Abstract
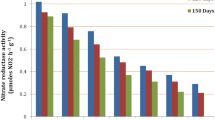
Sweet cherry is a temperate zone fruit tree grown under a wide range of climate conditions worldwide. Thus, understanding its responses to salt stress is important since cherry growing is influenced by salinity stress. In our study, many morphological, physiological and molecular responses were evaluated to investigate the influences of moderate salinity stress on sweet cherry. The study was conducted for 2 consecutive years. ‘0900 Ziraat’ sweet cherry plants (1 year old) grafted onto Mazzard (Prunus avium L.), MaxMa 14 (Mahaleb × Mazzard) and CAB-6P (Prunus cerasus L.) were planted in 13 L plastic containers filled with soil, peat and perlite (1:3:1). After about 1 month of acclimation, salinity application was started by irrigation with 35 mM NaCl solution except control plants. The saplings were treated by salinity for 4 months. At the end of the experiment, remarkable decreases in plant growth were noted under salinity conditions. 0900/Mazzard possessed remarkable decline in shoot diameter. 0900/Mazzard possessed higher declines in SPAD and stomatal conductivity values by 23.9 and 18.7%, respectively. The lowest increase in membrane permeability was found in 0900/CAB-6P. Total score analysis based on the morphological and physiological parameters revealed that 0900/CAB-6P with the lowest score was considered the most tolerant, whereas 0900/MaxMa 14 with the highest score was regarded as the most sensitive one. The expression of WRKY25, WRKY33 and WRKY38 genes in all plants was significantly increased by salinity. We consider that the increment in gene expression triggered a defense mechanism to salinity.


Similar content being viewed by others
References
Akçay D, Eşitken A (2017) MM106 anacı ve üzerine aşılı Golden Delicious elma çeşidine tuz stresinin etkileri. Selçuk Tarım Bilim Derg 3(2):228–232
Akrami M, Arzani A (2018) Physiological alterations due to field salinity stress in melon (Cucumis melo L.). Acta Physiol Plant 40:1–14
Aras S (2020) Silicon nutrition in alleviating salt stress in apple plant. Acta Sci Polonorum Hort Cultus 19(1):3–10
Aras S, Eşitken A (2019a) Dry matter partitioning and salt tolerance via salicylic acid treatment in strawberry plant under salt stress. Kahramanmaras Sütcü İmam Univ Doga Bilim Derg 22:337–341
Aras S, Eşitken A (2019b) Responses of apple plants to salinity stress. Yüzüncü Yil Univ J Agr Sci 29(2):253–257
Aras S, Keles H (2019) Evaluation of leaf properties of eight cherry cultivars grafted onto Maxma 14 rootstock. J Agric Stud 7(3):145–153
Aras S, Arslan E, Esitken A (2015) Biochemical and physiological responses of lemon plant under salt stress. Proceedings of 2nd International Conference on Sustainable Agriculture and Environment, Konya, September 30-October 3 (Oral presentation)
Balal RM, Ashraf MY, Khan MM, Jaskani MJ, Ashfaq M (2011) Influence of salt stress on growth and biochemical parameters of citrus rootstocks. Pak J Bot 43(4):2135–2141
Bankaji I, Sleimi N, Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM (2019) Identification and expression of the Cucurbita WRKY transcription factors in response to water deficit and salt stress. Sci Hortic 256:108562
Carillo P, Raimondi G, Kyriacou MC, Pannico A, El-Nakhel C, Cirillo V, Colla G, De Pascale S, Rouphael Y (2019) Morpho-physiological and homeostatic adaptive responses triggered by omeprazole enhance lettuce tolerance to salt stress. Sci Hortic 249:22–30
Chartzoulakis K, Loupassaki M, Bertaki M, Androulakis I (2002) Effects of NaCl salinity on growth, ion content and CO2 assimilation rate of six olive cultivars. Sci Hortic 96(1–4):235–247
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Cheng Y, Zhou Y, Yang Y, Chi YJ, Zhou J, Chen JY, Wang F, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z (2012) Structural and functional analysis of VQ motif containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol 159(2):810–825
Dai W, Wang M, Gong X, Liu JH (2018) The transcription factor Fc WRKY 40 of Fortunella crassifolia functions positively in salt tolerance through modulation of ion homeostasis and proline biosynthesis by directly regulating SOS 2 and P5 CS 1 homologs. New Phytol 219(3):972–989
