Abstract
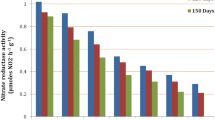
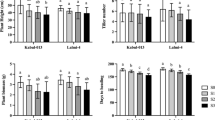
The productivity of important crops, particularly sugarcane, is greatly impacted by increased crop vulnerability to changing climatic conditions, including various abiotic stress factors like temperature, waterlogging, drought, etc. Salinity tolerance potential of five high yielding sugarcane genotypes was determined based on morpho-physiological, biochemical, and yield traits. The findings showed that morphological features (plant height, leaf area, stem diameter, number of internodes, and internodal length) were suppressed under salinity stress (ECiw 4, 8 and 10 dS/m). The relative water content (RWC) decreased by 4.4–12.5% as salinity level increased. Solute potential (Ψs) ranged from − 1.11 to − 2.27 MPa, whereas the water potential (Ψw) dropped from − 0.86 to − 1.99 MPa (from control to ECiw ~ 10 dS/m). Genotypes Co 13035 and Co 0118 maintained higher plant water status. There was a reduction in pigments and gas exchange traits due to increase in salinity in comparison to their respective control. Proline concentration increased up to seven times under salinity stress, with greatest accumulation in Co 0238 and Co 13035. The ionic (Na+/K+) ratio increased by 4, 6, and 8 times respectively under ECiw 4, 8 and 10 dS/m as compared to the control. The genotypes that were most resistant to salinity stress were Co 13035, Co 0238, and Co 0118, which had low Na+/K+ ratio. The results concluded that genotype Co 13035 had highest survival rate, low Na+/K+, maintained higher water content and osmolyte accumulation, better chlorophyll content, and single cane weight under salinity stress, thereby could be considered as tolerant to salinity.





Similar content being viewed by others
Data availability
The authors state that the data will be made accessible upon reasonable request.
References
Apon, T. A., Ahmed, S. F., Bony, Z. F., Chowdhury, M. R., Asha, J. F., & Biswas, A. (2023). Sett priming with salicylic acid improves salinity tolerance of sugarcane (Saccharum officinarum L.) during early stages of crop development. Heliyon, 9(5), e16030.
Azeem, M., Pirjan, K., Qasim, M., Mahmood, A., Javed, T., Muhammad, H., Yang, S., Dong, R., Ali, B., & Rahimi, M. (2023). Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Scientific Reports, 13, 2895.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Brindha, C., Vasantha, S., & Arunkumar, R. (2019). The response of sugarcane genotypes subjected to salinity stress at different growth phases. Journal of Plant Stress Physiology, 5, 28–33.
Chakraborty, K., Bhaduri, D., Meena, H. N., & Kalariya, K. (2016). External potassium (K+) application improves salinity tolerance by promoting Na+-exclusion, K+-accumulation and osmotic adjustment in contrasting peanut cultivars. Plant Physiology and Biochemistry, 103, 143–153.
Dhansu, P., Kulshreshtha, N., Kumar, R., Raja, A. K., Pandey, S. K., Goel, V., & Ram, B. (2021). Identification of drought-tolerant genotypes based on physiological traits, yield attributes and drought tolerance indices. Sugar Tech, 23, 741–767.
Dhansu, P., Kumar, R., Kumar, A., Vengavasi, K., Raja, A. K., Vasantha, S., Meena, M. R., Kulshreshtha, N., & Pandey, S. K. (2022a). Differential physiological traits, ion homeostasis and cane yield of sub-tropical sugarcane varieties in response to long-term salinity stress. Sustainability, 14(20), 13246.
Dhansu, P., Nandwal, A. S., Kumar, S., Chand, M., Rani, B., & Kulshreshtha, N. (2022b). Comparative evaluation of growth, yield and yield attributing traits in sugarcane (Saccharum officinarum) under different soil moisture regimes. Indian Journal of Agriculture Science, 92(8), 942–946.
Dionisio-Sese, M. L., & Tobita, S. (1998). Antioxidant responses of rice seedlings to salinity stress. Plant Science, 135(1), 1–9.
Gomathi, R., & Rakkiyapan, P. (2011). Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. International Journal of Plant Physiology and Biochemistry, 3(4), 67–74.
Gomathi, R., & Thandapani, P. (2014). Influence of salinity stress on growth parameters and yield of sugarcane. IOSR Journal of Pharmacy and Biological Sciences, 9(3), 28–32.
Gu, D. D., Wang, W. Z., Hu, J. D., Zhang, X. M., Wang, J. B., & Wang, B. S. (2016). Non-destructive determination of total chlorophyll content in maize using three-wavelength diffuse reflectance. Journal of Applied Spectroscopy, 83, 541–547.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Kaur, G., Sanwal, S. K., Sehrawat, N., Kumar, A., Kumar, N., & Mann, A. (2022). Getting to the roots of Cicer arietinum L. (chickpea) to study the effect of salinity on morpho-physiological, biochemical and molecular traits. Saudi Journal of Biological Sciences, 29(12), 103464.
