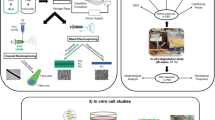
Abstract
Regeneration of cartilage and bone tissues remains challenging in tissue engineering due to their complex structures, and the need for both mechanical support and delivery of biological repair stimuli. Therefore, the goal of this study was to develop a composite scaffold platform for anatomic chondral and osteochondral repair using heparin-based hydrogels to deliver small molecules within 3D-printed porous scaffolds that provide structure, stiffness, and controlled biologic delivery. We designed a mold-injection system to combine hydrolytically degradable hydrogels and 3D-printed scaffolds that could be employed rapidly (< 30 min) in operating room settings (~23 °C). Micro-CT analysis demonstrated the effectiveness of our injection system through homogeneously distributed hydrogel within the pores of the scaffolds. Hydrogels and composite scaffolds exhibited efficient loading (~94%) of a small positively charged heparin-binding molecule (crystal violet) with sustained release over 14 days and showed high viability of encapsulated porcine chondrocytes over 7 days. Compression testing demonstrated nonlinear viscoelastic behavior where tangent stiffness decreased with scaffold porosity (porous scaffold tangent stiffness: 70%: 4.9 MPa, 80%: 1.5 MPa, and 90%: 0.20 MPa) but relaxation was not affected. Lower-porosity scaffolds (70%) showed stiffness similar to lower ranges of trabecular bone (4–8 MPa) while higher-porosity scaffolds (80% and 90%) showed stiffness similar to auricular cartilage (0.16–2 MPa). Ultimately, this rapid composite scaffold fabrication method may be employed in the operating room and utilized to control biologic delivery within load-bearing scaffolds.











Similar content being viewed by others
References
De Mori, A., et al. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers (Basel). 10(3):285, 2018.
De Witte, T.-M., et al. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regener. Biomater. 5(4):197–211, 2018.
Kuć, J., K. D. Szarejko, and M. Gołębiewska. The prevalence and overlaps of temporomandibular disorders in patients with myofascial pain with referral—a pilot study. Int. J. Environ. Res. Public Health. 18(18):9842, 2021.
Owsley, T. G., et al. Otoplastic surgery for the protruding ear. In: Peterson’s Principles of Oral and Maxillofacial Surgery, edited by M. Miloro, et al. Cham: Springer International Publishing, 2022, pp. 2259–2272.
Mladina, R., et al. Nasal septal deformities in ear, nose, and throat patients: an international study. Am. J. Otolaryngol. 29(2):75–82, 2008.
Fung, Y. C. Bone and cartilage. In: Biomechanics: Mechanical Properties of Living Tissues, edited by Y. C. Fung. New York: Springer, 1993, pp. 500–544.
Chen, X., et al. Determining tension-compression nonlinear mechanical properties of articular cartilage from indentation testing. Ann. Biomed. Eng. 44(4):1148–1158, 2016.
Danso, E., et al. Comparison of nonlinear mechanical properties of bovine articular cartilage and meniscus. J. Biomech. 47(1):200–206, 2014.
Jammalamadaka, U., and K. Tappa. Recent advances in biomaterials for 3D printing and tissue engineering. J Funct Biomater. 2018. https://doi.org/10.3390/jfb9010022.
Ballyns, J. J., et al. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng. A. 14(7):1195–1202, 2008.
Maletius, W., and K. Messner. The effect of partial meniscectomy on the long-term prognosis of knees with localized, severe chondral damage: a twelve-to fifteen-year followup. Am. J. Sports Med. 24(3):258–262, 1996.
Muzzarelli, R. A., et al. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohyd. Polym. 89(3):723–739, 2012.
Choe, R., et al. Biomechanical aspects of osteochondral regeneration: implications and strategies for 3D bioprinting. Tissue Eng. 2021:10, 2021.
Lonergan, A. R., and A. R. Scott. Autologous costochondral graft harvest in children. Int. J. Pediatr. Otorhinolaryngol.135:110111, 2020.
Read-Fuller, A. M., et al. The use of allogeneic cartilage for grafting in functional and reconstructive rhinoplasty. J. Oral Maxillofacial Surg. 76(7):15601–15607, 2018.
Wolford, L. M. Autologous fat grafts placed around temporomandibular joint (TMJ) total joint prostheses to prevent heterotopic bone. In: Autologous Fat Transfer, Springer, 2010, pp. 361–382.
