Abstract
Breeding success of birds depends on survival during two critical stages of the reproductive period: First, egg laying and incubation, and then nestling and fledgling. This essential element of avian life history mirrors an evolutionary adaptation of parents to existing environmental conditions. The main factors that determine nest survival involve weather, predation and sibling competition. Despite numerous studies documenting their impact on breeding success, only a few have analysed the issue with regard to ground-nesting birds and estimated their survival functions. Therefore, we decided to examine nest survival probability of the Crested Lark (Galerida cristata) in a population that occupies newly established roadside habitats in Central Poland. The analysis is based on 141 nests monitored between 2018 and 2022. We found that the mean survival probability of the whole nesting period was 0.376, and it was higher during the egg stage (0.836) than during the nestling period (0.392). The Cox proportional hazards model shows that nest survival in the nestling stage is mainly affected by the time of breeding and the distance from the road edge, and also by mean temperature of the whole nesting period. In other words, the probability of a brood failure in the nestling stage decreases as the breeding season advances, whereas it increases among nests located closer to the road edge. Furthermore, during the whole nesting period, nest failure decreases as the temperature rises. Our study suggests that the Crested Lark, being a multi-brood and thermophilic species inhabiting temperate regions, may benefit more if its nesting attempts begin later during the season when breeding conditions become more favourable. However, we also take into consideration a hypothesis that nesting in grassy habitats near road edges may prove to be an ecological trap, attracting high densities of nesting birds but leading to their low nest success.
Zusammenfassung
Überleben im Nest: Haubenlerchen (Galerida cristata) in intensiv genutzten Habitaten in Zentralpolen
Der Bruterfolg von Vögeln hängt vom Überleben in zwei kritischen Phasen während der Fortpflanzung ab: die erste ist die Eiablage und das Ausbrüten der Eier, die zweite die Aufzucht der geschlüpften Vögel bis zum Ausfliegen. Diese wesentlichen Merkmale im Leben eines Vogels spiegeln eine evolutionäre Anpassung der Eltern an bestehende Umweltbedingungen wider. Die wichtigsten, für das Überleben im Nest ausschlaggebenden Faktoren sind dabei das Wetter, Nesträuber und die Konkurrenz unter den Geschwistern. Trotz zahlreicher Studien über deren Auswirkungen auf den Bruterfolg haben sich nur wenige mit dieser Thematik bei bodenbrütenden Vögeln befasst und deren Überlebenschancen eingeschätzt. Deshalb wollten wir die Überlebenswahrscheinlichkeit im Nest bei einer Haubenlerchen-Population (Galerida cristata) in einem von neu angelegten Straßen durchzogenen Habitat in Zentralpolen untersuchen. Grundlage der Studie waren 141 Nester, die zwischen 2018 und 2022 überwacht wurden. Wir ermittelten über die gesamte Nistperiode hinweg eine durchschnittliche Überlebenswahrscheinlichkeit von 0,376, die mit 0,836 während des Eistadiums höher war als während der Nestlingszeit (0,392). Das Cox-Proportional-Hazards-Modell zeigt, dass das Überleben der Jungvögel im Nestlingsstadium hauptsächlich von der Dauer des Brütens, der Entfernung von den Straßenrändern sowie von der Durchschnittstemperatur während der gesamten Brutphase abhängt. Mit anderen Worten: die Wahrscheinlichkeit eines Brutausfalls im Nestlingsstadium nimmt mit fortschreitender Brutsaison ab, während sie bei Nestern näher am Straßenrand steigt. Außerdem nimmt die Zahl der Nestausfälle während der gesamten Brutzeit mit steigender Temperatur ab. Unsere Studie weist darauf hin, dass die Haubenlerche als mehrbrütige, wärmeliebende und in gemäßigten Regionen lebende Art eventuell eher davon profitiert, mit ihren Brutversuchen später in der Saison zu beginnen, wenn die Bedingungen dafür günstiger werden. Wir halten es aber auch für möglich, dass sich das Nisten in grasbewachsenen Lebensräumen in Straßennähe als ökologische Falle erweisen könnte, die zwar zu einer hohen Dichte nistender Vögel anlockt, ihnen aber nur einen geringen Nesterfolg beschert.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nest survival is a parameter of the adaptation of birds, reflecting their reproductive success, which subsequently regulates the dynamics of the population size (Newton 1998; Cresswell 2010). It can be governed by predation and weather as keys factors affecting nest failure, especially among ground-nesting species (Newton 1993; Wright et al. 2009; Martin et al. 2017; Reif et al. 2023). Since parents’ adaptation to ensure nest survival is under strong pressure from natural selection (Lack 1968; Martin 1995), it is not surprising that birds have developed many behavioural adaptations that ensure their reproductive success. Anti-predatory strategies of ground-nesting birds include a selection of a nesting place with sufficient concealment from predators (Mallord et al. 2007; MacDonald et al. 2016), active defence, attack and distraction of potential predators (Gómez-Serrano et al. 2017), repetition of broods in the case of loss (Lesiński 2009; Gates et al. 2013), and shorter time spent by chicks in the nest (Martin et al. 2011; Remeš et al. 2020). Nest survival is also influenced by weather (Chmura et al. 2018; Grudinskaya et al. 2022), because until nestlings develop their thermoregulatory ability (Starck and Ricklefs 1998), episodes of cold weather, such as a sharp temperature and/or precipitation drop, can significantly reduce the number of nestlings in the nest and consequently shape breeding success. Therefore, parental fitness is enhanced by well-hidden nests that attenuate the sounds of chicks begging for food, their smell and movement, and by their ability to balance the time spent on searching for food and warming chicks, especially during unfavourable weather (Auer and Martin 2017; Lejeune et al. 2019).
