to the improved interfacial interactions between the filler and the matrix. Based on the development of graphene-based composites, the use of Carbon-based fillers is very efficient and effective in improving the thermal, mechanical, and electrical properties and thus overcoming some of the disadvantages of the polymer matrix. Graphene and graphene derivatives like graphene flakes and graphene oxide can further improve the physical properties of graphene-based composites. Therefore, this family of graphene-based composite materials with excellent physical and functional properties shows promising results in both conventional and advanced applications ranging from sensors to electronic devices, to heat sinks, to the automotive industry, and many more. In the future, more studies should be done for better incorporation of graphene in polymer matrices where 3D graphene structures can be tested to make very conductive networks across polymer composites. Also, graphene can be added to self-healable polymers where their conductivity can be tested under harsh conditions.
Data availability
The authors confirm that the data supporting the findings of this study are available from the corresponding author and can be shared upon request.
References
Kim, H., Abdala, A.A., Macosko, C.W.: Graphene/Polymer Nanocomposites. Macromolecules 43, 6515–6530 (2010). https://doi.org/10.1021/ma100572e
Shen, X., Wang, Z., Wu, Y., Liu, X., He, Y.B., Kim, J.K.: Multilayer Graphene enables higher efficiency in improving Thermal Conductivities of Graphene/Epoxy Composites. Nano Lett 16, 3585–3593 (2016). https://doi.org/10.1021/acs.nanolett.6b00722
Li, A., Zhang, C., Zhang, Y.-F.: Thermal conductivity of Graphene-Polymer Composites: Mechanisms, Properties, and applications. Polym. (Basel) 9, 437 (2017). https://doi.org/10.3390/polym9090437
Tarhini, A.A., Tehrani-Bagha, A.R.: Graphene-based polymer composite films with enhanced mechanical properties and ultra-high in-plane thermal conductivity. Compos. Sci. Technol 184, 107797 (2019). https://doi.org/10.1016/j.compscitech.2019.107797
Balandin, A.A., Ghosh, S., Bao, W., Calizo, I., Teweldebrhan, D., Miao, F., Lau, C.N.: Superior Thermal Conductivity of single-layer graphene. Nano Lett 8, 902–907 (2008). https://doi.org/10.1021/nl0731872
Aryanfar, A., Medlej, S., Tarhini, A., Tehrani, B.: Elliptic percolation model for predicting the electrical conductivity of graphene–polymer composites. Soft Matter 17, 5258–5258 (2021). https://doi.org/10.1039/d0sm01950j
Ergoktas, M.S., Bakan, G., Steiner, P., Bartlam, C., Malevich, Y., Yenigun, E.O., He, G., Karim, N., Cataldi, P., Bissett, M., Kinloch, I.A., Novoselov, K.S., Kocabas, C.: Graphene-enabled adaptive infrared textiles. Nano Lett (2020). https://doi.org/10.1021/acs.nanolett.0c01694
Sayyar, S., Murray, E., Thompson, B.C., Chung, J., O, D.L., Gambhir, S.: Processable conducting graphene/chitosan hydrogels for tissue engineering. J. Mater. Chem. B. 481–490 (2015). https://doi.org/10.1039/c4tb01636j
Kumar, P., Yu, S., Shahzad, F., Hong, S.M., Kim, Y.H., Koo, C.M.: Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides. Carbon N Y 101, 120–128 (2016). https://doi.org/10.1016/j.carbon.2016.01.088