Del Amor F, Marcelis LFM (2003) Regulation of nutrient uptake, water uptake and growth under calcium starvation and recovery. J Hortic Sci Biotechnol 7:343–349
De Pascale S, Maggio A, Orsini F, Stanghellini C, Heuvelink E (2015) Growth response and radiation use efficiency in tomato exposed to short-term and long-term salinized soils. Sci Hortic 189:139–149
Du C, Zhao P, Zhang H, Li N, Zheng L, Wang Y (2017) The Reaumuria trigyna transcription factor RtWRKY1 confers tolerance to salt stress in transgenic Arabidopsis. J Plant Physiol 215:48–58
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA (2019) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem 137:144–153
Elsawy HI, Mekawy AMM, Elhity MA, Abdel-dayem SM, Abdelaziz MN, Assaha DV, Ueda A, Saneoka H (2018) Differential responses of two Egyptian barley (Hordeum vulgare L.) cultivars to salt stress. Plant Physiol Biochem 127:425–435
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146:359–388
Garcia-Legaz MF, López-Gómez E, Beneyto JM, Navarro A, Sánchez-Blanco MJ (2008) Physiological behaviour of loquat and anger rootstocks in relation to salinity and calcium addition. J Plant Physiol 165(10):1049–1060
Gonçalves B, Moutinho-Pereira J, Santos A, Silva AP, Bacelar E, Correia C, Rosa E (2006) Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol 26(1):93–104
Gong XQ, Hu JB, Liu JH (2014) Cloning and characterization of FcWRKY40, A WRKY transcription factor from Fortunella crassifolia linked to oxidative stress tolerance. Plant Cell Tissue Organ Cult 119(1):197–210
Goodacre R, Shann B, Gilbert RJ, Timmins EM, McGovern AC, Alsberg BK, Kell DB, Logan NA (2000) Detection of the dipicolinic acid biomarker in bacillus spores using Curie-point pyrolysis mass spectrometry and Fourier transform infrared spectroscopy. Anal Chem 72:119–127
Gucci R, Tattini M (1997) Salinity tolerance in olive. Hortic Rev 21:177–214
Hezaveh TA, Pourakbar L, Rahmani F, Alipour H (2019) Interactive effects of salinity and ZnO nanoparticles on physiological and molecular parameters of rapeseed (Brassica napus L.). Commun Soil Sci Plant Anal 50(6):698–715
Jia XM, Wang H, Svetla S, Zhu YF, Hu Y, Cheng L, Zhao T, Wang YX (2019) Comparative physiological responses and adaptive strategies of apple Malus halliana to salt, alkali and saline-alkali stress. Sci Hortic 245:154–162
Jiang Y, Deyholos MK (2009) Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol 69(1–2):91–105
Karabal E, Yucel M, Oktem HA (2003) Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci 164:925–930
Küçükyumuk C, Yildiz H, Ünlükara A (2014) Tuz Stresinin Farklı Anaçlar Üzerine Aşılı Kirazın Vejetatif Gelişimine Etkilerinin Belirlenmesi. Türk Tarimsal Arastirmalar Derg 1(2):154–161
Kumar J, Singh S, Singh M, Srivastava PK, Mishra RK, Singh VP, Prasad SM (2017) Transcriptional regulation of salinity stress in plants: a short review. Plant Gene 11:160–169
Li H, Guo Q, Lan X, Zhou Q, Wei N (2014) Comparative expression analysis of five WRKY genes from Tibetan hulless barley under various abiotic stresses between drought-resistant and sensitive genotype. Acta Physiol Plantarum 36(4):963–973
Li S, Fu Q, Huang W, Yu D (2009) Functional analysis of an Arabidopsis transcription factor WRKY25 in heat stress. Plant Cell Rep 28(4):683–693
Lin J, Dang F, Chen Y, Guan D, He S (2019) CaWRKY27 negatively regulates salt and osmotic stress responses in pepper. Plant Physiol Biochem 145:43–51
Liu QL, Zhong M, Li S, Pan YZ, Jiang BB, Jia Y, Zhang HQ (2013) Overexpression of a chrysanthemum transcription factor gene, DgWRKY3, in tobacco enhances tolerance to salt stress. Plant Physiol Biochem 69:27–33
Luo DL, Ba LJ, Shan W, Kuang JF, Lu WJ, Chen JY (2017) Involvement of WRKY transcription factors in abscisic-acid-induced cold tolerance of banana fruit. J Agric Food Chem 65:3627–3635
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Mahouachi J (2009) Changes in nutrient concentrations and leaf gas exchange parameters in banana plantlets under gradual soil moisture depletion. Sci Hortic 120:460–466