Although there are numerous studies documenting relationships between predation, weather and nest survival, there are no unambiguous patterns of these relationships with regard to ground-nesting birds living in open habitats. A good example is the family of larks (Alaudidae spp.), which may be an important indicator value of avian diversity not only in grassland, steppe or traditional habitats, but also in many previously degraded open spaces (Han et al. 2023). Lark broods are subject to strong predation and weather pressure (Donald et al. 2002; Praus et al. 2014; de Zwaan et al. 2019), as a result of which only 20% to 30% turn out to be successful (Suarez and Manrique 1992; de Juana et al. 2004; Praus and Weidinger 2010; Mwangi et al. 2018). Thus, despite these achievements in understanding nest survival of larks, all earlier studies quantified reproductive success exclusively as a binary success vs. failure variable and thus did not take gradual changes in egg or nestling number into account. This classic approach focuses only on nest success and nest failure, but it entirely disregards gradual declines in brood size that may result from predation and (even more likely) adverse weather. Nest survival may vary significantly during different nesting stages. Many studies on nesting passerines, including larks, suggest that during the eggs stage nests tend to have a higher survival rate than in the nestling period (Shkedy and Safriel 1992; Praus 2020; Golawski et al. 2023), but others have found the opposite (Mitrus and Soćko 2008), also showing differences occurred even within the same species (Mallord et al. 2008; Praus et al.2014). It is likely that multiple factors influence age-specific patterns of nest survival. Thus, estimating phase-specific (egg and nestling phases) survival and pinpointing the critical moment of survival during a nesting attempt may increase our understanding of breeding success limitations in a particular habitat and be essential for developing adequate conservation strategies (Zaremba et al. 2020). So far, no detailed research has been conducted on the Crested Lark (Galerida cristata) with regard to this aspect, and studies on this species focused mainly on breeding biology (Lesiński 2009; Praus 2020) and habitat selection (Šímová et al. 2015; Lisiecki et al. 2020; Chiatante 2022).
The Crested Lark is a small, mainly sedentary bird species belonging to larks Alaudidae family. This species, which features many subspecies, has a wide distribution ranging from north central Africa throughout Asia Minor to Central Asia and Europe (Guillaumet et al. 2006). Originally, it inhabited dry warm open areas with very low and sparse vegetation, for example semi-deserts and steppes, but it has also adapted to various human-modified landscapes, such as open farmland, urban settlements and outskirts, railway yards, airfields and roadsides (Roselaar 1988; BirdLife International 2023). At present, its population is decreasing across many parts of Europe (Birdlife International 2023) and a widespread decline was recorded during the second half of 20th and at the beginning of the twenty-first century (de Juana et al. 2004), a trend whose exact reasons are unknown and require urgent in-depth studies.
The Crested Lark is a ground-breeding open-cup nester. At our study site in Central Poland, the species breeds from the end of March to mid-August, having two to three broods per season. In this paper, we show the results of our analysis of the Crested Lark’s nest survival on the basis of regular nest inspections over a five-year period. We examine relationships between nest survival (eggs/chicks), weather conditions, and predatory pressure during a breeding season.
Our study aims to: (1) describe nest survival of the Crested Lark in intensively used habitats during the egg incubation and nestling stages; (2) establish the critical period of the reproductive cycle that influences reproductive success; (3) analyse how weather and predation pressure affect reproductive success during the incubation and nestling stages. In this analysis, we expect predation pressure to be relatively constant over years in monotonous landscapes (e.g., Kosicki et al. 2016). Moreover, since Crested Larks are evolutionarily adapted to relatively predictable weather on the continental steppe in spring and summer, they may be particularly sensitive to more fluctuating weather conditions in their recently occupied ranges in Central Europe, which are further exacerbated by increasing weather fluctuations resulting from climate change (Pérez-Granados et al. 2023; Tscharntke and Batáry 2023). Therefore, we hypothesise that the reproductive success of the Crested Lark is more dependent on weather conditions than on predation pressure.
Materials and methods
Study area
The study was conducted in Central Poland, a region which is dominated by flat landscape with the average elevation of 80 m to 100 m a.s.l. It is characterised by temperate continental climate with the average annual temperature of 8.2 °C and the so-called “rain shadow”, i.e. annual precipitation of about 500 mm, which is lower than in other regions (Kondracki 2009).
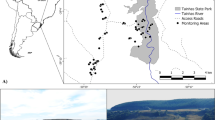
The surveyed population of the Crested Lark lives in roadside habitats along a bypass road round the town of Inowrocław (52o46′55N, 18°18′36E, Fig. 1). The study area of 4.6 km2 is parallel to a dual carriageway. Since the road was built between 2014 and 2019, it is a relatively new element of the landscape, which has changed from arable land to regularly mown grassland. Along its entire length (25.1 km), the immediate surroundings of the bypass include service roads, lanes of grassy vegetation interspersed with planted shrubs and small trees as well as adjacent arable fields with a varied crop structure, mainly winter wheat, rapeseed, maize and sugar beets.
Bird data
The data were collected from mid-March to mid-August in five consecutive breeding seasons between 2018 and 2022. Nests were spotted through a systematic search of the area along the road and associated structures by means of flushing incubating females, and observing adults carrying nesting material or food for the chicks. Once a nest was found, it was regularly inspected at intervals of two to four days to observe its content and status. During the five-year period, each nest was inspected on average (± SD) 5.96 ± 3.08 times. During the last inspection, nestlings were ringed with metal and coloured alphanumerical rings.