Tarhini, A., Tehrani-Bagha, A., Kazan, M., Grady, B.: The effect of graphene flake size on the properties of graphene‐based polymer composite films. J. Appl. Polym. Sci 138, 49821 (2021). https://doi.org/10.1002/app.49821
Tarhini, A., Tehrani-Bagha, A.R., Kazan, M.: Graphene‐based polymer composites with ultra‐high in‐plane thermal conductivity: A comparison study between optothermal Raman spectroscopy and laser flash method. J. Appl. Polym. Sci 137, 48927 (2020). https://doi.org/10.1002/app.48927
Aryanfar, A., Medlej, S., Tarhini, A., Damadi, S.R., Tehrani, B., Goddard, A.R.: 3D percolation modeling for predicting the thermal conductivity of graphene-polymer composites. Comput. Mater. Sci 197, 110650 (2021). https://doi.org/10.1016/j.commatsci.2021.110650
Marsden, A.J., Papageorgiou, D.G., Vallés, C., Liscio, A., Palermo, V., Bissett, M.A., Young, R.J., Kinloch, I.A.: Electrical percolation in graphene–polymer composites. 2d Mater. 6, (2018)
Bidsorkhi, H.C., D’Aloia, A.G., Bellis, G.: Nucleation effect of unmodified graphene nanoplatelets on PVDF/GNP film composites. Mater. Today Commun 11, 163–173 (2017). https://doi.org/10.1016/j.mtcomm.2017.04.001
Saeidijavash, M., Garg, J., Grady, B., Smith, B., Li, Z., Young, R.J., Tarannum, F., Bekri, B.: High thermal conductivity through simultaneously aligned polyethylene lamellae and graphene nanoplatelets. Nanoscale 9, 12867–12873 (2017). https://doi.org/10.1039/c7nr04686c
Shahil, K.M.F., Balandin, A.A.: Graphene-multilayer graphene nanocomposites as highly efficient thermal interface materials. Nano Lett 12, 861–867 (2012). https://doi.org/10.1021/nl203906r
Zhou, Y., Myant, C., Stewart, R.: Multifunctional and stretchable graphene/textile composite sensor for human motion monitoring. J. Appl. Polym. Sci. (2022). https://doi.org/10.1002/app.52755
De, S., Purcell, C., Murley, J., Flouda, P., Shah, S., Green, M., Lutkenhaus, J.: Spray-On reduced Graphene Oxide-Poly (vinyl alcohol) supercapacitors for flexible energy and power. Adv. Mater. Interfaces 1801237, 1–9 (2018). https://doi.org/10.1002/admi.201801237
Afroj, S., Karim, N., Wang, Z., Tan, S., He, P., Holwill, M., Ghazaryan, D., Fernando, A., Novoselov, K.S.: Engineering Graphene Flakes for Wearable Textile Sensors via highly scalable and ultrafast yarn dyeing technique. ACS Nano 13, 3847–3857 (2019). https://doi.org/10.1021/acsnano.9b00319
Cataldi, P., Ceseracciu, L., Athanassiou, A., Bayer, I.S.: Healable Cotton-Graphene Nanocomposite Conductor for Wearable Electronics. ACS Appl. Mater. Interfaces 9, 13825–13830 (2017). https://doi.org/10.1021/acsami.7b02326
Malekpour, H., Ramnani, P., Srinivasan, S., Balasubramanian, G., Nika, D.L., Mulchandani, A., Lake, R.K., Balandin, A.A.: Thermal conductivity of graphene with defects induced by electron beam irradiation. Nanoscale 8, 14608–14616 (2016). https://doi.org/10.1039/c6nr03470e
Sang, B., Li, Z., Li, X., hong, Yu, L., gui, Zhang, Z.: Graphene-based flame retardants: A review. J. Mater. Sci 51, 8271–8295 (2016). https://doi.org/10.1007/s10853-016-0124-0
Sattar, T.: Current Review on Synthesis. Springer International Publishing, Composites and Multifunctional Properties of Graphene (2019)
