Abstract
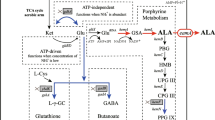
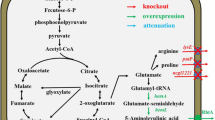
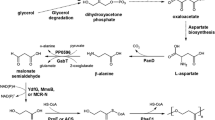
5-Aminolevulinic acid (ALA), the first committed intermediate for natural biosynthesis of tetrapyrrole compounds, has recently drawn intensive attention due to its broad potential applications. In this study, we describe the construction of recombinant Escherichia coli strains for ALA production from glucose via the C4 pathway. The hemA gene from Rhodobacter capsulatus was optimally overexpressed using a ribosome binding site engineering strategy, which enhanced ALA production substantially from 20 to 689 mg/L. Following optimization of biosynthesis pathways towards coenzyme A and precursor (glycine and succinyl-CoA), and downregulation of hemB expression, the production of ALA was further increased to 2.81 g/L in batch-fermentation.





Similar content being viewed by others
References
Ahn JH, Jang Y-S, Lee SY (2016) Production of succinic acid by metabolically engineered microorganisms. Curr Opin Biotechnol 42:54–66. doi:10.1016/j.copbio.2016.02.034
Akhtar J, Idris A, Abd Aziz R (2014) Recent advances in production of succinic acid from lignocellulosic biomass. Appl Microbiol Biotechnol 98:987–1000. doi:10.1007/s00253-013-5319-6
Bolt EL, Kryszak L, Zeilstraryalls J, Shoolinginjordan PM, Warren MJ (1999) Characterization of the Rhodobacter sphaeroides 5-aminolaevulinic acid synthase isoenzymes, HemA and HemT, isolated from recombinant Escherichia coli. Eur J Biochem 265:290–299. doi:10.1046/j.1432-1327.1999.00730.x
Bornscheuer UT (2001) Directed evolution of enzymes for biocatalytic applications. Biocatal Biotransform 19:85–97. doi:10.3109/10242420109003638
Butler JS, Springer M, Grunberg-Manago M (1987) AUU-to-AUG mutation in the initiator codon of the translation initiation factor IF3 abolishes translational autocontrol of its own gene (infC) in vivo. Proc Natl Acad Sci USA 84:4022–4025. doi:10.1073/pnas.84.12.4022
Catazaro J, Caprez A, Guru A, Swanson D, Powers R (2014) Functional evolution of PLP-dependent enzymes based on active-site structural similarities. Proteins 82:2597–2608. doi:10.1002/prot.24624
Farasat I, Collens J, Sails HM (2011) Efficient optimization of synthetic metabolic pathways with the RBS Library Calculator. Paper presented at the Abstr Pap Am Chem Soc, Mar
Feng L, Zhang Y, Fu J, Mao Y, Chen T, Zhao X, Wang Z (2016) Metabolic engineering of Corynebacterium glutamicum for efficient production of 5-aminolevulinic acid. Biotechnol Bioeng 113:1284–1293. doi:10.1002/bit.25886
Fu W, Lin J, Cen P (2007) 5-aminolevulinate production with recombinant Escherichia coli using a rare codon optimizer host strain. Appl Microbiol Biotechnol 75:777–782. doi:10.1007/s00253-007-0887-y
Grant GA, Schuller DJ, Banaszak LJ (1996) A model for the regulation of D-3-phosphoglycerate dehydrogenase, a V-max-type allosteric enzyme. Protein Sci 5:34–41. doi:10.1002/pro.5560050105
Gu P, Yang F, Su T, Li F, Li Y, Qi Q (2014) Construction of an l-serine producing Escherichia coli via metabolic engineering. J Ind Microbiol Biotechnol 41:1443–1450. doi:10.1007/s10295-014-1476-6
Jiang Y, Chen B, Duan C, Sun B, Yang J, Yang S (2015) Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl Environ Microbiol 81:2506–2514. doi:10.1128/AEM.04023-14
Kang Z, Wang X, Li Y, Wang Q, Qi Q (2012) Small RNA RyhB as a potential tool used for metabolic engineering in Escherichia coli. Biotechnol Lett 34:527–531. doi:10.1007/s10529-011-0794-2
Kang Z, Wang Y, Gu P, Wang Q, Qi Q (2011) Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose. Metab Eng 13:492–498. doi:10.1016/j.ymben.2011.05.003
Kang Z, Wang Y, Wang Q, Qi Q (2011) Metabolic engineering to improve 5-aminolevulinic acid production. Bioeng Bugs 2:1–4. doi:10.4161/bbug.2.6.17237
Kang Z, Zhang J, Zhou J, Qi Q, Du G, Chen J (2012) Recent advances in microbial production of delta-aminolevulinic acid and vitamin B12. Biotechnol Adv 30:1533–1542. doi:10.1016/j.biotechadv.2012.04.003
Lee JW, Kim TY, Jang YS, Choi S, Sang YL (2011) Systems metabolic engineering for chemicals and materials. Trends Biotechnol 29:370–378. doi:10.1016/j.tibtech.2011.04.001
Lee MJ, Chun S-J, Kim H-J, Kwon AS, Jun SY, Kang SH, Kim P (2012) Porphyrin derivatives from a recombinant Escherichia coli grown on chemically defined medium. J Microbiol Biotechnol 22:1653–1658. doi:10.4014/jmb.1208.08054
Lendrihas T, Hunter GA, Ferreira GC (2010) Targeting the active site gate to yield hyperactive variants of 5-aminolevulinate synthase. J Biol Chem 285:13704–13711. doi:10.1074/jbc.M109.074237
