Abstract
Antlers are formed anew each year to realise an optimal relationship between their size and weight and the physical body condition in Cervidae. This results in the objective to match fighting abilities with size and mechanical performance of the antlers, as well as to advertise these correlated abilities to other males and females. The resulting variation in individual antler characteristics from year to year can show considerable differences. To characterise and understand these differences is important in hunting, game management and deer breeding, as well as potentially to assess the habitat quality. However, relatively few traits of the antler have been scientifically tested for this purpose, and only a few studies were conducted on the same individual in free-ranging red deer over the years. The objective of the present study was to quantify the influence of the individual (repeatability), the age and the site on the expression of 125 antler characteristics. For this purpose, we collected 35 stags with an average of about 10 consecutive antlers per individual (confirmed by genetic analysis), a total of 355 antlers. The antlers were scanned 3-dimensionally and measured semi-automatically. Numbers, lengths, distances, circumferences, bending, curvatures, angles, forms and CIC (International Council for Game and Wildlife Conservation) characteristics were compiled and evaluated in a generalised linear mixed model adapted to the distribution of the characteristics. The complete model explained 1.6 to 83% of character variation. Mean repeatability of the characteristics varied between 2.7 and 74.4%. The stags’ age explained 0 to 36.4%, and the side explained 0 to 2.5% of the variability. Some characteristics of burr, signet, beam and the lower tines reached the highest repeatability; the highest variability was found in characteristics of the crown. Values of 11 features that are frequently used in other studies corresponded very well with the present study. However, some features reached higher repeatability every year, whereas others varied more closely with age. Such characteristics might be selectively included into further research or practical applications to increase informative value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After antlers from the previous year are shed in February to April, male red deer start immediately growing the new antlers every year, in about 4 to 5 months (Gaspar-López et al. 2010). Thus, antlers are among the fastest growing tissues in the entire animal kingdom (Price and Allen 2004; Price et al. 2005; Landete-Castillejos et al. 2019). The reason is to adapt antler strength to body size and to repair antler damage (Garcia et al. 2010). And more importantly, because it is a dead structure, it cannot be repaired. Damage would increase over the years and make it easier for pathogens to enter the body. Therefore, they must be renewed shortly after their annual use ends. The antlers are used for intraspecific fights, especially during the rut (Clutton-Brock 1982), as a weapon against enemies, and as a demonstration of power, as well as attraction for females (Lincoln 1972; Garcia et al. 2010). Deer with large antlers have greater reproductive success than deer with smaller antlers (Clutton-Brock 1982; Kruuk et al. 2002; Bartos and Bahbouh 2006; Vanpé et al. 2010).
The size and shape of the antlers change every year. While the lower antler traits seem to be relatively constant over time (Kruuk et al. 2002) with the exception of the bay (or bez) tine (Fierro et al. 2002), the shape of the crown is especially variable (Azorit et al. 2002; García et al. 2002). The weight, length and number of tines are often used to evaluate antler quality. In Europe, detailed assessment is usually carried out using the international CIC formula (International Council for Game and Wildlife Conservation; Trense et al. 1981).
Antler traits are used to identify individuals for hunting purposes (recognition, Kruuk et al. 2002; age estimation, Azorit et al. 2002; Mysterud et al. 2005; Martínez Salmerón 2014); to assess individual quality (Clutton-Brock 1982; Kruuk et al. 2002; Schmidt et al. 2001) in the context of management (Lockow 1991; Landete-Castillejos et al. 2013) and even for selection of free range (Clutton-Brock and Albon 1989) or farmed red deer (Van den Berg and Garrick 1997); to estimate the degree of inbreeding (Pérez-González et al. 2010); to predict the expected antler quality in older individuals (Degmecic and Florijancic 2014); to assess habitat quality (Kolejanisz et al. 2012), for the classification of cervid systematics (Samejima and Matsuoka 2020; Heckeberg et al. 2022); and, last but not the least, to elaborate the genetic architecture of antler traits for basic research (Peters et al. 2022). And, one of the most important characteristics of an antler is its mechanical performance. An antler is a tube made of bone filled with foam made of bone, and as such, its mechanical performance depends on diameter, cortical thickness and the mechanical quality of the bone material (Landete-Castillejos et al. 2007, 2010). Especially the diameter of the beam is directly related to the mechanical performance of the antler.
For all these purposes, the assessment of antler trait constancy is of great importance. It is essential to know to what extent certain traits vary depending on age (Clements et al. 2010; Ludwig and Vocke 1990; Drechsler 1992), genetics (Johnston et al. 2017), environmental effects (Bubenik et al. 1987; Azorit et al. 2002; Putman and Staines 2004; Degmečić and Florijančić 2014) and mechanical performance (Landete-Castillejos et al. 2019).
Red deer offer good possibilities to investigate these issues based on the repeatability of the traits, i.e. the proportion of the total variance that is explained by the differences between the various stags (Kruuk et al. 2002) which can be estimated by comparing cast antler lines from the same individual.
However, so far, only few studies are available that investigate the repeatability of antler traits (Bartos et al. 2007), especially in free-range individuals of red deer (Kruuk et al. 2002). At the same time, only a limited number of traits have been evaluated, mainly focussing on antler weight and length, eventually including the number of tines and circumferences measured at different points of the main beam.
The aim of the present study was to significantly extend the existing knowledge on the variability/constancy of antler traits in red deer, including effects of the stag, age and side. The results will be used to optimise existing antler traits for the identification of individuals and for conducting elaborate scientific studies with large numbers of animals.
Materials and methods
The basis for this study was a total of 377 cast antlers and 10 trophies from a total of 35 different red deer stags originating from six different regions in Germany. Drill samples of all cast antlers and trophies were examined to ensure that they belonged to the same stag. The samples were taken on the signet (abscission area), of cast antlers and the occiput of trophy skulls.
The sampling area
Stags with rows of different antlers were sampled in the area of Germany between longitudes 7.84424 and 14.08447 and latitudes 49.42527 and 52.33533 with a north–south extension of 330 km and a west–east extension of 380 km. The area comprises a mosaic of different types of land use, predominantly forest, pastures and agriculture. In order to be able to collect stags with as many as possible cast antlers, stags were used from different regions within the sampling area and only few (not representative) stags per origin were available. However, the aim was explicitly to characterise the variability of antlers within individuals, also depending on age and side. Therefore, the origin was considered as a random effect and the effects of the individual origins on antler characteristics were not calculated.