Laraba, S.R., Luo, W., Rezzoug, A., Zahra, Q., ul ain, Zhang, S., Wu, B., Chen, W., Xiao, L., Yang, Y., Wei, J., Li, Y.: Graphene-based composites for biomedical applications. Green. Chem. Lett. Rev 15, 724–748 (2022). https://doi.org/10.1080/17518253.2022.2128698
Tripathi, S.N., Rao, G.S.S., Mathur, A.B., Jasra, R.: Polyolefin/graphene nanocomposites: A review. Royal Soc. Chem. 23615–23632 (2017). https://doi.org/10.1039/c6ra28392f
Han, Z., Fina, A.: Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog Polym. Sci 36, 914–944 (2011). https://doi.org/10.1016/j.progpolymsci.2010.11.004
Ebbesen, T.W., Lezec, H.J., Hiura, H., Bennett, J.W., Ghaemi, H.F., Thio, T.: Electrical conductivity of individual carbon tubes. Nature 382, 54–56 (1996). https://doi.org/10.1038/382054a0
Boden, A., Boerner, B., Kusch, P., Firkowska, I., Reich, S.: Nanoplatelet size to Control the Alignment and Thermal Conductivity in copper – graphite composites. Nano Lett 6, 3640–3644 (2014). https://doi.org/10.1021/nl501411g
Luo, T., Wang, Q.: Effects of Graphite on Electrically Conductive Cementitious Composite Properties. A review (2021)
Costa, L.C., Henry, F.: DC electrical conductivity of carbon black polymer composites at low temperatures. J. Non Cryst. Solids 357, 1741–1744 (2011). https://doi.org/10.1016/j.jnoncrysol.2010.11.119
Pantea, D., Darmstadt, H., Kaliaguine, S., Sümmchen, L., Roy, C.: Electrical conductivity of thermal carbon blacks: Influence of surface chemistry. Carbon N Y 39, 1147–1158 (2001). https://doi.org/10.1016/S0008-6223(00)00239-6
Zhao, Q., Zhang, K., Zhu, S., Xu, H., Cao, D., Zhao, L., Zhang, R., Yin, W.: Review on the electrical resistance/conductivity of carbon fiber reinforced polymer (2019)
Fang, T., Lee, Z., Chang, W., Huang, C.: Determining porosity e ff ect on the thermal conductivity of single-layer graphene using a molecular dynamics simulation. Phys. E Low Dimens Syst Nanostruct 106, 90–94 (2019). https://doi.org/10.1016/j.physe.2018.10.017
Du, J., Cheng, H.: The fabrication, properties, and uses of graphene/polymer composites. Macromolecular Chem. Phys. 213, 1060–1077 (2012). https://doi.org/10.1002/macp
Sharma, A., Thakur, M., Bhattacharya, M., Mandal, T., Goswami, S.: Commercial application of cellulose nano-composites – a review. Biotechnol. Rep 21, e00316 (2019). https://doi.org/10.1016/j.btre.2019.e00316
Liu, L., Bian, X., Tang, J., Xu, H., Hou, Z., Song, W.: RSC advances properties in tunable all-graphene papers †. 75239–75247 (2015). https://doi.org/10.1039/c5ra15533a
Huang, Y., Kormakov, S., He, X., Gao, X., Zheng, X., Liu, Y.: Conductive polymer composites from renewable Resources: An overview of Preparation, Properties, and applications. Polym. (Basel). 1–32 (2019). https://doi.org/10.3390/polym11020187
Cao, Y., Liang, M., Liu, Z.: Enhanced thermal conductivity for poly(vinylidene fluoride) composites with nano-carbon fillers. Royal Soc. Chem 6, 68357–68362 (2016). https://doi.org/10.1039/c6ra11178e
Yu, J., Huang, X., Wu, C., Jiang, P.: Permittivity, Thermal Conductivity and Thermal Stability of Poly (vinylidene fluoride)/ graphene Nanocomposites. IEEE Trans. Dielectr. Electr. Insul 18, 478–484 (2011). https://doi.org/10.1109/TDEI.2011.5739452
Tehrani-Bagha, A.R.: Waterproof breathable layers – A review. Adv. Colloid Interface Sci (2019). https://doi.org/10.1016/j.cis.2019.03.006