When a nest was found during the egg-laying stage, we calculated the time of the clutch initiation on the basis of the number of eggs, assuming that one egg per day was laid (Roselaar 1988; de Juana et al. 2004). When a nest was discovered during incubation, the date of the first egg laying was determined by back-dating (since hatching) using a 12-day incubation period. If a nest was found during the advancement brood, we weighed and measured the nestlings and estimated their age according to the age–body weight relationship (Shkedy and Safriel 1992). We assumed that the maximal brood duration was 30 days, including the egg-laying and incubation period (17 days) and the nestling stage (13 days) (Roselaar 1988; de Juana et al. 2004).
By nesting success, we meant a nest that produced at least one fledgling aged at least nine days, which is a minimum age for leaving the nest (de Juana et al. 2004). Also, when the last inspection revealed adult birds feeding the chicks outside the nest or showing anxiety if an observer appeared in the nest’s vicinity, we considered it as a nesting success. In our analysis, we included successful nests, nests that were totally lost, i.e. all eggs had failed or all nestlings had died, as well as those whose number of nestlings decreased between inspections. We excluded from the analysis nests with a brood destroyed by a predator as documented by trail cameras (two cases) and also nests that were destroyed (nine) or abandoned (ten) at the incubation stage without the possibility of determining the approximate date of clutch initiation. Thus, our dataset consisted of 141 broods out of the total of 162 recorded ones. However, excluding nests from the analysis may overestimate survival at the egg stage. Therefore, we conducted an alternative survival analysis (see Electronic Supplementary Material), assuming that the day the nest was found was the first day of incubation. The average breeding density in this population was 5.4 pairs per 1 km2.
During each nest control, a five-minute-point counting of potential nest predators was made within 50 m of the nest. The mean (± SD) number of predatory birds per control was 2.84 ± 2.72 individuals (range: 0–14), and it differed between the years (except 2018 vs. 2019; Electronic Supplementary Material), while the mean (± SD) number of predatory mammals was 0.18 ± 0.44 individuals (range: 0–2), and in that case we found no differences between the years (Electronic Supplementary Material)).
The shortest distance from the nest to the edge of the nearest road was measured with a tape measure (to the nearest 0.1 m).
To assess the effect of weather conditions on nest survival, climatic data were obtained from meteorological stations of the Institute of Meteorology and Water Management—National Research Institute, located in the vicinity of the bypass road. Temperature data were obtained from a station in Kołuda Wielka, located 12 km away from the center of the study plot, whereas precipitation was provided by stations in Pakość, Jaksice and Więcławice, located 15.1 km NW, 11.3 km N and 7.1 km NE, respectively, from the center of the study plot, so accurate rainfall values were provided for the entire study area. The mean (± SD) temperature during the study period (from 22nd March to 15th August) was 13.35 ± 6.17 °C (range: −0.63 to 28.60) and it differ between the years (except 2018 vs. 2019, see Electronic Supplementary Material), while the average (± SD) daily precipitation was 1.54 ± 4.15 mm (range: 0–40.4), and was different between years (except 2018 vs. 2020 and 2018 vs. 2021, see Electronic Supplementary Material).
Data processing and analysis
Nest survival was calculated as a change in the number of active nests or their contents during each breeding season (Kleinbaum 1996). We analysed the data according to life tables, which are the most universal method for estimating survival functions, where the life history of each brood, i.e. from brood initiation to the offspring leaving the nest/destruction of the nest during a particular breeding season is known. When analysing life tables, survival is considered to be a function of time (Kleinbaum 1996; Kosicki 2012; Langowska and Zduniak 2020), contrary to the most frequently analysed cases when breeding success is expressed as a simple proportion of surviving broods. Thus, in our approach, we were able to calculate critical time points during the breeding season of the studied population (Zduniak 2010; Zduniak et al. 2011; Kosicki 2012; Golawski et al. 2023). We estimated the survival time of each nest during the whole nesting period, i.e. from first egg laying in the nest to the date of the last visit, but also separately for the egg stage (egg laying and incubation) and the nestling stage (form hatching to fledging). The day when eggs or nestlings failed was considered as a halfway point between two consecutive nest controls.
To analyse the impact of weather conditions, predatory pressure and breeding parameters on nest survival, we used the Cox proportional hazards model (Cox 1972; Muenchow 1986; Moya-Larano and Wise 2000; Reino et al. 2009), where time of life and status (survival/mortality) were used as input parameters of the response variable. In this approach, the hazard ratio, i.e. the probability that an individual that survived till the beginning of a given interval will die before the end of that interval is a function of independent variables. Accordingly, it is possible to estimate the regression of coefficients (and test them to see if they are significantly different from zero) for independent variables. When a regression coefficient for a given independent variable is positive and statistically significant, it shows decreased survival (higher risk of failure), and—vice versa—a statistically significant but negative coefficient is associated with increased survival (lower risk of death).
This approach let us test several environmental components that might influence nest survival, such as temperature, precipitation, distance between the nest and the road, the number of bird and mammal predators, as well as the date of the brood onset, i.e. the date of the first egg laying. This variable is expressed as the number of days after 1st January (first day) and it was median-centred for the particular breeding season. The number of bird and mammal predators was also mean-centred for a particular breeding season. Weather conditions were expressed as mean daily values of air temperature and mean sum of precipitation (from three nearest meteorological stations) calculated for duration time of each nest stage, separately for eggs, nestlings and whole nesting period (Kosicki 2012).
Due to the fact that the survival rate of the egg stage was relatively high (see results), we developed the Cox proportional hazards model for: (1) the nestling period, and (2) the whole nesting period (from egg laying to the fledglings’ departure from the nest).