The genetic analysis to identify cast antlers from dead hunted deer was based on antler samples for cast antlers, and from the skull in the dead animal. Drilling samples from antlers were taken from the signet and those from the skulls from the occipital bone. All bone samples were stored at − 20 °C until further use and are available from our laboratory. We used samples from 35 legally harvested animals and already collected cast antlers from the same stags that were provided by the hunters. No animals were killed specifically for the study. No living animals were sampled, and no cast antlers were sought or collected for the study. All antlers remained with their respective owner.
DNA analysis and genotyping
DNA was extracted by using a commercially available kit (Instant Virus RNA Kit, Analytik Jena, Germany). For this purpose, 30 to 50 mg of tissue were processed according to the manufacturer’s instructions. DNA concentration was determined photometrically and adjusted to 5 ng/μl with RNase-free water. The presence of high molecular weight DNA was confirmed by agarose gel electrophoresis. Sixteen microsatellites were used to genotype red deer as described in detail by Willems et al. (2016). Primers were combined in four multiplex PCRs (Reiner et al. 2021). PCR was performed in a volume of 10 μl consisting of 5 μl of 2× Multiplex Master Mix (Qiagen, Germany), 4 μl of primer mix and 1 μl (5 ng) of extracted DNA. DNA was amplified after an initial denaturing step of 15 min in 26 cycles of denaturing at 94 °C for 30 s, annealing at 56 °C (multiplex PCR 4 at 50 °C) for 90 s and extension at 72 °C for 30 s. After a final step at 60 °C for 30 min, PCRs were cooled down to 4 °C.
Capillary electrophoresis
One microliter of the fluorescently labelled PCR product and 0.375 μl DNA Size Standard 500 Orange (Nimagen, Netherlands) were added to 12 μl Hi-Di formamide (Thermo Fisher Scientific, Germany) and electrophoresed on an ABI PRISM 310 automatic sequencer. Allele sizes were determined with the Peak Scanner Software 2.0 (Thermo Fisher Scientific, Germany) within the framework of the study by Reiner et al. (2021).
Antler general information
Three hundred fifty-five antlers from 35 stags were included in the study. The antlers were shed between 2004 and 2019. The age of the stags when shedding was 8.46 ± 2.24 years (5 to 14) (Table 1). Stags of younger and older stags were excluded from the analysis because they were clearly out of range. All antlers and trophies were accompanied by the stag’s number, the stag’s age when casting, the antler side and the stag’s origin. The stags’ age was determined from trophy, based on abrasions of the teeth of the mandibles by an experienced expert team. Unclear cases were further assessed by serial sections through dental cement layers (Schatz 1987). Antlers were weighted with a luggage scale with a precision of 10 g. For trophies, 0.7 kg for the skull was subtracted and the remaining weight was divided by 2 to receive the single antler’s weight. The first, second and third tines were named brow tine, bay tine (synonymous to bez tine) and tray tine (synonymous for trez tine), respectively. Features as the presence of bay tines (0/1), the crown form (1–15), the number of tines per antler (CIC, > 2 cm), the number of additional tines (smaller than 2 cm), the presence of beam processes (0/1) and the number of crown tines were recorded (Table 1).
Further antler characteristics
The burr and signet (Table 2), the beam (Table 3), the main tines (Table 4) and the crown (Table 5) were investigated. Diverse lengths, distances and circumferences were measured. Numbers were counted. Curvatures, bendings, outgoing angles of tines, beam angles and splitting of tines were recorded. These data were supplemented by 13 CIC characteristics (Table 5). Due to the high complexity and variety of features, abbreviations are deliberately omitted in the following.
Lengths, distances, circumferences, angles, bending, splitting and curvature were semi-automatically analysed in the antlers’ 3D models as described in the following section.
Acquisition of detailed antler characteristics
A hand-held scanner Artec Eva Lite (Artec 3D, Luxembourg) was used to create 3D models of trophies and cast antlers. Artec Eva Lite is a portable white light scanner that works with an accuracy of up to 0.1 mm and a resolution of up to 0.5 mm. The scanner was connected to a standard notebook equipped with Artec Studio 13 Professional software. The antler was placed on a rotating table with lines and numbers for orientation. It was scanned from two different sides. The 3D models were finished in different consecutive steps using the same software. Errors bigger than 0.5 mm were sorted out, and background recording was deleted. Then, the 3D models were transferred to CATIA V5 CAD software, where a total of 93 features was measured or calculated for each single antler.
An axis through the centre of the beam and each tine was virtually drawn as the basis for all measurements using the “splinefunction” of the CAD software (Fig. 1).
The base of each tine was defined as the point, where its virtual centre line crossed the centre line of the beam. The coordinates of tine bases and tine tips were used to determine distances between the tines at their base and the tip and between the base and tip of different tines and the crown base. They were also used to predict the length of tines. Each length was measured in three different ways: (i) along the central line as a circular arc from base to tip, (ii) as the direct line from base to tip (secant) and (iii) along the longer outer side of the tine (CIC). The relative length of brow (first tine), bay (second tine) and tray (third tine) was assessed as the ratio of the tine length and the main beam length (Fig. 2).
The lengths of tines were measured along the central line (orange) as a circular arc from base to tip, as the direct line from base to tip (yellow line; secant), and along the longer outer side according to CIC. The maximal height of the circular segment represented by the tine, to the secant (line from tine base to tine tip), which is called the sagitta (green line) was used to quantify the curvature of brow, bay and tray tines
The maximal height of the circular segment represented by the tine, to the secant (line from tine base to tine tip), which is called the sagitta was used to quantify the curvature of brow, bay and tray tines. Accordingly, the curvature of the beam was determined measuring the sagitta to the secant (line from the signet to the crown base; Fig. 2).
Outgoing angles of brow, bay and tray tines were measured at their base, relative to the centre line of the beam (Supplemental Fig. 1). The beam angles at the bases of brow, bay and tray tines were also measured. Any angular measurements were done within the virtual 3D model, based on the central lines of beam and tines. Bending of the beam was evaluated in a semi-quantitative way from three directions (front, side, top), where no bending (0), single bending (1) or double bending (“S-shape”) (2) was considered. Similarly, bending of the three main tines was examined from three directions (front, side, top). Local bends in the axis of beam and main tines were scored with 0 (no bend), 1 (slight bend) and 2 (pronounced bend).