Rea, D. N. B. W. The temporomandibular joint implant controversy: a review of autogenous/alloplastic materials and their complications. J. Nutrit. Environ. Med. 8(3):289–300, 1998.
Bell, R. B., et al. Staged reconstruction of the severely atrophic mandible with autogenous bone graft and endosteal implants. J. Oral Maxillofacial Surg. 60(10):1135–1141, 2002.
Brodland, D. G. Auricular reconstruction. Dermatol. Clin. 23(1):23–41, 2005.
Nguyen, L. H., et al. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 32(29):6946–6952, 2011.
Yang, F., et al. A synthetic hydrogel composite with the mechanical behavior and durability of cartilage. Adv. Funct. Mater. 30(36):2003451, 2020.
Kunisch, E., et al. StarPEG/heparin-hydrogel based in vivo engineering of stable bizonal cartilage with a calcified bottom layer. Biofabrication.11(1):015001, 2018.
Korpayev, S., et al. Chitosan/collagen based biomimetic osteochondral tissue constructs: a growth factor-free approach. Int. J. Biol. Macromol. 156:681–690, 2020.
Yang, J., et al. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 57:1–25, 2017.
Liao, J., et al. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 5(1):1–15, 2017.
Drury, J. L., and D. J. Mooney. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 24(24):4337–4351, 2003.
Spicer, C. D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 11(2):184–219, 2020.
Jo, H., et al. Recent strategies in fabrication of gradient hydrogels for tissue engineering applications. Macromol. Biosci. 20(3):1900300, 2020.
Kim, H. D., et al. Chondroitin sulfate-based biomineralizing surface hydrogels for bone tissue engineering. ACS Appl. Mater. Interf. 9(26):21639–21650, 2017.
Kim, S., et al. Enhanced skull bone regeneration by sustained release of BMP-2 in interpenetrating composite hydrogels. Biomacromolecules. 19(11):4239–4249, 2018.
Peppas, N., et al. Physicochemical foundations and structural design of hydrogels in medicine and biology. Ann. Rev. Biomed. Eng. 2(1):9–29, 2000.
Van de Wetering, P., et al. Poly (ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J. Control. Release. 102(3):619–627, 2005.
Flynn, J., et al. Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J. Mater. Chem. B. 8(18):4029–4038, 2020.
Hudalla, G. A., T. S. Eng, and W. L. Murphy. An approach to modulate degradation and mesenchymal stem cell behavior in poly (ethylene glycol) networks. Biomacromolecules. 9(3):842–849, 2008.
Peng, Y., L. E. Tellier, and J. S. Temenoff. Heparin-based hydrogels with tunable sulfation & degradation for anti-inflammatory small molecule delivery. Biomater Sci. 4(9):1371–1380, 2016.
Tellier, L., et al. Localized SDF-1α delivery increases pro-healing bone marrow-derived cells in the supraspinatus muscle following severe rotator cuff injury. Regener. Eng. Transl. Med. 4(2):92–103, 2018.
Tellier, L. E., et al. Hydrolysis and sulfation pattern effects on release of bioactive bone morphogenetic protein-2 from heparin-based microparticles. J. Mater. Chem. B. 3(40):8001–8009, 2015.
Hettiaratchi, M. H., et al. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 6(1):e1240, 2020.
Ao, Q., et al. Fibrin glue/fibronectin/heparin-based delivery system of BMP2 induces osteogenesis in MC3T3-E1 cells and bone formation in rat calvarial critical-sized defects. ACS Appl. Mater. Interf. 12(11):13400–13410, 2020.
Krieger, J., et al. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1α-releasing hydrogels enhances microvascular network remodeling. Biomaterials. 77:280–290, 2016.
Sadir, R., et al. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J. Biol. Chem. 279(42):43854–43860, 2004.
Purcell, B. P., et al. Synergistic effects of SDF-1α chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials. 33(31):7849–7857, 2012.
Danek, C. Recent advances and future challenges in the additive manufacturing of hydrogels. Polymers. 14(3):494, 2022.
Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 5(8):1480–1490, 2017.
Cohen, B. P., et al. Long-term morphological and microarchitectural stability of tissue-engineered, patient-specific auricles in vivo. Tissue Eng. A. 22(5–6):461–468, 2016.
Gauvin, R., et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 33(15):3824–3834, 2012.
Hollister, S. J. Porous scaffold design for tissue engineering. Nat. Mater. 4(7):518–524, 2005.
Liao, E., et al. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 13(3):537–550, 2007.