Malekpour, H., Chang, K.H., Chen, J.C., Lu, C.Y., Nika, D.L., Novoselov, K.S., Balandin, A.A.: Thermal conductivity of graphene laminate. Nano Lett 14, 5155–5161 (2014). https://doi.org/10.1021/nl501996v
Sarac, M.F., Anderson, B.D., Pearce, R.C., Railsback, J.G., Oni, A.A., White, R.M., Hensley, D.K., Lebeau, J.M., v Melechko, A., Tracy, J.B.: Airbrushed Nickel Nanoparticles for large-area growth of vertically aligned Carbon Nano fi bers on metal (Al, Cu, Ti) Surfaces. Appl. Mater. Interfaces (2013). https://doi.org/10.1021/am401889t
Bondavalli, P., Soc, J.E., Bondavalli, P., Delfaure, C., Legagneux, P., Pribat, D.: Supercapacitor Electrode based on mixtures of Graphite and Carbon Nanotubes Deposited using a dynamic air-brush deposition technique Supercapacitor Electrode based on mixtures of Graphite and Carbon Nanotubes Deposited using a Dynamic Air-Brush deposition. J. Electrochem. Soc (2013). https://doi.org/10.1149/2.048304jes
Lee, S.J., Yoon, S.J., Jeon, I.-Y.: Graphene/Polymer Nanocomposites: Preparation, Mechanical Properties, and application. Polym. (Basel) 14, 4733 (2022). https://doi.org/10.3390/polym14214733
Singh, V., Joung, D., Zhai, L., Das, S.: Graphene based materials: Past, present and future. Prog Mater. Sci 56, 1178–1271 (2011). https://doi.org/10.1016/j.pmatsci.2011.03.003
Zeranska, K., Filak, K., Wilczyński, K., Siemion, A., Palka, N., Godziszewski, K., Yashchyshyn, Y., Zdrojek, M.: Graphene-Based thermoplastic Composites as extremely Broadband and frequency-dependent EMI Absorbers for multifunctional applications. ACS Appl. Electron. Mater 4, 4463–4470 (2022). https://doi.org/10.1021/acsaelm.2c00722
Jung, H., Yu, S., Bae, N.S., Cho, S.M., Kim, R.H., Cho, S.H., Hwang, I., Jeong, B., Ryu, J.S., Hwang, J., Hong, S.M., Koo, C.M., Park, C.: High through-plane thermal conduction of Graphene Nanoflake filled Polymer Composites Melt-Processed in an L-Shape kinked tube. ACS Appl. Mater. Interfaces 7, 15256–15262 (2015). https://doi.org/10.1021/acsami.5b02681
Burger, N., Laachachi, A., Ferriol, M., Lutz, M., Toniazzo, V., Ruch, D.: Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog Polym. Sci 61, 1–28 (2016). https://doi.org/10.1016/j.progpolymsci.2016.05.001
Huang, X., Yin, Z., Wu, S., Qi, X., He, Q., Zhang, Q., Yan, Q., Boey, F., Zhang, H.: Graphene-Based materials: Synthesis, characterization, Properties, and applications. Small 7, 1876–1902 (2011). https://doi.org/10.1002/smll.201002009
Song, S.H., Park, K.H., Kim, B.H., Choi, Y.W., Jun, G.H., Lee, D.J., Kong, B., Paik, K., Jeon, S.: enhanced thermal conductivity of epoxy – graphene composites by using non-oxidized graphene flakes with non-covalent functionalization. Adv. Mater. 732–737 (2013). https://doi.org/10.1002/adma.201202736
Li, W.M., Fang, W., Wang, S.: Non-covalently modified reduced graphene oxide / polyurethane nanocomposites with good mechanical and thermal properties. J. Mater. Sci 49, 562–571 (2014). https://doi.org/10.1007/s10853-013-7736-4
Kim, H., Lee, S.: Electrical Properties of Graphene / Waterborne polyurethane Composite Films. 18, 1304–1313 (2017). https://doi.org/10.1007/s12221-017-7142-7
Tang, L., Zhao, L., Qiang, F., Wu, Q., Gong, L.: Mechanical Properties of Rubber Nanocomposites Containing Carbon Nanofillers. Elsevier Inc (2019)