Kumar, A., Mann, A., Lata, C., Kumar, N., & Sharma, P. C. (2019). Salinity-induced physiological and molecular responses of halophytes. Research developments in saline agriculture, 331–356.
Kumar, A., Mann, A., Kumar, A., Kumar, N., & Meena, B. L. (2021). Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. International Journal of Phytoremediation, 23(10), 1041–1051.
Kumar, R., Meena, M. R., Dhansu, P., Karuppaiyan, R., Appunu, C., Kulshreshtha, N., Kaushik, P., & Ram, B. (2022). Winter tolerance potential of genetically diverse sugarcane clones under subtropical climate of Northern India. Sustainability, 14(18), 11757.
Kumar, A., Sheoran, P., Mann, A., Yadav, D., Kumar, A., Devi, S., Kumar, N., Dhansu, P., & Sharma, D. K. (2023a). Deciphering trait associated morpho-physiological responses in pearlmillet hybrids and inbred lines under salt stress. Frontiers in Plant Science, 14, 1121805.
Kumar, R., Dhansu, P., Kulshreshtha, N., Meena, M. R., Kumaraswamy, M. H., Appunu, C., Chhabra, M. L., & Pandey, S. K. (2023b). Identification of salinity tolerant stable sugarcane cultivars using AMMI, GGE and some other stability parameters under multi environments of salinity stress. Sustainability, 15(2), 1119.
Kumar, R., Meena, M. R., Kulshreshtha, N., Kumar, A., & Ram, B. (2017). Genotypic response of recently evolved sugarcane Co-clones under different levels of saline irrigation water. Journal of Sugarcane Research, 7(2), 159–168.
Kumar, R., Sagar, V., Verma, V. C., Kumari, M., Gujjar, R. S., Goswami, S. K., Kumar, S., Pandey, H., Dubey, A. K., Srivastava, S., Singh, S. P., Mall, A. K., Pathak, A. D., Singh, H., Jha, P. K., & Prasad, P. V. V. (2023c). Drought and salinity stress induced physio-biochemical changes in sugarcane: An overview of tolerance mechanism and mitigating approaches. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2023.1225234
Lata, C., Kumar, A., Mann, A., Soni, S., Meena, B. L., & Rani, S. (2022). Mineral nutrient analysis of three halophytic grasses under sodic and saline stress conditions. Indian Journal of Agriculture Science, 92(9), 1051–1055.
Lichtenthaler, H.K., & Buschmann, C. (2001). Chlorophylls and Carotenoids: Measurement UNIT F4.3 and Characterization by UV-VIS Spectroscopy. Current Protocols in Food Analytical Chemistry, F4.3.1-F4.3.8.
Mahadevaiah, C., Vignesh, P., Appunu, C., Valarmathi, R., Dhansu, P., Kumar, A., Dharshini, S., Padmanabhan, T. S. S., Narayan, J. A., & Selvamuthu, K. (2023). Physiological characterization of tripidium arundinaceum and sugarcane (Saccharum spp.) germplasm for salinity stress tolerance at the formative stage. Sustainability, 15, 6962.
Mann, A., Bishi, S.K., Mahatma, M.K., & Kumar, A. (2015). Metabolomics and salt stress tolerance in plants. Managing Salt Tolerance in Plants: Molecular and Genomic Perspectives, Taylor and Francis Group LLC. (2015), pp. 251–266 1.
Mann, A., Lata, C., Kumar, N., Kumar, A., Kumar, A., & Sheoran, P. (2023). Halophytes as new model plant species for salt tolerance strategies. Frontiers in Plant Science, 14, 1137211.
Mittal, S., Kumari, N., & Sharma, V. (2012). Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiology and Biochemistry, 54, 17–26.
Plaut, Z., Meinzer, F. C., & Federman, E. (2000). Leaf development, transpiration and ion uptake and distribution in Sugarcane cultivars- grown under salinity. Plant and Soil, 218, 59–69.
Pooja, A., & Sharma, K. D. (2016). Salicylic acid induced amelioration of salinity stress in mungbean. KG, Germany: Scholars Press Omni Scriptum GmbH & Co.
Pooja, Nandwal, A. S., Chand, M., Pal, A., Kumari, A., Rani, B., Goel, V., & Kulshreshtha, N. (2020). Soil moisture deficit induced changes in antioxidative defense mechanism of sugarcane (Saccharum officinarum) varieties differing in maturity. Indian Journal of Agriculture Science, 90, 507–512.
Pooja, Nandwal, A. S., Chand, M., Singh, K., Mishra, A. K., Kumar, A., Kumari, A., & Rani, B. (2019). Varietal variation in physiological and biochemical attributes of sugarcane varieties under different soil moisture regimes. Indian Journal of Experimental Biology, 57, 721–732.