Boere, K. W. M., et al. Biofabrication of reinforced 3D-scaffolds using two-component hydrogels. J. Mater. Chem. B. 3(46):9067–9078, 2015.
Williams, J. M., et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 26(23):4817–4827, 2005.
Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Boil. Eng. Comput. 51(3):245–256, 2013.
Patel, J. J., C. L. Flanagan, and S. J. Hollister. Bone morphogenetic protein-2 adsorption onto poly-ɛ-caprolactone better preserves bioactivity in vitro and produces more bone in vivo than conjugation under clinically relevant loading scenarios. Tissue Eng. C: Methods. 21(5):489–498, 2015.
Morrison, R. J., et al. Treatment of severe acquired tracheomalacia with a patient-specific, 3D-printed, permanent tracheal splint. JAMA Otolaryngol. Head Neck Surg. 143(5):523–525, 2017.
Morrison, R. J., et al. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 7(285):285ra64, 2015.
Ramaraju, H., et al. Evaluating directional dependency of selective laser sintered patient specific biodegradable devices to improve predictive modeling and design verification. Ann. Biomed. Eng. 49(9):2579–2589, 2021.
Woodruff, M. A., and D. W. Hutmacher. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog. Polym. Sci. 35(10):1217–1256, 2010.
Hahn, M. S., et al. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 27(12):2519–2524, 2006.
Seto, S. P., T. Miller, and J. S. Temenoff. Effect of selective heparin desulfation on preservation of bone morphogenetic protein-2 bioactivity after thermal stress. Bioconjug. Chem. 26(2):286–293, 2015.
Yang, L., C. Yan, and Y. Shi. Fracture mechanism analysis of Schoen gyroid cellular structures manufactured by selective laser melting. In: 2017 International Solid Freeform Fabrication Symposium,University of Texas at Austin, 2017.
Hollister, S. J., et al. Design control for clinical translation of 3D printed modular scaffolds. Ann. Biomed. Eng. 43(3):774–786, 2015.
Hollister, S. J., et al. Integrating image-based design and 3D biomaterial printing to create patient specific devices within a design control framework for clinical translation. ACS Biomater. Sci. Eng. 2(10):1827–1836, 2016.
Chang, B., et al. Hybrid three-dimensional–printed ear tissue scaffold with autologous cartilage mitigates soft tissue complications. Laryngoscope. 131(5):1008–1015, 2021.
Lee, C. H., et al. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Eng. A. 15(12):3923–3930, 2009.
Keys, K. B., F. M. Andreopoulos, and N. A. Peppas. Poly (ethylene glycol) star polymer hydrogels. Macromolecules. 31(23):8149–8156, 1998.
Gao, G., et al. Bioprinting cartilage tissue from mesenchymal stem cells and PEG hydrogel. Cell Cult. Methods Protocols. 2017:391–398, 2017.
Qiu, Y., et al. PEG-based hydrogels with tunable degradation characteristics to control delivery of marrow stromal cells for tendon overuse injuries. Acta Biomater. 7(3):959–966, 2011.
Hao, Y., and C. C. Lin. Degradable thiol-acrylate hydrogels as tunable matrices for three-dimensional hepatic culture. J. biomed. Mater. Res. A. 102(11):3813–3827, 2014.
Chang, B., et al. Evaluation of human nasal cartilage nonlinear and rate dependent mechanical properties. J. Biomech.100:109549, 2020.
Inoue, Y., and K. Nagasawa. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohyd. Res. 46(1):87–95, 1976.
Nakamura, S., et al. Controlled release of fibroblast growth factor-2 from an injectable 6-O-desulfated heparin hydrogel and subsequent effect on in vivo vascularization. J. Biomed. Mater. Res. A. 78(2):364–371, 2006.
Orr, T., et al. Compressive properties of cancellous bone defects in a rabbit model treated with particles of natural bone mineral and synthetic hydroxyapatite. Biomaterials. 22(14):1953–1959, 2001.
Liu, F., et al. Mechanical properties of optimized diamond lattice structure for bone scaffolds fabricated via selective laser melting. Materials. 11(3):374, 2018.
Little, C. J., N. K. Bawolin, and X. Chen. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. B: Rev. 17(4):213–227, 2011.
Mansour, J. M. Kinesiology: the mechanics and pathomechanics of human movement. Biomech. Cartilage. 2:66–79, 2003.
Chiu, L. L., et al. Comparisons of auricular cartilage tissues from different species. Ann. Otol. Rhinol. Laryngol. 126(12):819–828, 2017.