Circumferences (and eventually diameters) of beam and tines were measured at different locations: at the signet and the burr, at the smallest circumference between tines for the beam and exactly halfway along the tines. The circumference of the signet was measured in the notch between signet and burr. Concavity/convexity of the signet was classified on a scale from −3 (concave) to +3 (convex). Splitting of the main tines was recorded, and the depth of the splitting was measured in mm.
The CIC characteristics (length of the main beam, brow tine, bay tine and tray tine, burr circumference, lower and upper circumferences of the main beam, number of tines ≥ 2 cm and crown tines ≥ 2 cm, length of crown tines, quality of colour, pearls and tips) were measured according to the CIC formula (Trense et al. 1981).
Splitting of the main tines was scored with 0 (no splitting), 1 (fork) and 2 (three tine tips). The quality of splitting was scored with 0 (no splitting), 1 (less than 2 cm deep splitting) and 2 (≥ 2 cm deep splitting).
The crown
The base of the crown was defined as the virtual junction of the central beam line with the central lines of the first two crown branches (Fig. 3). This point was further addressed as the first branching level of the crown. Further up in both branches, the next bifurcation (with two or more new branches) defined the second branching level, and so on. The last branching level that could be found in the present study was level 5. Each bifurcation was additionally numbered in ascending order starting with the crown base (1) preceding to the next bifurcation of the front crown branch (2), to next bifurcation of the hind crown branch (3) and so on. The maximum number of bifurcations that was found in the present study was 7. Branching level (1–5) and bifurcation number (1–7) were combined (11, 22, 23, 24, 25, 33,…, 46, 47, 55, 56), where the first digit is the branching level and the second digit is the bifurcation number (Fig. 3). Any branching event could lead to two or more new branches. These branches could end further up as free tines (crown tines), or they could end in another branching; in the latter case, they were designated as crown beams. Numbers and lengths of crown beams and tines were measured. For statistical analysis, beams and tines were evaluated together and also separately according to their level. Crown tines were counted if (i) they were visible at all (number of crown tines) and if they were at least 2 cm long (CIC). When crown tines had kinks or lamellar joints, they were recorded. The shape of the crown was considered in 13 different forms (Supplemental Fig. 2). The percentages of crown forms were counted, and it was recorded, if different forms were available in the same stag, either in different years or at different sides of the antler. The height of the crown was measured from the crown base point to the tip of the highest crown tine (Supplemental Fig. 3). The crowns’ length was defined as the distance between the two most distant crown tine tips in the same direction as the main tines (brow and tray). The crowns’ width was measured as the distance between the most distant two crown tine tips vertically to the length (Supplemental Fig. 4).
The base of the crown was defined as the virtual junction of the central beam line with the central lines of the first two crown branches (red dot). Branching levels and bifurcations of the crown. Branching levels: 1 = red; 2 = green; 3 = blue; 4 = orange. Yellow dots: tips of free crown ends. Numbers: bifurcations 1 to 6, starting with the lowest branching level, front. In combination, the numbering leads to 22, 23, 34, 35 and 46 (branching level and bifurcation number)
Statistics
Statistical analysis was done with the program package IBM SPSS (V27) (Munich, Germany). Descriptive statistics were calculated including mean, standard deviation (SD), minimum and maximum. Coefficients of variation were calculated as SD/mean. Percentage values were calculated from binary data (present, absent), whereby variation was not considered.
Repeatability was calculated as the effect of the stag on the variability of antler characteristics (the amount of the total variance within the antler traits that is explained by the stag). High percentage of repeatability means low (intraspecific/interindividual) variance. Effects of stag, age, antler side (left or right) and the origin of the stag (6 distinct regions) on antler characteristics were calculated, applying a general linear model, where the distribution of characteristics (metric, ordinal or binary) was included.
The model is as follows:
where Yij is the antler score of stag j from region i, y is the average trait score of the antler, β1age is the fixed effect of the age (4 to 14 years), β2side is the fixed effect of the side (left or right), Ri is the random effect of the region (1–6), Sij is the random effect of the stag j from region I (1–35) and eijk is the residual variance.
Characteristics were sorted in descending order by their effect sizes and presented in separate figures with respect to repeatability (effect of stag on antler characteristics), age and antler side. The regions were not considered, because stag numbers in the different regions were not representative.
Subsequently, all characteristics were split into their components and the effects of the components on the variability of antler characteristics were estimated. For example, the feature length of tray tine was divided into the following components: length, tray tine and tine. Regression coefficients (B) for all components were calculated using multiple linear regression with simultaneous inclusion of all variables.
The regression model is as follows:
where, for i = no. of observations, Yi is the dependent variable (percentage of variance of antler characteristics explained by stag, age and side), xi is the explanatory variables (numbers, lengths, circumferences, distances, angles, curvature, bending, splitting, crown form, kinks, CIC, quality, crown level, crown volume (length, height, broad), signet, burr, beam, tines, brow, bay tine (bez tine), tray tine (trez tine), crown), β0 is the model constant, β1xi1–β22xi22 is the slope coefficients for each explanatory variable and ei is the residual.
The respective regression coefficients were added to the model constant and presented together with their standard errors as a bar chart.
Results
Antlers weighed between 1 and 4.4 kg. They had 3 to 12 tines, including 0 to 6 tines shorter than 2 cm (Table 1). The number of tines below 2 cm had the highest coefficient of variation.
Characteristics of burr and signet were available from 331 to 355 antlers. The diameter of the burr was 245.85 ± 20.95 mm (Table 5), and that of the signet was 184.8 ± 18.8 mm. Coefficients of variation were extremely low for burr and signet characteristics, except for the signet convexity (mean: 0.4; SD: 1.2; min: − 3; max: 3).
Beam lengths differed between 67.5 and 119.8 cm (Table 3). Only 1.7% of the beams had a beam process. Some measures were only available, if the bay tine was present (n = 285) or not present (n = 69), as 20% of the antlers had no bay tine (Table 4). Beam kinks were regularly found in the region of the tray tine base. The degree of beam curvature had the highest coefficient of variation among the beam characteristics. More characteristics can be found in Table 4.