In order to verify whether full models (with all predictors) could be simplified, the Akaike information criterion (AIC; Burnham and Anderson 2002) was employed. By adding or removing predictors, it generated all possible combinations of candidate models. The model with the lowest AIC value and thus the highest Akaike weight was considered to be the best and the most parsimonious (Burnham and Anderson 2002).
Results
We found 162 nests located mainly in roadside areas covered with grassy vegetation (83%) and in adjacent arable fields (17%) (Fig. 2). The mean distance from the nest to the road was 13.48 ± 12.45 m.
Nests of the Crested Lark (Galerida cristata) located in different micro-habitat patches with plant cover at various stages of development. A arable field at the beginning of growing season; B grassy area at the beginning of growing season; C grassy area at the peak of growing season; D Red Clover (Trifolium pratense); E Rapeseed (Brassica napus); F Common Bean (Phaseolus vulgaris)
The mean survival ratio for the whole nesting period (30 days from egg laying to fledgling) was 0.376 (95% CL: 0.244–0.581, n = 141, Fig. 3a) and did not differ over the five-year study period (Chi2 = 2.8, df = 4, p = 0.6). The mean survival during the eggs stage (egg laying + incubation = 17 days) was 0.836 (95% CL: 0.746–0.937), and it also did not differ significantly between years (Chi2 = 1.2, df = 4, p = 0.9), but the result was statistically higher than during the whole nesting period (Gehan–Wilcoxon test, test value = 2.31, p < 0.01). Finally, the mean survival ratio for the nestling period was 0.392 (95% CL: 0.212–0.727), and it also did not differ in particular study years (Chi2 = 2.1, df = 4, p = 0.7). Besides, there was no difference between this period and the whole nesting period (Gehan–Wilcoxon test, test value = 0.73, p < 0.54).
According to the hazard ratio, the most critical moment for the survival of eggs/chicks occurs at the end of the incubation period and during the first days of the nestlings’ life. The highest hazard ratio was observed from the 15th day of egg stage, i.e. just before hatching, to the ninth day of nestlings’ life (Fig. 3b). Therefore, the analysis of the impact of environmental factors on survival was performed separately for the whole nesting period (from egg laying to nest leaving) and for the nestling period (from hatching to nest leaving).
Out of all analysed Cox proportional hazards models (Table 1) for the nestling period, the most parsimonious model included only two predictors, i.e. the hatching day and the distance between the nest and the road (Table 1, model 1A). This model turned out to be slightly better than the second model in our candidate set (evidence ration = 2.69), which additionally contained temperature as a predictor (Table 1, model 2A).
The best supported model was statistically significant (likelihood ratio test = 50.09, p < 0.0001, r2 = 0.335) and it showed that the risk of chicks’ death, expressed as a hazard ratio, decreased during the breeding season (β for Hazard ratio (± SE) = -0.639 (± 0.09), Wald statistic = −6.64, p < 0.001, Fig. 4a). In other words, the later in the season birds started to breed, the lower the risk of their chicks’ death. What’s more, we showed that the risk of chicks’ death decreased with the increase in the distance from the nest to the road (β for hazard ratio (± SE) = −0.342 (± 0.07), Wald statistic = −5.64, p < 0.02, Fig. 4b). According to the second model, our candidate set could also suspect that the temperature will be high statistically significant impact on probability of nestling death, but this factor was not significant (β for hazard ratio (± SE) = −0.04 (± 1.00), Wald statistic = 0.140, p < 0.88, Fig. 4c).
The most parsimonious Cox proportional hazards model for the whole nesting period (from first egg laying to fledge) (Table 1) was also significant (Likelihood ratio test = 41.78, p < 0.0001, r2 = 0.253). It included three predictors, i.e. the time of breeding, temperature and the distance from the nest to the road. This model was slightly better than the second model, which also included the number of bird predators and may be considered a valid alternative in our candidate set (evidence ratio = 1.80).
According to the best supported model, we found that the death risk of the content of the nest (eggs or chicks) decreased during the breeding season. In other words, the later the breeding attempt was undertaken, the better survival of the offspring (β for hazard ratio = −0.134, Wald statistic = −1.382, p = 0.045, Fig. 4d). A similar tendency was established with regard to temperature: The risk of death decreased with the increase of air temperature (β for hazard ratio = −0.179, Wald statistic = −3.038, p = 0.002, Fig. 4e). Finally, the highest survival rate, i.e. the lowest hazard rate, was found for nests located the furthest from the road (β for hazard ratio = −0.013, Wald statistic = −2.323, p = 0.02, Fig. 4f). In the second model in our candidate set, we also found that probability of nestlings death increase proportionally to the number of bird predators, but it should be noted that this relationship is on the border of statistical significance (β for Hazard ratio = 0.010, Wald statistic = 1.03, p = 0.05, Fig. 4g). So the time of breeding, distance from the edge of the road and temperature explain most cases of nest success, even though bird predators may also play a role in the process.
Discussion
Our study is the first of this kind to have estimated nest survival of the Crested Lark during different brood stages. It also tested the impact of a variety of factors that potentially affected survival. We found that the survival rate in the egg stage was twice as high as in the nestling stage, a result which is consistent with earlier studies on birds nesting on the ground (Mallord et al. 2007; Pérez-Granados 2017). We also found similar tendency in our extended analysis (see Electronic Supplementary Material), which also included broods for which there was uncertainty about the start date of laying. Thus, the nest survival pattern of the Crested Lark is generally consistent with that of ground-nesting birds, showing overall low survival in comparison with species using other nesting niches (Shochat et al. 2005).