Zopf, D. A., et al. Biomechanical evaluation of human and porcine auricular cartilage. Laryngoscope. 125(8):E262–E268, 2015.
Pappa, A. K., et al. A pilot study comparing mechanical properties of tissue-engineered cartilages and various endogenous cartilages. Clin. Biomech. 50:105–109, 2017.
Griffin, M., et al. Biomechanical characterisation of the human auricular cartilages; implications for tissue engineering. Ann. Biomed. Eng. 44(12):3460–3467, 2016.
Staudenmaier, R., et al. Flap prefabrication and prelamination with tissue-engineered cartilage. J. Reconstr. Microsurg. 21(07):555–564, 2004.
Staudenmaier, R., et al. Tissue-engineered cartilage in a prefabricated free skin flap. HNO. 52:510–517, 2004.
Bomhard, A. V., et al. Prefabrication of 3D cartilage contructs: towards a tissue engineered auricle–a model tested in rabbits. PloS ONE. 8(8):e71667, 2013.
Redenski, I., et al. Engineered vascularized flaps, composed of polymeric soft tissue and live bone, repair complex tibial defects. Adv. Funct. Mater. 31(44):2008687, 2021.
Bae, S. E., et al. Controlled release of bone morphogenetic protein (BMP)-2 from nanocomplex incorporated on hydroxyapatite-formed titanium surface. J. Control. Release. 160(3):676–684, 2012.
Hettiaratchi, M. H., et al. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 35(25):7228–7238, 2014.
Olthof, M. G., et al. Bone morphogenetic protein-2 release profile modulates bone formation in phosphorylated hydrogel. J. Tissue Eng. Regener. Med. 12(6):1339–1351, 2018.
DeFail, A. J., et al. Controlled release of bioactive TGF-β1 from microspheres embedded within biodegradable hydrogels. Biomaterials. 27(8):1579–1585, 2006.
Yoon, J. P., et al. Sustained delivery of transforming growth factor β1 by use of absorbable alginate scaffold enhances rotator cuff healing in a rabbit model. Am. J. Sports Med. 46(6):1441–1450, 2018.
Scheiner, K. C., et al. Sustained release of vascular endothelial growth factor from poly (ε-caprolactone-PEG-ε-caprolactone)-b-poly (l-lactide) multiblock copolymer microspheres. ACS Omega. 4(7):11481–11492, 2019.
Davies, N., et al. The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials. 29(26):3531–3538, 2008.
Temenoff, J. S., and A. G. Mikos. Biomaterials: The Intersection of Biology and Materials Science. London: Pearson/Prentice Hall, 2008.
Lee, S., X. Tong, and F. Yang. The effects of varying poly (ethylene glycol) hydrogel crosslinking density and the crosslinking mechanism on protein accumulation in three-dimensional hydrogels. Acta Biomater. 10(10):4167–4174, 2014.
Holloway, J. L., et al. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J. Control. Release. 191:63–70, 2014.
Parlato, M., et al. Poly (ethylene glycol) hydrogels with adaptable mechanical and degradation properties for use in biomedical applications. Macromol. Biosci. 14(5):687–698, 2014.
Roman, M., and W. T. Winter. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules. 5(5):1671–1677, 2004.
Chu, Y., et al. pH-induced swelling kinetics of polyelectrolyte hydrogels. J. Appl. Polym. Sci. 58(12):2161–2176, 1995.
Dalton, E., Z. Morris, and N. Ayres. Synthesis and characterization of sulfated-lactose polyurethane hydrogels. Polym. Chem. 13(20):2933–2940, 2022.
Roy, S., et al. Bioactivity screening of partially desulfated low-molecular-weight heparins: a structure/activity relationship study. Glycobiology. 21(9):1194–1205, 2011.
Ladet, S., L. David, and A. Domard. Multi-membrane hydrogels. Nature. 452(7183):76–79, 2008.
Zucchelli, A., et al. Electrospun nanofibers for enhancing structural performance of composite materials. Polym. Adv. Technol. 22(3):339–349, 2011.
Nguyen, K. T., and J. L. West. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 23(22):4307–4314, 2002.
Jang, C. H., Y. Koo, and G. Kim. ASC/chondrocyte-laden alginate hydrogel/PCL hybrid scaffold fabricated using 3D printing for auricle regeneration. Carbohyd. Polym.248:116776, 2020.