First hints on a higher variability of the crown characteristics were found with the coefficients of variation (Table 5), and 350 crowns were available. As any branching was characterised, starting from the level 1 (i.e. the first branching of the beam), up to 5 branching levels could be reached. The numbers of bifurcations realised on the respective level could be 2 to 7. On average, antlers reached 2.7 branching levels (2–5). Forty-three percent of antlers reached branching levels of 2 or 3. Higher branching levels were found to be more seldom. Some branching developed into crown beams which led to further branching or they ended directly as tines. One branching could lead to 1 to 3.5 tines. Altogether, 633 crown beams and 1061 crown tines were available for measuring. The length of the crown beams varied from 16 to 299 mm and the length of crown tines from 13 to 480 mm, depending on the branching level. Crown beams were reduced from 114 mm at level 1 to 93 mm at level 4. Crown tines were reduced from 261 mm (level 1) to 119 mm (level 4) and 72 mm (level 5; only 2 examples). These lengths showed high degrees in coefficients of variation.
The arrangement of the antlers’ crown beams and tines at the different levels led to the distinction of 13 different crown forms (Supplemental file). Some forms that had been expected were not found among the samples. Forms 6 and 7 were the most frequent crowns, followed by forms 15 and 1.
The crown’s volume of a single antler was characterised by its height, length and width which reached up to 49.7 cm, 63.3 cm and 45.6 cm, respectively. Crown tine kinks were found in 3% of the antlers, and 11% showed lamellar joints.
CIC characteristics of the two antlers on the skull were available in too low numbers. Thus, they were not taken into consideration. Besides tine quality, colour, pearls and bay tine length, these values had relatively low coefficients of variation (Table 5).
The mean repeatability of the individual antler characteristics, i.e. the characteristic’s variance explained by the stag, was 44.3% (2.7–74.4%; Fig. 4). Zero to 36.4% of the characteristic’s variation were explained by the age of the stag (mean: 6.9%). Only 0 to 2.5% (mean 0.3%) were explained by the side of the antler.
The highest repeatability was found for the distances between the tips of brow-crown junction, brow-tray junction, brow-bay junction, absolute and relative bay tine and tray tine lengths (CIC) and the number of tines above 2 cm (CIC). The highest variability with no repeatability was found for the existence of kinks within crown tines. The constancy of most crown forms and available branching levels had also a low repeatability as they varied clearly with the stags’ age. All antler characteristics sorted by repeatability are shown in (Fig. 4).
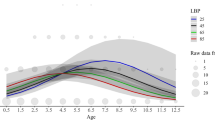
If the individual characteristics are broken down into their components, repeatability was significantly higher when numbers, curvature, lengths, circumferences, the crown’s outreach, distances and forms were considered, while considering other characteristics of the crown, the branching level and the existence of kinks led to significantly decreasing effects on repeatability (Fig. 5). Measures in the areas of tray and bay tines also increased repeatability. As an example, bay tine lengths (CIC) were similar in all antlers of a distinct stag, while they differed extremely between the stags (Fig. 6). In fact, the length varied between 2.6 and 41 cm. The 95% confidence intervals of different individuals mostly showed only a slight overlap. Pairwise significances between different stags are not presented, but after Bonferroni correction, 45% of all pairwise comparisons were statistically significant.
Effects of different antler trait components on the variance of antler characteristics explained by the stag (repeatability), shown as the regression coefficient (B) expressed as a positive or negative deviation from the mean repeatability of 40.5%. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. High percentage of repeatability means low (intraspecific/interindividual) variance
Effects of the age were especially visible for burr and signet circumference, direct or indirect length of the brow tine, where 28 to 36% of variance were explained. The stags’ age had no effect on some crown forms, brow tine bending, splitting and other characteristics (Fig. 7).
Circumferences and the existence of branching levels were more greatly affected by age than by other characteristics, while crown characteristics, counted numbers, signet, bay tine characteristics, bending and splitting were least affected by age (Fig. 8).
Effects of different antler trait components on the variance of antler characteristics explained by age. This figure shows the regression coefficient (B) in the form of a positive or negative deviation from the mean variance explained by the age of the stag at casting (5.6%). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
Only 0 to 3% of variation were explained by the antler side. The highest effects of the side were found for crown length, some angles and bends (Figs. 9 and 10).
The overall model explained 51.2% of the variance of antler characteristics (between 3.7 and 83%; Fig. 10). Burr and signet diameter/circumference, bay tine length, antler weight, beam length and many more characteristics had the highest coefficients of determination. Crown form including realised branching levels showed the highest degrees of variation that could not be explained by any of the studied effects.
Characteristics based on crown width, circumferences, lengths, curvatures, distances and numbers had significantly higher coefficients in multiple regression analysis, and any other crown characteristics, including the realised branching levels, had significantly lower coefficients than other characteristics (Fig. 10).
Eleven antler characteristics, seven of them defined by CIC, are frequently used in other studies. Five to 68 of the characteristics measured in the present study had higher repeatability (Fig. 11). Among them, the number of tines (according to CIC) had the highest repeatability. Only brow tine circumference, bay tine length (CIC), the ratio of bay tine length to beam length, the distance between brow tine tip and tray tine tip and the distance between brow tine tip and crown base had a higher repeatability.
Relative repeatability of the most frequently used antler characteristic related to the characteristics of the present study. Brow tine = first tine; bay tine = second tine (bez tine); tray tine = third tine (trez tine). High percentage of repeatability means low (intraspecific/interindividual) variance
Discussion
Constant antler characteristics are of great importance for the correct identification of individual deer and for a wide variety of scientific studies. Thus, traits with low variability could be used to more accurately estimate gene-environment interactions and genetic principles of antler development than those with high variation. Studies on the phylogenetic evolution of red deer (Samejima and Matsuoka 2020; Heckeberg et al. 2022) would also benefit from distinguishing traits of high from those of low variability and using them in a more targeted manner. Early facts on antler tine anatomy, homology and terminology can be found in Brooke (1878) and Pocock (1933). Antlers are generally considered relatively constant over time in an individual (Azorit et al. 2002; Fierro et al. 2002; Garcia et al. 2002; Kruuk et al. 2002), especially antler weight and length, and the number of tines is used to address individuals for hunting and selecting purposes. The issues raised in this context, which are estimated on the basis of antler shape, are extraordinarily diverse. However, the present study shows a considerable variation in antler traits among individuals from year to year. The repeatability of antler characteristics has been studied mostly in only a few traits. It ranged from 51% (Peters et al. 2022, free range), 53% (present study), 57% (Kruuk et al. 2002, free range), 64% (Van den Berg and Garrick 1997, captive) and 75% (Bartos et al. 2007, captive) for antler weight in red deer and 75.2% (Bartos et al. 2007) and 61% (present study) for antler length, respectively. Antler length repeatability ranged from 59 to 82% for white-tailed deer (Lukefahr and Jacobsen 1998; Foley et al. 2012). In the present study, the assumption of relatively high repeatability for weight and length as well as for end numbers (Bartos et al. 2007: 70.1% vs. 68% in the present study) was confirmed, as is the markedly high variability of crown characteristics. However, among the crown characteristics, their spatial extent or outreach, in particular the length of the projection of the crown of the individual cast antler in three-dimensional space, was found to be remarkably constant (repeatability 65%), a characteristic not previously considered in other studies. The number of crown tines also showed high repeatability (59.7%), especially when considering adult deer between 5 and 14 years of age, as in the present study (Mysterud et al. 2005; Martínez Salmerón 2014). The high agreement of the values of essential characteristics with the results from various other studies shows that the current study is built on a solid database.