The overall nest survival during the whole nesting period (0.376) of the Crested Lark is similar to the value (0.367) obtained for a population of this species studied in Czechia (Praus 2020), but higher than among birds breeding in the Negev Desert (0.238) (Shkedy and Safriel 1992). The differences may result from different conditions in Central Europe and the Near East. As regards other lark species from habitats in temperate regions, the results we obtained for whole nesting period were comparable or slightly higher. In Kazakhstan, the mean probability of the Calandra Lark’s (Melanocorypha calandra) nest to survive the entire nesting period in the steppe and among abandoned crops was 0.207 (Lameris et al. 2016), whereas in the case of the Woodlark (Lullula arborea) nesting in heathlands in southern England the probability was 0.470 (Mallord et al. 2007), while for the Eurasian Skylark (Alauda arvensis) in farmlands of southern England, it was 0.242 (Donald et al. 2002), and 0.322 for the Horned Lark (Eremophila alpestris) in the alpine environment of Canada (MacDonald et al. 2016).
Our results show that two factors, such as the time of breeding and the distance from the road, affect the probability of nestlings’ survival. Besides, the whole nesting cycle (from egg laying to nest leaving) is also affected by temperature. Thus, the probability of nestling failure decreases as the breeding season advances, and increases when nests are closer to the road. What is more, during the whole nesting period, nest failure decreases as the temperature increases. The survival probability is higher during the egg stage than in the nestling period. It can be explained by increased parental feeding activity and nestlings’ begging calls for food. These two types of visual and acoustic cues may disclose the location of the nest and attract potential predators (Martin et al. 2000; MacDonald et al. 2009; Ibáñez-Álamo et al. 2012).
Our findings on the time of brood initiation are surprising, because opposite results have been described many times. Generally, pairs that breed earlier grant lower nest mortality than pairs beginning to breed later (Siikamaki 1998; Currie et al. 2000; Morrison et al. 2019). Additionally, this relationship has a strong theoretical basis, as studies show that birds that start breeding earlier are more experienced and they are the first to occupy better territories (Weggler 2000; Kokko et al. 2006). However, this assumption may not be applied to sedentary and multi-brood species that originally inhabited steppe and semi-desert landscapes with much warmer and arid climate than the climate in temperate regions. We speculate that our contrasting results may reflect a reproductive strategy of the studied species in the face of changing weather conditions, which occur regularly during the whole breeding season (from late March to mid-August). As a multi-brood species, the Crested Lark may potentially compensate for the loss of earlier nests by several nesting attempts throughout an extended breeding season. The Crested Lark nests on the ground, building nests lined mainly by grass in a shallow depression under the shelter of tussock or—less frequently—shrub. At the beginning of the breeding season in the third decade of March (23/03/2018), when the first egg was laid in the studied population, vegetation on the ground was relatively low and sparse, thus making the nest more visible to the predator. This hypothesis may be supported by a study conducted on the Negev Desert (Shkedy and Safriel 1992), where survival in the eggs stage was 0.368 as compared to 0.836 obtained in our study. The differences may result both from the structure of the desert vegetation cover providing poorer nest concealment as well as the character and activity of nest predators (Shkedy and Safriel 1992). During the whole nesting period, the second model selected by the AIC showed that bird predators may also play a role in determining breeding output. There may be several potential explanations for why predation pressure was not included in the most parsimonious model, despite the fact that this factor was also marginally statistically significant. First, it is plausible that the five-minute-point counting of potential predators is not long enough and may not reflect their real impact on nest survival. Secondly, the obtained field data could be biased as only diurnal predators were observed, whereas nocturnal animals, e.g. like Red Foxes (Vulpes vulpes), Feral Cats (Felis catus), hedgehogs (Erinaceus sp.), Stone Martens (Martes foina), rodent Rodentia sp., could be potentially more responsible for nest losses. This explanation can be supported by results obtained from studies on the Eurasian Skylark and the Woodlark in the Netherlands, where nest predation occurred in the dark in 55% cases (Praus et al. 2014). Alternatively, due to the fact that the area we study is a linear habitat running along the motorway, it is also likely that potential predators, which are opportunists, benefit from the carcass on the road and thus scavenge their food, while bird nests remain an alternative source of food (Pescador and Peris 2007). Still, due to specific features of the study area and methodological limitations, the actual impact of predators on nest survival may have been masked in favour of weather conditions, especially temperature, which may have influenced the breeding success of the Crested Lark. Moreover, local frosts that occur at the beginning of the breeding season can pose a problem for this typically steppe species. During the incubation period, this factor is less important, because the brood is predominantly incubated by the female (with short feeding breaks), which maintains a constant temperature of the nest (Hartley 1946; own data). Still, low air temperature right after hatching may impact nestling survival in two ways. First, it happens directly, because days with low temperatures are dangerous for small nestlings whose homeothermic regulation has not developed. In the period just before hatching, embryos and nestlings soon after hatching have reduced thermoregulation so they suffer from cooling (Rodriguez and Barba 2016). Second, which is a more probable explanation, air temperature in spring and summer indirectly affects potential food resources, such as beetles Coleoptera, grasshoppers Acrididae, ants Formicidae, caterpillars, snails Gastropoda and spiders Araneae (de Juana et al. 2004) that adults feed on, whereas the offspring are fed mainly with insects, especially caterpillars and small Orthoptera (Roselaar 1988). These organisms, which are the main element of the Crested Lark’s diet, are poikilothermic, so they cannot regulate their body temperature and their activity largely depends on thermal conditions of the surrounding environment (Lehmann 1999; Zhang et al. 2020; Hannigan et al. 2023). Thus, when it is cold, invertebrates are scarce, which may lead to nestling starvation, their reduced growth rate and general poor condition. Additionally, adverse weather forces adult birds to increase their energy expenditure and foraging activity to provide the same amount of food for nestlings. Consequently, when parents need more time for foraging, their chicks are exposed to hypothermia, especially in the first few days after hatching. Also, reduced food availability and severe weather constitute a stress factor, which is often connected with parasite infections (Newton 1998), additionally increasing the mortality of nestlings. Finally, we found that the further from the road, the higher survivability of the brood. Higher survival rates of nests located further from the road can stem from the fact that they are located mostly on agricultural fields, where denser vegetation provides more stable temperature and better nest concealment. Our findings are consistent with results from studies on the Eurasian Skylark, whose nests in cereal crops had a significantly higher survival rate than those located in other field types (Donald et al. 2002). Meanwhile, the lower survival rate of nests located closer to the road edge may result from increased human disturbance in this zone, including agricultural machines movements to adjacent fields and mowing grass works.