Mare C, Mazzucotelli E, Crosatti C, Francia E, Cattivelli L (2004) Hv-WRKY38: a new transcription factor involved in cold-and drought-response in barley. Plant Mol Biol 55(3):399–416
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Plant Biol 59:651–681
Mustard J, Renault S (2004) Effects of NaCl on water relations and cell wall elasticity and composition of red-osier dogwood (Cornus stolonifera) seedlings. Physiol Plant 121:265–271
Nam TN, Sao DM, Tuan NV (2017) Overexpression of NbWRKY79 enhances salt stress tolerance in Nicotiana benthamiana. Acta Physiol Plantarum 39(6):121
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742
Okubo M, Sakuratani T (2000) Effects of sodium chloride on survival and stem elongation of two Asian pear rootstock seedlings. Sci Hortic 85(1–2):85–90
Papadakis IE, Veneti G, Chatzissavvidis C, Sotiropoulos TE, Dimassi KN, Therios IN (2007) Growth, mineral composition, leaf chlorophyll and water relationships of two cherry varieties under NaCl-induced salinity stress. Soil Sci Plant Nutr 53(3):252–258
Richardson SG, McCree KJ (1985) Carbon balance and water relations of sorghum exposed to salt and water stress. Plant Physiol 79:1015–1020
Ruiz D, Martínez V, Cerdá A (1997) Citrus response to salinity: growth and nutrient uptake. Tree Physiol 17(3):141–150
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101
Shekhawat UKS, Ganapathi TR, Srinivas L (2011) Cloning and characterization of a novel stress-responsive WRKY transcription factor gene (MusaWRKY71) from Musa spp. cv. Karibale Monthan (ABB group) using transformed banana cells. Mol Biol Rep 38:4023–4035
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260
Soliman M, Elkelish A, Souad T, Alhaithloul H, Farooq M (2020a) Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol Mol Biol Plants 26(3):501–511
Soliman MH, Abdulmajeed AM, Alhaithloul H, Alharbi BM, El-Esawi MA, Hasanuzzaman M, Elkelish A (2020b) Saponin biopriming positively stimulates antioxidants defense, osmolytes metabolism and ionic status to confer salt stress tolerance in soybean. Acta Physiol Plantarum 42(7):1–13
Sotiropoulos TE (2007) Effect of NaCl and CaCl2 on growth and contents of minerals, chlorophyll, proline and sugars in the apple rootstock M 4 cultured in vitro. Biol plant 51(1):177–180
Strommer J, Gregerson R, Vayda M (1993) Methods in plant molecular biology and biotechnology. CRC Press, Boca Raton, pp 49–65
Sumathi M, Shivashankar S (2017) Metabolic profiling of sapota fruit cv. Cricket ball grown under foliar nutrition, irrigation and water deficit stress. Sci Hortic 215:1–8
Ülker B, Somssich IE (2004) WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7(5):491–498
Wu QS, Zou YN (2009) Adaptive responses of birch-leaved pear (Pyrus betulaefolia) seedlings to salinity stress. Not Bot Horti Agrobot Cluj Napoca 37(1):133–138
Yin R, Bai T, Ma F, Wang X, Li Y, Yue Z (2010) Physiological responses and relative tolerance by Chinese apple rootstocks to NaCl stress. Sci Hortic 126:247–252
Zhou QY, Tian AG, Zou HF, Xie ZM, Lei G, Huang J, Wang CM, Wang HW, Zhang JS, Chen SY (2008) Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J 6(5):486–503
Zhu D, Hou L, Xiao P, Guo Y, Deyholos MK, Liu X (2019) VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci 280:132–142
Zrig A, Tounekti T, Vadel AM, Mohamed HB, Valero D, Serrano M, Chtara C, Khemira H (2011) Possible involvement of polyphenols and polyamines in salt tolerance of almond rootstocks. Plant Physiol Biochem 49(11):1313–1322
Acknowledgements
This study was supported by grants from the University of Selcuk with OYP Research Programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Aras, A. Eşitken and Y. Karakurt declare that they have no competing interests.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Aras, S., Eşitken, A. & Karakurt, Y. The Comprehensive Responses of Young Sweet Cherry Trees Under Moderate Saline Conditions Depending on the Different Rootstocks. Erwerbs-Obstbau 65, 443–451 (2023). https://doi.org/10.1007/s10341-023-00838-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-023-00838-3