Repeatability of antler characteristics of the present study can be compared especially well with the study by Peters et al. (2022) which was based on 10 antler characteristics. Repeatabilities (Peters et al. 2022 vs. this study) of tray length (66.1% vs. 65.0%), upper beam circumference (63.7% vs. 58%), antler weight (51.4% vs. 53%), coronet circumference (55.4% vs. 45%), lower beam circumference (48.3% vs. 54.4%), distances between coronet and brow, brow and tray (55–56% vs. 45–74%), brow length (48.1% vs. 47%) and form (number of crown tines) (54.1% vs. 59.7%) corresponded very well, except for antler length (38.5% vs. 61%), although the higher values corresponded well with other data from Cervidae (Lukefahr and Jacobsen 1998; Foley et al. 2012). Additionally, the present study identified some characteristics with even higher repeatability of 67 to 74% that might be useful for future research.
Variation in antler characteristics increased to a large extent from proximal to distal depending on the stag’s age, but also unsystematically, randomly. Repeatability was highest for the positions and distances between the bases of brow, bay and tray tine on the beam and for the lengths and circumferences of bay tine, tray tine and beam and the tine number. These characteristics were especially suitable to identify individual stags. The number of tines and lengths of tray tine were also investigated in other studies (Lukefahr and Jacobsen 1998; Bartos et al. 2007; Foley et al. 2012). Only four to 22 of the 125 characteristics of the present study had a higher repeatability than these two characteristics. However, 24 to 68 of the 125 characteristics of the present study showed a higher repeatability when compared to the remaining nine characteristics that are frequently used in other studies. Thus, it might be interesting to include those with the highest repeatability in further studies or field work, where unsystematic variation of characteristics might reduce information values, e.g. in genetic studies.
Effects of the stag age on antler characteristics
Strength, length and end richness of antlers increase rapidly with age at first, but only slowly from age 7 to 8 years, and the strongest antlers develop between ages 8 and 14 years (Garcia et al. 2010). Thereafter, antlers regress, especially in terms of their end richness and the expression of the branches (Montoya 1999; Carranza 1999; Azorit et al. 2002; Garcia et al. 2010). The weight, circumference and length of the main beam decrease only slightly (Bokor et al. 2013). The number of tines is subject to marked variation. It can range from 2 to 12 tines in 2-year-old stags and from 2 to 16 tines in 6–12-year-old stags (Mysterud et al. 2005; Martínez Salmerón 2014). The most end-rich antlers occur at 8 to 9 years of age (Martínez Salmerón 2014). These findings were also true for the current study.
Degmečić and Florijančić (2014) were particularly interested in the similarity of antlers in animals under and over 5 years of age, and they described a significant developmental jump. Thus, we excluded the younger age classes for the present study. Therefore, in principle, only a few antlers of the young age class were collected (but not included into analysis). Antlers increase markedly in mass, length and expression with age until 8 to 10 years of age (Lockow 1991; present study). In some characteristics, repeatability could be increased, if the age of the stag was known (Ludwig and Vocke 1990; Drechsler 1992; Clements et al. 2010). This applies to the circumference/diameter of burr and signet; the lengths and circumferences of beam, brow tine, bay tine and tray tine; and the antler weight. Burr and signet and length and circumferences of beam, brow and bay tine and the antler weight are especially dependent on the stag age. Other characteristics that changed clearly with age were the pearls, tine quality and colour according to CIC.
Effect of the side on antler characteristics
The vanishingly small effect of the side on the expression of the characteristics is well known (Mateos et al. 2008; Schoenebeck et al. 2013). Only for the crown shape, numerous asymmetries could be detected. Stronger expressions could be observed in the same stag on the left as well as on the right side. Only in one stag, all traits examined in lateral comparison were always greater on the right than on the left. Such a preference of the right antler side was also described in the literature (Alvarez 1995; Martínez Salmerón 2014).
Effect of the stag’s origin on antler characteristics
The origin of the stags was only considered as a random effect, since the selection of the animals in the field was not done deliberately to estimate differences and effects on antler characteristics in this regard. Such origin effects have been described in detail in the literature and explained by genetic differences and environmental factors (Lowe 1971; Mitchell 1967; Mitchell et al. 1986; Beccu 1989; Mattioli 1993; Bokor et al. 2013; Mattioli and Ferretti 2014).
Number of tines
The current study confirms that the number of tines is not a suitable feature for age estimation, especially between 5 and 14 years (Clutton-Brock et al. 1980; McCorquodale et al. 1989; Azorit et al. 2002; Gaspar-López et al. 2010; Šmehýl et al. 2018; Mysterud et al. 2005; Drechsler 1992). The effect of age also did not play a significant role in the number of additional tines (< 2 cm).
The occurrence of beam processes was explained to a considerable extent by the stag, but only a few deer actually had beam processes every year. Thus, they can actually only serve to identify stags to a limited extent. Comparable data are not available.
Burr
Burr and signet were inevitably particularly constant characteristics within a stag. The strong age influence of burr circumference can be explained by the fact that this characteristic is predetermined by the burr diameter, which shows a positive correlation with age up to the age of 13 (Drechsler 1992). The results for burr circumference according to CIC are also consistent with the literature (Fierro et al. 2002; Bokor et al. 2013). That older stags have more concave signets than young ones because they shed their antlers earlier (Gaspar-López et al. 2010) was confirmed by our results.