Conclusion
The breeding success of the Crested Lark nesting in road margins depends mainly on the micro-habitat and climatic conditions. Our study suggests that in temperate regions the Crested Lark, which is a multi-brood and thermophilic species, may benefit more from later nesting attempts when breeding conditions, such as higher temperature and peak of food abundance, have improved. Therefore, the ongoing climate changes may contribute to better survival of broods and a recovery of its populations in Northern and Central Europe.
Our findings also demonstrate the importance of nest location, because it is a factor that might seriously reduce or increase the number of fledglings. Nesting in grassy habitats near road edges may prove to be “ecological traps”, attracting high densities of nesting birds, but leading to low nest success due to increased nest failure. That is why colonisation of new man-made linear habitats, such as roadsides, may be detrimental for the Crested Lark. However, we are aware that our assessment method of nest predators is limited and the data regard mainly diurnal predators, so our conclusions may not reflect the real impact of predators on nest survival. Hence, future studies using trail cameras should be initiated to identify nest predators, predator abundance, and predators’ activity patterns throughout the breeding season in different types of habitats occupied by the Crested Lark. Last but not least, further research is necessary to expand our knowledge on how factors related to human activity, e.g. field work and lawn moving affect nest survival of this ground-nesting species.
Data availability
The data is available directly from the author, Rafał Sandecki rafal.sandecki@amu.edu.pl
References
Auer SK, Martin TE (2017) Parental care mitigates carry-over effects of poor early conditions on offspring growth. Behav Ecol 28:1176–1182
BirdLife International (2023) Species factsheet: Galerida cristata. Downloaded from http://datazone.birdlife.org/species/factsheet/crested-lark-galerida-cristata on 20/07/2023
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Chiatante G (2022) Habitat use and niche overlap of ground-nesting steppic birds. Avian Biol Res 15:180–193
Chmura HE, Krause JS, Pérez JH, Asmus A, Sweet SK, Hunt KE, Meddle SL, McElreath R, Boelman NT, Gough L, Wingfield JC (2018) Late-season snowfall is associated with decreased offspring survival in two migratory arctic-breeding songbird species. J Avian Biol 49:e01712. https://doi.org/10.1111/jav.01712
Cox DR (1972) Regression models and life tables. J R Stat Soc 34:187–220
Cresswell W (2010) Predation in bird populations. J Ornithol 152:251–263
Currie D, Thompson D, Burke T (2000) Patterns of territory settlement and consequences for breeding success in the Northern Wheatear Oenanthe oenanthe. Ibis 142:389–398
de Juana E, Suarez F, Ryan PG (2004) Family Alaudidae (larks). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the birds of the world. Vol. 9. Contingas to pipits and wagtails. Lynx Edicions, Barcelona, pp 496–601
de Zwaan DR, Camfield AF, MacDonald EC, Martin K (2019) Variation in offspring development is driven more by weather and maternal condition than predation risk. Funct Ecol 33:447–456
Donald PF, Evans AD, Muirhead LB, Buckingham DL, Kirby WB, Schmitt SIA (2002) Survival rates, causes of failure and productivity of Skylark Alauda arvensis nests on lowland farmland. Ibis 144:652–664
Gates HR, Lanctot RB, Powell AN (2013) High renesting rates in Arctic-breeding Dunlin (Calidris alpina): a clutch-removal experiment. Auk 130:372–380
Golawski A, Mroz E, Golawska S, Parapura A, Zduniak P (2023) Brood survival in the Red-backed Shrike Lanius collurio in eastern Poland. J Ornithol 164:921–929
Gómez-Serrano MA, López-López P (2017) Deceiving predators: linking distraction behavior with nest survival in a ground-nesting bird. Behav Ecol 28:260–269
Grudinskaya V, Samsonov S, Galkina E, Grabovsky A, Makarova T, Vaytina T, Fedotova S, Shitikov D (2022) Effects of spring weather on laying dates, clutch size, and nest survival of ground-nesting passerines in abandoned fields. ACE 17:8
Guillaumet A, Pons JM, Godelle B, Crochet PA (2006) History of the Crested Lark in the Mediterranean region as revealed by mtDNA sequences and morphology. Mol Phylogenet Evol 39:645–656
Han Z, Yang X, Zhao X, Jiguet F, Tryjanowski P, Wang H (2023) Mongolian Lark as an indicator of taxonomic, functional and phylogenetic diversity of steppe birds. Avian Res 14:100124. https://doi.org/10.1016/j.avrs.2023.100124
Hannigan S, Nendel C, Krull M (2023) Effects of temperature on the movement and feeding behaviour of the large Lupine Beetle, Sitona gressorius. J Pest Sci 96:389–402
Hartley PHT (1946) Notes on the breeding biology of the Crested Lark. Brit Birds 39:142–144
Ibáñez-Álamo JD, Arco L, Soler M (2012) Experimental evidence for a predation cost of begging using active nests and real chicks. J Ornithol 153:801–807