Ahlfeld, T., et al. Design and fabrication of complex scaffolds for bone defect healing: combined 3D plotting of a calcium phosphate cement and a growth factor-loaded hydrogel. Ann. Biomed. Eng. 45(1):224–236, 2017.
Van Belleghem, S., et al. Hybrid 3D printing of synthetic and cell-laden bioinks for shape retaining soft tissue grafts. Adv. Funct. Mater. 30(3):1907145, 2020.
Visscher, D. O., et al. Design and fabrication of a hybrid alginate hydrogel/poly (ε-caprolactone) mold for auricular cartilage reconstruction. J. Biomed. Mater. Res. B: Appl. Biomater. 107(5):1711–1721, 2019.
Hernandez, I., A. Kumar, and B. Joddar. A bioactive hydrogel and 3D printed polycaprolactone system for bone tissue engineering. Gels. 3(3):26, 2017.
She, Y., et al. 3D printed biomimetic PCL Scaffold as framework interspersed with collagen for long segment tracheal replacement. Front. Cell Develop. Biol. 2021. https://doi.org/10.3389/fcell.2021.629796.
Chen, C. H., et al. Selective laser sintered poly-epsilon-caprolactone scaffold hybridized with collagen hydrogel for cartilage tissue engineering. Biofabrication.6(1):015004, 2014.
Lee, M. Y., et al. Laser sintered porous polycaprolacone scaffolds loaded with hyaluronic acid and gelatin-grafted thermoresponsive hydrogel for cartilage tissue engineering. Biomed. Mater. Eng. 23(6):533–543, 2013.
Bas, O., et al. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication.9(2):025014, 2017.
Hutmacher, D. W., et al. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 55(2):203–216, 2001.
Dong, L., et al. 3D-printed poly (ε-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering. Sci. Rep. 7(1):1–9, 2017.
Fazeli, N., et al. 3D-printed PCL scaffolds coated with nanobioceramics enhance osteogenic differentiation of stem cells. ACS Omega. 2021. https://doi.org/10.1021/acsomega.1c04015.
Seyedsalehi, A., et al. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 10(1):1–14, 2020.
Abueidda, D. W., et al. Mechanical properties of 3D printed polymeric Gyroid cellular structures: experimental and finite element study. Mater. Design.165:107597, 2019.
Jung, Y., and S. Torquato. Fluid permeabilities of triply periodic minimal surfaces. Phys. Rev. E.72(5):056319, 2005.
Liu, F., et al. Additively manufactured continuous cell-size gradient porous scaffolds: pore characteristics, mechanical properties and biological responses in vitro. Materials. 13(11):2589, 2020.
Kapfer, S. C., et al. Minimal surface scaffold designs for tissue engineering. Biomaterials. 32(29):6875–6882, 2011.
Torres-Rendon, J. G., et al. Bioactive gyroid scaffolds formed by sacrificial templating of nanocellulose and nanochitin hydrogels as instructive platforms for biomimetic tissue engineering. Adv. Mater. 27(19):2989–2995, 2015.
Gao, J., P. M. Crapo, and Y. Wang. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 12(4):917–925, 2006.
Bryant, S. J., et al. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials. 28(19):2978–2986, 2007.
Johnson, T., et al. Fabrication of highly porous tissue-engineering scaffolds using selective spherical porogens. Bio-med. Mater. Eng. 20(2):107–118, 2010.
Hu, K., et al. Effects of condylar fibrocartilage on the biomechanical loading of the human temporomandibular joint in a three-dimensional, nonlinear finite element model. Med. Eng. Phys. 25(2):107–113, 2003.
Griffin, M., et al. Comparison of the compressive mechanical properties of auricular and costal cartilage from patients with microtia. J. Biomech.103:109688, 2020.
Soufivand, A. A., et al. Prediction of mechanical behavior of 3D bioprinted tissue-engineered scaffolds using finite element method (FEM) analysis. Addit. Manuf.33:101181, 2020.
Alkan, Z., et al. Tensile characteristics of costal and septal cartilages used as graft materials. Archiv. Facial Plastic Surg. 13(5):322–326, 2011.
Griffin, M., et al. Biomechanical characterization of human soft tissues using indentation and tensile testing. J. Visualized Exp.118:e54872, 2016.
Al Dayeh, A. A., and S. W. Herring. Compressive and tensile mechanical properties of the porcine nasal septum. J. Biomech. 47(1):154–161, 2014.
Tanaka, E., et al. Strain-rate effect on the biomechanical response of bovine temporomandibular joint disk under compression. J. Biomed. Mater. Res. A. 67(3):761–765, 2003.