Beam
The age-dependent increase in antler weight was consistent with literature findings (Clutton-Brock 1982; Clements et al. 2010; Drechsler 1992; Bokor et al. 2013). However, in contrast to Šmehýl et al. (2018), a regress of weight was observed as early as 14 years of age. Beam length results also agreed well with literature data (Ludwig and Vocke 1990; Drechsler 1992; Bokor et al. 2013; Šmehýl et al. 2018).
Also, characteristics of the lower beam and main tines showed a relatively high constancy of expression. The beams of older stags should be more curved distally than those of younger stags. This assumption was confirmed in our study, in agreement to Martínez Salmerón (2014). The curvature of the main beam has been found to be a highly constant feature and is therefore suited for distinguishing stags from each other.
Main tines
Consistent with the literature, there was a strong age dependence of the lengths of the brow tine and tray tine according to CIC (Drechsler 1992; Bokor et al. 2013). The greater curvature of brow tine and tray tine in older stags was consistent with the results of Martínez Salmerón (2014).
The distances between the bases of the main tines hardly changed with age. This finding was also consistent with the results of other investigators (Fierro et al. 2002). In contrast, the fact that the tines attach more proximally at the beam with age (Martínez Salmerón 2014; Lehmann 1959) could not be confirmed with our study. However, such differences between studies may be due to different genetics and origin of the stags studied.
According to Martínez Salmerón (2014), brow tine and tray tine are less curved in younger deer than in older ones. Such age dependence could not be confirmed with the present study. This discrepancy indicates the possibility of existing differences between different red deer subspecies. Further studies are needed to conclusively resolve this issue. Bay tines may be absent or present in a deer at different time points. They occur more frequently in older stags (Fierro et al. 2002; Azorit et al. 2002; Martínez Salmerón 2014), which is also largely consistent with the current study. Nevertheless, bay tine characteristics showed a relatively high repeatability with only small age effects. Thus, there appear to be individuals with predisposition and those without predisposition to bay tines, although the penetrance of the trait does not reach 100%. It is thought that a correlation between the lower beam circumference and the occurrence of bay tines exists. However, the present study could not confirm such a correlation in the present samples.
The curvature of the main tines is variable due to its great age and origin dependence, and consequently, it is less suitable to distinguish stags from each other in practice. These results are consistent with those found in the literature, which indicate that the brow tine and tray tine are straighter in younger stags and more curved in older ones (Martínez Salmerón 2014).
It is often assumed that the presence of a divided brow tine and tray tine is dependent on age. The present study, however, shows that the division of the brow tine is highly dependent on the individual and little on age. In the case of tray tine division, a strong dependence on origin was prominent, which, in turn, may have environmental or genetic causes.
Crown
The highest variabilities were found for the architecture of the crown, including kinks, branching levels and form which changes dramatically from year to year and even between sides. Thus, crown parameters can hardly be used if individuals have to be identified (Azorit et al. 2002; García et al. 2002). This aspect should also be considered with regard to studies on historical and prehistoric antlers. The only exception is the number of crown tines (25/125), crown length (41/125) and crown height (57/125). The number of crown tines (CIC) was employed in other studies, and the outreach of the crown in single cast antlers has not been described so far. Repeatability of crown characteristics could also not be improved, if the age was considered, because they could change dramatically from year to year.
The appearance of the crown is also described in the literature as highly diverse and variable, apart from the number of crown tines (Azorit et al. 2002; García et al. 2002; Martínez Salmerón 2014), and only few comparable studies are available. Environmental factors and age, in particular, are considered to be causes of the high variability in crown shape (Martínez Salmerón 2014). In our own study, however, age showed only minor effects on crown shape. Different authors report differences in the onset of the reduction of the number of crown tines, which already occurred at 10–12 years of age (Ludwig and Vocke 1990; Martínez Salmerón 2014) or only at an age of 14 years (Azorit et al. 2002; present study).
About 60% of the stags had asymmetric crown forms, i.e. different forms on the right and left sides in the same year. Since the same genes are influencing antler development in both sides, left–right asymmetry does not seem to be genetically influenced. If the crown was symmetrical in shape, it was mostly step crowns or branch crowns. These were also the most common, along with crowns with a single front end and multiple forked rear tines. Most stags exhibited 4 to 7 different crown shapes during the life span studied. Different crown shapes occurred more frequently at differing ages. There was no general trend towards more or less different crown shapes with increasing age.
The pronounced variation in the results of different studies is based on the examination of different red deer populations, the inclusion of free-living animals and of farmed deer, the comparison of cast antlers and trophies and, last but not least, on different habitats, whereby the influences are overlapping and fluid. In particular, factors such as population density, food supply and nutrient supply (Lukefahr and Jacobson 1998), but also climate (Smith 1998) and rainfall, are cited here (Kruuk et al. 2002; Azorit et al. 2002; Peláez et al. 2018). Supplemental winter feeding improves antler growth (Hamilton and Suttie 1983; Kozak et al. 1994), and mild winters have a positive effect on tine number (Smith 1998; Degmečić and Florijančić 2014). Habitat improvements are known to have a positive effect on crown tine number (Mattioli et al. 2003; Mattioli and Ferretti 2014). That the number of crown tines reached higher repeatabilities in the present study, in contrast to other studies (Drechsler 1992; Garcia et al. 2010; Mysterud et al. 2005), suggests that there are relatively constant environmental conditions in the study area. At the same time, the current data confirm that crown tines are also not a good indicator for age determination (Drechsler 1992; Garcia et al. 2010; Mysterud et al. 2005).