Clingerman, M.L., King, J.A., Schulz, K.H., Meyers, J.D.: Evaluation of Electrical Conductivity Models for Conductive Polymer Composites. J. Appl. Polym. Sci 83, 1341–1356 (2002). https://doi.org/10.1002/app.10014
Qian, Y., Wu, H., Yuan, D., Li, X., Yu, W., Wang, C.: In situ polymerization of polyimide-based nanocomposites via covalent incorporation of functionalized graphene nanosheets for enhancing mechanical, thermal, and electrical properties. J. Appl. Polym. Sci 132, 1–10 (2015). https://doi.org/10.1002/app.42724
Yu, J., Qian, R., Jiang, P.: Enhanced thermal conductivity for PVDF composites with a hybrid functionalized graphene sheet-nanodiamond filler. Fibers Polym 14, 1317–1323 (2013). https://doi.org/10.1007/s12221-013-1317-7
Li, Y.C., Tjong, S.C., Li, R.K.Y.: Electrical conductivity and dielectric response of poly (vinylidene fluoride)– graphite nanoplatelet composites. Synth. Met 160, 1912–1919 (2010). https://doi.org/10.1016/j.synthmet.2010.07.009
Varrla Eswaraiah, K.B.: One-pot synthesis of conducting graphene–polymer composites and their strain sensing application. Nanoscale. 1258–1262 (2012). https://doi.org/10.1039/c2nr11555g
Kumar, P., Shahzad, F., Yu, S., Hong, S.M., Kim, Y.H., Koo, C.M.: Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon N Y 94, 494–500 (2015). https://doi.org/10.1016/j.carbon.2015.07.032
Xia, X., Wang, Y., Zhong, Z., Weng, G.J.: A theory of electrical conductivity, dielectric constant, and electromagnetic interference shielding for lightweight graphene composite foams. J. Appl. Phys. 120 (2016). https://doi.org/10.1063/1.4961401
Mazaheri, M., Payandehpeyman, J., Khamehchi, M.: A developed theoretical model for effective electrical conductivity and percolation behavior of polymer-graphene nanocomposites with various exfoliated filleted nanoplatelets. Carbon N Y 169, 264–275 (2020). https://doi.org/10.1016/j.carbon.2020.07.059
Manta, A., Gresil, M., Soutis, C.: Predictive model of Graphene Based Polymer Nanocomposites: Electrical performance. Appl. Compos. Mater 24, 281–300 (2017). https://doi.org/10.1007/s10443-016-9557-5
Zhang, H., bin, Zheng, W.G., Yan, Q., Yang, Y., Wang, J.W., Lu, Z.H., Ji, G.Y., Yu, Z.Z.: Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polym. (Guildf) 51, 1191–1196 (2010). https://doi.org/10.1016/j.polymer.2010.01.027
Du, J., Zhao, L., Zeng, Y., Zhang, L., Li, F., Liu, P., Liu, C.: Comparison of electrical properties between multi-walled carbon nanotube and graphene nanosheet/high density polyethylene composites with a segregated network structure. Carbon N Y 49, 1094–1100 (2011). https://doi.org/10.1016/j.carbon.2010.11.013
Henry, A.: Thermal transport in polymers. Annual Rev. Heat Transf. 17 (2014)
Choy, C.L.: Thermal conductivity of polymers. Polym. (Guildf) 18, 984–1004 (1977). https://doi.org/10.1016/0032-3861(77)90002-7
Xu, P., Loomis, J., Bradshaw, R.D., Panchapakesan, B.: Load transfer and mechanical properties of chemically reduced graphene reinforcements in polymer composites. Nanotechnology. (2012). https://doi.org/10.1088/0957-4484/23/50/505713
Liu, L., Bian, X.M., Tang, J., Xu, H., Hou, Z.L., Song, W.L.: Exceptional electrical and thermal transport properties in tunable all-graphene papers. RSC Adv. (2015). https://doi.org/10.1039/c5ra15533a