Kleinbaum DG (1996) Survival analysis. Springer, New York
Kokko H, Gunnarsson TG, Morrell LJ, Gill JA (2006) Why do female migratory birds arrive later than males? J Anim Ecol 75:1293–1303
Kondracki J (2009) Geografia regionalna Polski. Wydawnictwo Naukowe PWN, Warszawa
Kosicki JZ (2012) Effect of weather conditions on nestling survival in the White Stork Ciconia ciconia population. Ethol Ecol Evol 24:140–148
Kosicki JZ, Zduniak P, Ostrowska M, Hromada M (2016) Are predators negative or positive predictors of farmland bird species community on a large geographical scale? Ecol Indic 62:259–270
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lameris TK, Fijen TPM, Urazaliev R, Pulikowa G, Donald PF, Kamp J (2016) Breeding ecology of the endemic Black Lark Melanocorypha yeltoniensis on natural steppe and abandoned croplands in post-Soviet Kazakhstan. Biodivers Conserv 25:2381–2400
Langowska A, Zduniak P (2020) No direct contact needed for drones to shorten workers lifespan in honey bee. J Apic Res 59:88–94
Lehmann FO (1999) Ambient temperature affects free-flight performance in the fruit fly Drosophila melanogaster. J Comp Physiol B 169:165–171
Lejeune L, Savage JL, Bründl AC, Thiney A, Russell AF, Chaine AS (2019) Environmental effects on parental care visitation patterns in Blue Tits Cyanistes caeruleus. Front Ecol Evol 7:356. https://doi.org/10.3389/fevo.2019.00356
Lesiński G (2009) Breeding biology and population decline of Crested lark Galerida cristata in Warsaw, Poland. Ornis Hungarica 17–18:1–11
Lisiecki M, Dylewski Ł, Kistowska BE, Tobółka M (2020) The Crested Lark Galerida cristata as an example of a bird species that benefits from agricultural management in western Poland. Bird Study 67:197–205
MacDonald PG, Wilson DR, Evans C (2009) Nestling begging increases predation risk, regardless of spectral characteristics or avian mobbing. Behav Ecol 20:821–829
MacDonald EC, Camfield AF, Martin M, Wilson S, Martin K (2016) Nest-site selection and consequences for nest survival among three sympatric songbirds in an alpine environment. J Ornithol 157:393–405
Mallord JW, Dolman PM, Brow A, Sutherland WJ (2007) Nest-site characteristics of Woodlarks Lullula arborea breeding on heathlands in southern England: are there consequences fornest survival and productivity? Bird Study 54:307–314
Mallord JW, Dolman PM, Brown A, Sutherland WJ (2008) Early nesting does not result in greater productivity in the multi-brooded Woodlark Lullula arborea. Bird Study 55:145–151
Martin TE (1995) Avian life-history evolution in relation to nest sites, nest predation and food. Ecol Monogr 65:101–127
Martin TE, Scott J, Menge C (2000) Nest predation increases with parental parental activity: separating nest site and parental activity effects. P Roy Soc B 267:2287–2293
Martin TE, Lloyd P, Bosque C, Barton DC, Biancucci AL, Cheng Y, Ton R (2011) Growth rate variation among passerine species in tropical and temperate sites: an antagonistic interaction between parental food provisioning and nest predation risk. Evolution 65:1607–1622
Martin K, Wilson S, MacDonald EC, Camfield AF, Martin M, Trefry SA (2017) Effects of severe weather on reproduction for sympatric songbirds in an alpine environment: interactions of climate extremes influence nesting success. Auk 134:696–709
Mitrus C, Soćko B (2008) Breeding success and nest-site characteristics of Red-breasted Flycatchers Ficedula parva in a primeval forest. Bird Study. 55:203–208
Morrison CA, Alves JA, Gunnarsson TG, Pórisson B, Gill JA (2019) Why do earlier-arriving migratory birds have better breeding success? Ecol Evol 9:8856–8864
Moya-Larano J, Wise DH (2000) Survival regression analysis: a powerful tool for evaluating fighting and assessment. Anim Behav 60:307–313
Muenchow G (1986) Ecological use of failure time analysis. Ecology 67:246–250
Mwangi J, Ndithia HK, Kentie R, Muchai M, Tieleman BI (2018) Nest survival in year-round breeding tropical Red-capped Larks Calandrella cinerea increases with higher nest abundance but decreases with higher invertebrate availability and rainfall. J Avian Biol 49:e01645. https://doi.org/10.1111/jav.01645
Newton I (1993) Predation and limitation in birds number. Curr Ornithol 11:143–198
Newton I (1998) Population limitation in birds. Academic, London
Pérez-Granados C, Bota G, Gómez-Catasús J, Pla M, Barrero A, Sáez-Gómez P, Reverter M, López-Iborra GM, Giralt D, Bustillo-dela Rosa D, Zurdo J, Traba J (2023) Short-term impact of an extreme weather event on the threatened Dupont’s Lark Chersophilus duponti. Bird Conserv Int 33:e53. https://doi.org/10.1017/S0959270923000035
Pérez-Granados C, López-Iborra GM, Garza V, Traba J (2017) Breeding biology of the endangered Dupont’s Lark Chersophilus duponti in two separate Spanish shrub-steppes. Bird Study. 64:328–338
Pescador S, Peris S (2007) Influence of roads on bird nest predation: an experimental study in the Iberian Peninsula. Landsc Urban Plan 82:66–71