Conclusions
The reliability of the expression of antler characteristics, independent of age, side or random processes, is of great importance for the characterisation of individual stags in practice and research. The present study reveals a pronounced agreement in 11 antler features frequently used by other studies. However, several of the remaining 105 characteristics examined were less variable and thus achieved a higher repeatability. Other characteristics were more closely correlated with the age. In larger studies, it is hardly possible to characterise the antlers as comprehensively as in the present study. The presented results facilitate the reasonable selection of phenotypes that are as constant as possible for studies with high animal numbers (e.g. genomic studies), but also for studies with limited animal numbers (e.g. taxonomic studies) and practical questions with the need for detection and selection of individuals. Additionally, some assumptions about the constancy and variability of antler traits could be confirmed or rejected in the context of the studied stag cohort.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alvarez F (1995) Functional directional asymmetry in fallow deer (Dama dama) antlers. J Zool 236:563–569
Azorit C, Analla M, Carrasco R, Munoz-Coro J (2002) Influence of age and environment on antler traits in Spanish red deer (Cervus elaphus hispanicus). Zeitschr Jagdwissensch 48:137–144
Bartos L, Bahbouh R (2006) Antler size and fluctuating asymmetry in red deer (Cervus elaphus) stags and probability of becoming a harem holder in rut. Biol J Linn Soc 87:59–68
Bartos L, Bahbouh R, Vach M (2007) Repeatability of size and fluctuating asymmetry of antler characteristics in red deer (Cervus elaphus) during ontogeny. Biol J Linn Soc 91:215–226
Beccu E (1989) II cervo sardo. In: Delfino Editore, Sassari, pp. 1–167
Bokor J, Bokor A, Nagy J, Horn P, Nagy I (2013) Summary of comments on analysis of Hungarian red deer trophies by means of principal component analysis in two different counties. J Central Europ Agri 14:452–466
Brooke V (1878) On the classification of the Cervidae, with a synopsis of the existing species. Proc Zool Soc 883–928
Bubenik GA, Schams D, Coenen G (1987) The effect of artificial photoperiodicity and antiandrogen treatment on the antler growth and plasma levels of LH, FSH, testosterone, prolactin and alkaline phosphatase in the male white-tailed deer. Comp Biochem Physiol 87A:551–559
Carranza J (1999) Aplicationes de la Etología al manejo de las poblaciones de ciervo del suroeste de la Península Ibérica: produccíon y conservación. Etología 7:5–18
Clements MN, Clutton-Brock TH, Albon ST, Pemberton JM, Kruuk LEB (2010) Getting the timing right: antler growth phenology and sexual selection in a wild red deer population. Oecologia 164:357–368
Clutton-Brock T, Albon SD (1989) Red deer in the highlands. Blackwell Scientific Publications, Oxford, UK
Clutton-Brock TH (1982) The functions of antlers. Behaviour 79:108–125
Clutton-Brock TH, Albon SD, Harvey PH (1980) Antlers, body size and breeding group size in the Cervidae. Naturae 285:565–567
Degmečić D, Florijančić T (2014) The impact of climate and hydrological factors in development of red deer (Cervus elaphus, L.) antlers in Croatian part of Baranja Danube region. Šumarski List 9–10:451–461
Drechsler H (1992) Über die Zusammenhänge zwischen verschiedenen Körper- und Geweihmerkmalen der Rothirsche und dem Alter. Zeitschr Jagdwissensch 38:101–106
Fierro Y, Gortazar C, Landete-Castillejos T, Vicente J, García A, Gallego L (2002) Baseline values for cast antlers of Iberian red deer (Cervus elaphus hispanicus). Zeitschr Jagdwissensch 48:244–251
Foley AM, DeYoung RW, Lukefahr SD, Lewis JS, Hewitt DG, Hellickson MW, Draeger DA, DeYoung CA (2012) Repeatability of antler characteristics in mature white-tailed deer in South Texas: consequences of environmental effects. J Mammal 93:1149–1157
García AJ, Landete-Castillejos T, Garde JJ, Gallego L (2002) Reproductive seasonality in female Iberian red deer (Cervus elaphus hispanicus). Theriogen 58:1553–1562
Garcia AJ, Gaspar-López E, Estévez JA, Gómez JA, Ceacero F, Olguín A (2010) El trofeo en los cérvidos: caracterizatión funcional del crecimiento de la cuerna usando como modelo el ciervo. In: Santiago M. und López S. (Hrsg.): Ungulados silvestres de España, pp 267–295
Gaspar-López E, Landete-Castillejos T, Estevez JA, Ceacero F, Gallego L, García AJ (2010) Biometrics, testosterone, cortisol and antler growth cycle in Iberian red deer stags (Cervus elaphus hispanicus). Reprod Domest Anim 45:243–249
Hamilton WJ, Suttie JM (1983) The effect of winter nutrition on growth of young Scottish Red deer (Cervus elaphus). J Zool 201:153–161
Heckeberg NS, Zachos FE, Kierdorf U (2022) Antler tine homologies and cervid systematics: a review of past and present controversies with special emphasis on Elaphus davidianus. Anat Rec 1–24
Johnston SE, Huisman J, Ellis PA, Pemberton JM (2017) A high-density linkage map reveals sexual dimorphism in recombination landscapes in red deer (Cervus elaphus). G3 (Bethesda. Md.) 7:2859–2870
Kolejanisz T, Sonkoly K, Csányi S (2012) Age-dependent changes of antler size in red deer in two contrasting habitats in Hungary. Rev Agricult Rural Developm 1:314–320
Kozak HM, Hudson RJ, Renecker LA (1994) Supplemental winter feeding. Rangelands 16:153–156
Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guinness F, Clutton-Brock T (2002) Antler size in red deer: heritability and selection but no evolution. Int J Organic Evol 56:1683–1695
Landete-Castillejos T, Currey JD, Estevez JA, Gaspar-Lopez E, Garcia AJ, Gallego L (2007) Influence of physiological effort of growth and chemical composition on antler bone mechanical properties. Bone 41:794–803
Landete-Castillejos T, Currey JD, Estevez JA, Fierro Y, Calatayud A, Caecero F, Garcia AJ, Gallego L (2010) Do drastic weather effects on diet influence changes in chemical composition, mechanical properties and structure in deer antlers? Bone 47:815–825
Landete-Castillejos T, Estevez F, Ceacero JA, Garcia AJ, Gallego L (2013) Effects of public vs. private management on deer antler composition, mechanical and structural variables. Eur J Wildl Res 59:519–529
Landete-Castillejos T, Kierdorf U, Gomez S, Luna S, Garcia AJ, Cappelli J, Perez-Serrano M, Perez-Barberia J, Gallego L, Kierdorf U (2019) Antlers - evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone 128:115046
Lincoln GA (1972) The role of antlers in the behaviour of red deer. J Exper Zool 182:233–249