Huang, X., Jiang, P., Tanaka, T.: A review of Dielectric Polymer Composites with High Thermal Conductivity. IEEE Electr. Insul. Mag 27, 8–16 (2011). https://doi.org/10.1109/MEI.2011.5954064
Qian, R., Wu, Y.U.J.: Alumina-coated graphene sheet hybrids for electrically insulating polymer composites with high thermal conductivity. Royal Soc. Chem 3, 17373–17379 (2013). https://doi.org/10.1039/c3ra42104j
Alam, F.E., Dai, W., Yang, M., Du, S., Li, X., Yu, J.: In situ formation of a cellular graphene framework thermal conductivity. Royal Soc. Chem 5, 6164–6169 (2017). https://doi.org/10.1039/c7ta00750g
Hwang, B., Yun, G.: Stretchable and patchable composite electrode with trimethylolpropane formal acrylate-based polymer. Compos. Part B 163, 185–192 (2019). https://doi.org/10.1016/j.compositesb.2018.11.009
Kang, B., Park, B., Ha, T.: Highly sensitive wearable glucose sensor systems based on functionalized single-wall carbon nanotubes with glucose oxidase-nafion composites. Appl. Surf. Sci 470, 13–18 (2019). https://doi.org/10.1016/j.apsusc.2018.11.101
Lv, L., Dai, W., Li, A., Cheng-Te, L.: Graphene-Based thermal interface materials: An application-oriented perspective on Architecture Design. Polym. (Basel). (2018). https://doi.org/10.3390/polym10111201
Song, Z., Xu, T., Gordin, M.L., Jiang, Y., Bae, I., Xiao, Q., Zhan, H., Liu, J., Wang, D.: Polymer - graphene Nanocomposites as Ultrafast-Charge and - discharge cathodes for rechargeable Lithium batteries. Nano Lett. (2012).
Wu, Q., Xu, Y., Yao, Z., Liu, A., Shi, G.: Supercapacitors based on flexible Graphene/Polyaniline Nanofiber Composite Films. ACS Nano 4, 1963–1970 (2010)
Tarhini, A., Alchamaa, M.W., Khraiche, M., Kazan, M., Tehrani-Bagha, A.: The effect of temperature on the electrical and thermal conductivity of graphene-based polymer composite films. J. Appl. Polym. Sci. (2021). https://doi.org/10.1002/app.51896
Ghosh, S., Calizo, I., Teweldebrhan, D., Al, E.: Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett 92, 1–4 (2008). https://doi.org/10.1063/1.2907977
Balandin, A.A.: In-plane and cross-plane thermal conductivity of graphene: Applications in thermal interface materials. In: Carbon Nanotubes, Graphene, and Associated Devices IV (2011)
Bahamonde, J.P., Nguyen, H.N., Fanourakis, S.K., Rodrigues, D.F.: Recent advances in graphene based biosensor technology with applications in life sciences. J Nanobiotechnol. 1–17 (2018). doi: 10.1186/s12951-018-0400-z.
Dong, H.S., Qi, S.J.: Realising the potential of graphene-based materials for biosurfaces – A future perspective. In: Biosurface and Biotribology. pp. 229–248 (2015)
Kaur, G., Adhikari, R., Cass, P., Bown, M., Gunatillake, P.: Electrically conductive polymers and composites for biomedical applications. Royal Soc. Chem. 37553–37567 (2015). https://doi.org/10.1039/c5ra01851j
Funding
Open Access funding provided by Aalto University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarhini, A., Tehrani-Bagha, A.R. Advances in Preparation Methods and Conductivity Properties of Graphene-based Polymer Composites. Appl Compos Mater 30, 1737–1762 (2023). https://doi.org/10.1007/s10443-023-10145-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10443-023-10145-5