Praus L (2020) Breeding ecology of the Crested Lark (Galerida cristata) in the Czech Republic. Sylvia 56:49–71
Praus L, Weidinger L (2010) Predators and nest success of Sky Larks Alauda arvensis in large arable fields in the Czech Republic. Bird Study 57:525–530
Praus L, Hegemann A, Tieleman BI, Weidinger K (2014) Predators and predation rates of Skylark Alauda arvensis and Woodlark Lullula arobrea nest in semi-natural area in the Netherlands. Ardea 102:87–94
Reif J, Koleček J, Morelli F, Benedetti Y (2023) Population trends of ground-nesting birds indicate increasing environmental impacts from Eastern to Western Europe: different patterns for open-habitat and woodland species. Front Environ Sci 11:1156360
Reino L, Moya-Larano J, Antonio Heitor AC (2009) Using survival regression to study patterns of expansion of invasive species: will the Common Waxbill expand with global warming? Ecography 32:237–246
Remeš V, Matysioková B, Vrána J (2020) Adaptation and constraint shape the evolution of growth patterns in passerine birds across the globe. Front Zool 17:29. https://doi.org/10.1186/s12983-020-00377-7
Rodriguez S, Barba E (2016) Effects of cool nest microclimates on nestling development: an experimental study with Mediterranean Great Tits Parus major. Ardeola 63:251–260
Roselaar CS (1988) Galerida cristata Crested Lark. In: Cramp S (ed) Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic, vol 5. Oxford University Press, pp 145–163
Shkedy Y, Safriel UN (1992) Nest predation and nestling growth rate of two lark species in the Negev Desert, Israel. Ibis 134:268–272
Shochat E, Wolfe DH, Patten MA, Reinking DL, Sherod SK (2005) Tallgrass prairie management and bird success along roadside. Biol Cons 121:399–407
Siikamaki P (1998) Limitation of reproductive success by food availability and breeding time in pied flycatchers. Ecology 79:1789–1796
Šímová P, Šťastný K, Šálek M (2015) Refugial role of urbanized areas and colonization potential for declining Crested Lark (Galerida cristata) populations in the Czech Republic, Central Europe. J Ornithol 156:915–921
Starck JM, Ricklefs RE (1998) Avian growth and development. Evolution within the altricial precocial spectrum. Oxford University Press, New York
Suarez F, Manrique J (1992) Low breeding success in Mediterranean shrubsteppe passerines: Thekla Lark Galerida theklae, Lesser Short-toed Lark Calandrella rufescens, and Black-eared Wheatear Oenanthe hispanica. Ornis Scand 23:24–28
Tscharntke T, Batáry P (2023) Agriculture, urbanization, climate, and forest change drive bird declines. Proc Natl Acad Sci USA 120(22):e2305216120. https://doi.org/10.1073/pnas.2305216120
Weggler M (2000) Reproductive consequences of autumnal singing in Black Redstarts (Phoenicurus ochruros). Auk 117:65–73
Wright LJ, Hoblyn RA, Green RE, Bowden CGR, Mallord JW, Sutherland WJ, Dolman PM (2009) Importance of climatic and environmental change in the demography of a multi-brooded passerine, the Woodlark Lullula arborea. J Animal Ecol 78:1191–1202
Zaremba U, Kasprzykowski Z, Golawski A (2020) Effect of nest age and habitat variables on nest survival in Marsh Harrier (Circus aeruginosus) in a fishpond habitat. PeerJ 8:e9929. https://doi.org/10.7717/peerj.9929
Zduniak P (2010) Water conditions influence nestling survival in a Hooded Crow Corvus cornix wetland population. J Ornithol 151:45–50
Zduniak P, Czechowski P, Jędro G (2011) The effect of nesting habitat on reproductive output of the Barn Swallow (Hirundo rustica). A comparative study of populations from atypical and typical nesting habitats in western Poland. Belg J Zool 141:38–43
Zhang P, van Leeuwen C, Bogers D, Poelma M, Xu J, Bakker E (2020) Ectothermic omnivores increase herbivory in response to rising temperature. Oikos 129:1028–1039
Acknowledgements
We thank Justyna Grześkowiak for her linguistic assistance. We are also grateful to two anonymous reviewers for their numerous and valuable comments. This study was funded by Adam Mickiewicz University, Poznań, ID-UB Doctoral mini-grants No. 017/02/SNP/0027. The study are comply with the current laws of the Poland. All experiments were conducted with the permission of the Local Ethical Committee for Animal Experiments in Poznań (No. 25/2018) and Bird Ringing Permit of Polish Ornithological Scheme (No.343/2023).
Author information
Authors and Affiliations
Contributions
Conceptualization: Rafał Sandecki, Jakub Kosicki; Methodology: Jakub Kosicki, Rafał Sandecki; Formal analysis and investigation: Jakub Kosicki, Rafał Sandecki; Writing—original draft preparation: Rafał Sandecki, Jakub Kosicki; Writing—review and editing: Rafał Sandecki, Jakub Kosicki.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sandecki, R., Kosicki, J.Z. Nest survival of Crested Lark (Galerida cristata) in intensively used habitats in Central Poland. J Ornithol 165, 947–958 (2024). https://doi.org/10.1007/s10336-024-02183-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-024-02183-y