Ouyang, J., et al. Biomechanical characteristics of human trabecular bone. Clin. Biomech. 12(7–8):522–524, 1997.
van Rietbergen, B., et al. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J. Biomech. 28(1):69–81, 1995.
Rath, A., S. Mathesan, and P. Ghosh. Nanomechanical characterization and molecular mechanism study of nanoparticle reinforced and cross-linked chitosan biopolymer. J. Mech. Behav. Biomed. Mater. 55:42–52, 2016.
Zhang, J., et al. Assigning viscoelastic and hyperelastic properties to the middle-ear soft tissues for sound transmission. Biomech. Model. Mechanobiol. 19(3):957–970, 2020.
Allen, K. D., and K. A. Athanasiou. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J. Biomech. 39(2):312–322, 2006.
Li, L., M. Buschmann, and A. Shirazi-Adl. Strain-rate dependent stiffness of articular cartilage in unconfined compression. J. Biomech. Eng. 125(2):161–168, 2003.
Bredbenner, T. L., and D. T. Davy. The effect of damage on the viscoelastic behavior of human vertebral trabecular bone. J. Biomech. Eng. 128:473, 2006.
Quaglini, V., V. La Russa, and S. Corneo. Nonlinear stress relaxation of trabecular bone. Mech. Res. Commun. 36(3):275–283, 2009.
Gandhi, N. S., and R. L. Mancera. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim. Biophys. Acta Proteins Proteom. 1824(12):1374–1381, 2012.
McCaffrey, T. A., D. J. Falcone, and B. Du. Transforming growth factor-β1 is a heparin-binding protein: identification of putative heparin-binding regions and isolation of heparins with varying affinity for TGF-β1. J. Cell. Physiol. 152(2):430–440, 1992.
Fairbrother, W. J., et al. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure. 6(5):637–648, 1998.
Kim, K., et al. Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J. Control. Release. 168(2):166–178, 2013.
Wang, H., et al. Osteogenic effect of controlled released rhBMP-2 in 3D printed porous hydroxyapatite scaffold. Coll. Surfaces B Biointerf. 141:491–498, 2016.
Jeon, O., et al. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J. Control. Release. 154(3):258–266, 2011.
Tae, G., et al. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J. Biomater. Sci. Polym. Ed. 17(1–2):187–197, 2006.
Schirmer, L., et al. StarPEG-heparin hydrogels to protect and sustainably deliver IL-4. Adv. Healthcare Mater. 5(24):3157–3164, 2016.
Freeman, I., A. Kedem, and S. Cohen. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 29(22):3260–3268, 2008.
Tellier, L. E., et al. Intra-articular TSG-6 delivery from heparin-based microparticles reduces cartilage damage in a rat model of osteoarthritis. Biomater. Sci. 6(5):1159–1167, 2018.
Seto, S. P., M. E. Casas, and J. S. Temenoff. Differentiation of mesenchymal stem cells in heparin-containing hydrogels via coculture with osteoblasts. Cell Tissue Res. 347(3):589–601, 2012.
Lei, J., W. L. Murphy, and J. S. Temenoff. Combination of heparin binding peptide and heparin cell surface coatings for mesenchymal stem cell spheroid assembly. Bioconjugate Chem. 29(4):878–884, 2018.
Bandyopadhyay, A., B. B. Mandal, and N. Bhardwaj. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J. Biomed. Mater. Res. A. 110(4):884–898, 2022.
Zhu, S., et al. 3D-printed extracellular matrix/polyethylene glycol diacrylate hydrogel incorporating the anti-inflammatory phytomolecule honokiol for regeneration of osteochondral defects. Am. J Sports Med. 48(11):2808–2818, 2020.
Acknowledgments
The authors would like to thank Sarah Jo Tucker for her assistance in printing and cleaning the 3D-printed scaffolds and Dr. Laxminarayanan Krishnan for his guidance in performing µCT scans and analysis. This work was supported by the Carol Ann and David D. Flanagan Professorship and Patsy and Alan Dorris Chair in Pediatric Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
This work was supported by the Carol Ann and David D. Flanagan Professorship and Patsy and Alan Dorris Chair in Pediatric Technology. The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brown, N.E., Ellerbe, L.R., Hollister, S.J. et al. Development and Characterization of Heparin-Containing Hydrogel/3D-Printed Scaffold Composites for Craniofacial Reconstruction. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03530-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03530-z