Lockow KW (1991) Vorhersage der Geweihentwicklung des Rothirschs – eine Entscheidungshilfe für Wahlabschuß und Hege. Z Jagdwiss 37:24–34
Lowe VPW (1971) Some effects of a change in estate management on a deer population. In: E. Duffey and A. S. Watt. Blackwell (eds) The scientific management of animal and plant communities for conservation. pp. 435–456
Ludwig J, Vocke G (1990) Die Vereinfachung einer Wachstumsfunktion. dargestellt am Rothirschgeweih. Zeitschr Jagdwissensch 36:119–225
Lukefahr SD, Jacobson HA (1998) Variance component analysis and heritability of antler traits in white-tailed deer. J Wildl Managem 62:262–268
Martínez Salmerón D (2014) Three-dimensional study of the Iberian red deer antler (Cervus elaphus hispanicus): application of geometric morphometrics techniques and other methodologies. Thesis University Barcelona
Mateos C, Alarcos S, Carranza J, Sánchez-Prieto CB, Valencia J (2008) Fluctuating asymmetry of red deer antlers negatively relates to individual condition and proximity to prime age. Animal Behav 75:1629–1640
Mattioli S (1993) Antler conformation in red deer of the Mesola Wood, Northern Italy. Acta Theriol 38:443–450
Mattioli S, Fico R, Lorenzini R, Nobili G (2003) Mesola red deer: Physical characteristics, population dynamics and conservation perspectives. Ital J Mammal 14:87–94
Mattioli S, Ferretti F (2014) Morphometric characterization of Mesola red deer Cervus elaphus italicus (Mammalia: Cervidae). Ital J Zool 81:144–154
McCorquodale M, Eberhardt LE, Sargeant GA (1989) Antler characteristics in a colonizing elk population. J Wildl Managem 53:618–621
Mitchell B (1967) Growth layers in dental cement for determining the age of red deer (Cervus elaphus L.). J Anim Ecol 36:279–293
Mitchell B, McCowan D, Parish T (1986) Performance and population dynamics in relation to management of red deer Cervus elaphus at Glenfeshie, inverness-shire, Scotland. Biol Conserv 37:237–267
Montoya JM (1999) El ciervo y el monte. Manejo y conservaciòn (Cervus elaphus L.). Madrid: Editorial Mundi-prensa, p 308
Mysterud A, Meisingset E, Langvatn R, Yoccoz NG, Stenseth NC (2005) Climate-dependent allocation of resources to secondary sexual traits in red deer. Nordic Society Oikos 111:245–252
Peláez M, Perea R, Díaz M, San Miguel A, Rodríguez-Vigal C, Côté SD (2018) Use of cast antlers to assess antler size variation in red deer populations: effects of mast seeding, climate and population features in Mediterranean environments. J Zool 306:8–15
Pérez-González J, Carranza J, Torres-Porras J, Fernández-García JL (2010) Low heterozygosity at microsatellite markers in Iberian red deer with small antlers. J Hered 101:553–561
Peters L, Huisman J, Kruuk LEB, Pemberton JM, Johnston SE (2022) Genomic analysis reveals a polygenic architecture of antler morphology in wild red deer (Cervus elaphus). Mol Ecol 31:1281–1298
Pocock RI (1933) The homologies between the branches of the antlers of the Cervidse based on the theory of dichotomous growth. J Zool 103:377–406
Price J, Allen S (2004) Exploring the mechanisms regulating regeneration of deer antlers. Phil Trans Royal Soc London B. Biol Sci 359:809–822
Price JS, Allen S, Faucheux C, Althnaian T, Mount JG (2005) Deer antlers: a zoological curiosity or the key to understanding organ regeneration in mammals? J Anatomy 207:603–618
Putman RJ, Staines BW (2004) Supplementary winter feeding of wild red deer Cervus elaphus in Europe and North America: justifications. feeding practice and effectiveness. Mammals Rev 34:285–306
Reiner G, Klein C, Lang M, Willems H (2021) Human-driven genetic differentiation in a managed red deer population. Eur J Wildl Res 67:29
Samejima Y, Matsuoka H (2020) A new point on antlers reveals the evolutionary history of deer (Cervidae, Mammalia). Sci Rep 10:8910
Schatz A (1987) Serienschnitt Technik im MultiSegment TM - Verfahren zur Altersbestimmung bei Cerviden. Online verfügbar unter https://www.schatz-zahnschliff.com/multisegmenttm-verfahren-von-a-schatz-seit-1987/
Schmidt KT, Stien A, Albon SD, Guinness FE (2001) Antler length of yearling red deer is determined by population density. weather and early life-history. Oecologia 127:191–197
Schoenebeck CW, Peterson BC, Obermiller JA (2013) Accuracy of antler metrics in predicting age of white-tailed deer and mule deer. Great Plains Res 23:33–37
Šmehýl P, Hlavatý J, Slamečka J (2018) Evaluation of European red deer (Cervus elaphus hippelaphus) antlers traits in district of Čadca. Anim Sci Biotechnol 51:70–75
Smith BL (1998) Antler size and winter mortality of elk: effects of environment, birth year, and parasites. J Mammal 79:1038–1044
Trense W, de Boislambert AJH, Whitehead GK (1981) Die Jagdtrophaen der Welt; Les trophées de chasse du monde; The game-trophies of the world. Germany: Hamburg and Berlin: P. Parey
Van den Berg GHJ, Garrick DJ (1997) Inheritance of adult velvet antler weights and live weights in farmed red deer. Livestock Prod Sci 49:287–295
Vanpé C, Gaillard JM, Kjellander P, Liberg O, Delorme D, Hewison AJ, Mark, (2010) Assessing the intensity of sexual selection on male body mass and antler length in roe deer Capreolus capreolus: is bigger better in a weakly dimorphic species? Oikos 119:1484–1492
Von Lehmann E (1959) Zur Homologie der unteren Geweihsprossen. Zeitschr Säugetierk 24:54–69
Willems H, Welte J, Hecht W, Reiner G (2016) Temporal variation of the genetic diversity of a German red deer population between 1960 and 2012. Eur J Wildl Res 62:277–284
Acknowledgements
The authors greatly appreciate the help of the hunters and active members of the red deer conservation societies for the provision of cast antlers and antlers.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
KB collected the samples, elaborated the 3D technology and recorded the antler characteristics. KB also took the photos. HW performed the laboratory tests. GR performed the statistical analysis and prepared the texts. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
No animal experiments were performed in the present study. Only samples of stags shot and cast antlers already collected before the study, by authorised persons during legalised hunting and collecting within the framework of red deer management, were used. No animals were killed specifically for the study. No living animals were sampled, and no cast antlers were sought or collected for the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bils, K., Willems, H. & Reiner, G. Variation of antlers in individual red deer (Cervus elaphus) stags: repeatability, age and side effects. Eur J Wildl Res 69, 27 (2023). https://doi.org/10.1007/s10344-023-01646-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01646-6