Pooja, Sharma, K. D., & Kumar, A. (2012). Improvement in plant water relation and photosynthetic activity of mungbean (Vigna radiata L.) in response to salicylic acid under salinity stress. Indian Journal of Plant Physiology, 17(3), 268–274.
Ran, X., Wang, X., Gao, X., Liang, H., Liu, B., & Huang, X. (2021). Effects of salt stress on the photosynthetic physiology and mineral ion absorption and distribution in white willow (Salix alba L.). PLoS ONE, 16(11), e0260086.
Rao, V. P., Sengar, R. S., Singh, S., & Sharma, V. (2015). Molecular and metabolic perspectives of sugarcane under salinity stress pressure. Progressive Agriculture, 15, 77–84.
Santana, M. J., Carvalho, J. A., Souza, K. J., Sousa, A. M. G., Vasconcelos, C. L., & Andrade, L. A. B. (2007). Effects of irrigation water salinity on sprouting and initial development of sugarcane (Saccharum sp.) and in soils with different textural levels. Ciência e Agrotecnologia, 31, 1470–1476.
Sengar, K., Sengar, R. S., & Singh, A. (2013). Biotechnological and genomic analysis for salinity tolerance in sugarcane. International Journal of Biotechnology and Bioengineering Research, 4, 407–414.
Shabala, S., & Cuin, T. A. (2008). Potassium transport and plant salt tolerance. Physiologia Plantarum, 133(4), 651–669.
Sharma, P.C., Kumar, A., & Mann, A. (2021). Physiology of salt tolerance in crops. Sustainable Agriculture, 199.
Sheoran, P., Kamboj, P., Kumar, A., Kumar, A., Singh, R. K., Barman, A., Prajapat, K., Mandal, S., Yousuf, D. J., Narjary, B., & Kumar, S. (2023). Matching N supply for yield maximization in salt–affected wheat agri–food systems: On-farm participatory assessment and validation. Science of the Total Environment, 875, 162573.
Simões, W. L., de Oliveira, A. R., Tardin, F. D., de Oliveira, C. P. M., de Morais, L. K., Teodoro, L. P. R., & Teodoro, P. E. (2023). Saline stress affects the growth of Saccharum complex genotypes. Journal of Agronomy and Crop Science. https://doi.org/10.1111/jac.12647
Soni, S., Kumar, A., Sehrawat, N., Kumar, A., Kumar, N., Lata, C., & Mann, A. (2021). Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi Journal of Biological Sciences, 28, 2510–2517.
Thiem, D., Tyburski, J., Golebiewski, M., & Hrynkiewicz, K. (2020). Halotolerant fungi stimulate growth and mitigate salt stress in Alnus glutinosa Gaertn. Dendrobiology, 83, 30.
Vasantha, S., & Gomathi, R. (2012). Growth and development of sugarcane under salinity. Journal of Sugarcane Research, 2(1), 1–10.
Vasantha, S., Venkataramana, S., Rao, P. N. G., & Gomathi, R. (2010). Long term salinity effect on growth, photosynthesis and osmotic characteristics in sugarcane. Sugar Tech, 12, 5–8.
Verma, K. K., Song, X. P., Verma, C. L., Malviya, M. K., Guo, D. J., Rajput, V. D., Sharma, A., Wei, K. J., Chen, G. L., & Solomon, S. (2021). Prediction of photosynthetic leaf gas exchange of sugarcane (Saccharum spp) leaves in response to leaf positions to foliar spray of potassium salt of active phosphorus under limited water irrigation. ACS Omega, 6, 2396–2409.
Verma, K. K., Song, X. P., Zeng, Y., Li, D. M., Guo, D. J., Rajput, V. D., Chen, G. L., Barakhov, A., Minkina, T. M., & Li, Y. R. (2020). Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega, 5, 24145–24153.
Verma, K., Kumar, R., Kumar, A., Bhardwaj, A. K., & Verma, R. C. (2023). Host plant regulates growth processes, ion homeostasis, and salinity tolerance of sandalwood (Santalum album L.). J Plant Growth Regul, 1–13. https://doi.org/10.1007/s00344-023-10906-3.
Weatherley, P. E. (1950). Studies in the water relations of the cotton plant I.The field measurements of water deficit in leaves. New Phytologist, 49, 81–97.
Acknowledgements
We appreciate ICAR-SBI, RC, Karnal and MM (DU), Mullana, Ambala for the help with logistics that allowed us to finish this study.
Funding
This research received no grant in the form external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Preet, K., Dhansu, P., Sehrawat, N. et al. Morpho-physiological analysis of salinity tolerance in sugarcane genotypes. Plant Physiol. Rep. (2024). https://doi.org/10.1007/s40502-024-00782-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40502-024-00782-8