Leonardi R, Zhang YM, Rock CO, Jackowski S (2005) Coenzyme A: back in action. Prog Lipid Res 44:125–153. doi:10.1016/j.plipres.2005.04.001
Li F, Wang Y, Gong K, Wang Q, Liang Q, Qi Q (2014) Constitutive expression of RyhB regulates the heme biosynthesis pathway and increases the 5-aminolevulinic acid accumulation in Escherichia coli. FEMS Microbiol Lett 350:209–215. doi:10.1111/1574-6968.12322
Lin J, Fu W, Cen P (2009) Characterization of 5-aminolevulinate synthase from Agrobacterium radiobacter, screening new inhibitors for 5-aminolevulinate dehydratase from Escherichia coli and their potential use for high 5-aminolevulinate production. Bioresour Technol 100:2293–2297. doi:10.1016/j.biortech.2008.11.008
Liu XX, Wang L, Wang YJ, Cai LL (2010) D-glucose enhanced 5-aminolevulinic acid production in recombinant Escherichia coli culture. Appl Biochem Biotechnol 160:822–830. doi:10.1007/s12010-009-8608-x
Lou J, Zhu L, Wu M, Yang L, Lin J, Cen P (2014) High-level soluble expression of the hemA gene from Rhodobacter capsulatus and comparative study of its enzymatic properties. J Zhejiang Univ Sci B 15:491–499. doi:10.1631/jzus.B1300283
Mauzerall D, Granick S (1956) The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem 219:446
Meng Q, Zhang Y, Ju X, Ma C, Ma H, Chen J, Zheng P, Sun J, Zhu J, Ma Y, Zhao X, Chen T (2016) Production of 5-aminolevulinic acid by cell free multi-enzyme catalysis. J Biotechnol 226:8–13. doi:10.1016/j.jbiotec.2016.03.024
Mundhada H, Schneider K, Christensen HB, Nielsen AT (2016) Engineering of high yield production of l-serine in Escherichia coli. Biotechnol Bioeng 113:807–816. doi:10.1002/bit.25844
Neidle EL, Kaplan S (1993) Expression of the Rhodobacter sphaeroides hemA and hemT genes, encoding two 5-aminolevulinic acid synthase isozymes. J Bacteriol 175:2292–2303. doi:10.1128/jb.175.8.2292-2303.1993
Peters AE, Bavishi A, Cho H, Choudhary M (2012) Evolutionary constraints and expression analysis of gene duplications in Rhodobacter sphaeroides 2.4.1. BMC Res Notes 5:192. doi:10.1186/1756-0500-5-192
Pranawidjaja S, Choi S-I, Lay BW, Kim P (2015) Analysis of heme biosynthetic pathways in a recombinant Escherichia coli. J Microbiol Biotechnol 25:880–886. doi:10.4014/jmb.1411.11050
Ramzi AB, Hyeon JE, Kim SW, Park C, Han SO (2015) 5-Aminolevulinic acid production in engineered Corynebacterium glutamicum via C5 biosynthesis pathway. Enzyme Microb Technol 81:1–7. doi:10.1016/j.enzmictec.2015.07.004
Rock CO, Calder RB, Karim MA, Jackowski S (2000) Pantothenate kinase regulation of the intracellular concentration of coenzyme A. J Biol Chem 275:1377–1383. doi:10.1074/jbc.275.2.1377
Rock CO, Park HW, Jackowski S (2003) Role of feedback regulation of pantothenate kinase (CoaA) in control of coenzyme A levels in Escherichia coli. J Bacteriol 185:3410–3415. doi:10.1128/jb.185.11.3410-3415.2003
Sasaki K, Watanabe M, Tanaka T, Tanaka T (2002) Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl Microbiol Biotechnol 58:23–29. doi:10.1007/s00253-001-0858-7
Sasikala C, Ramana CV (1995) Biotechnological potentials of anoxygenic phototrophic bacteria. I. Production of single-cell protein, vitamins, ubiquinones, hormones, and enzymes and use in waste treatment. Adv Appl Microbiol 41:173–226. doi:10.1016/s0065-2164(08)70310-1
Thakker C, Martínez I, Li W, San KY, Bennett GN (2015) Metabolic engineering of carbon and redox flow in the production of small organic acids. J Ind Microbiol Biotechnol 42:403–422. doi:10.1007/s10295-014-1560-y
Vadali RV, Bennett GN, San KY (2004) Cofactor engineering of intracellular CoA/acetyl-CoA and its effect on metabolic flux redistribution in Escherichia coli. Metab Eng 6:133–139. doi:10.1016/j.ymben.2004.02.001
Vallari DS, Jackowski S, Rock CO (1987) Regulation of pantothenate kinase by coenzyme A and its thioesters. J Biol Chem 262:2468–2471
Vuoristo KS, Mars AE, Sanders JPM, Eggink G, Weusthuis RA (2016) Metabolic engineering of TCA cycle for production of chemicals. Trends Biotechnol 34:191–197. doi:10.1016/j.tibtech.2015.11.002
Yang P, Liu W, Cheng X, Wang J, Wang Q, Qi Q (2016) A new strategy for production of 5-aminolevulinic acid in recombinant Corynebacterium glutamicum with high yield. Appl Environ Microbiol 82:2709–2717. doi:10.1128/AEM.00224-16
Yu X, Jin H, Cheng X, Wang Q, Qi Q (2016) Transcriptomic analysis for elucidating the physiological effects of 5-aminolevulinic acid accumulation on Corynebacterium glutamicum. Microbiol Res 192:292–299. doi:10.1016/j.micres.2016.08.004
Yu X, Jin H, Liu W, Wang Q, Qi Q (2015) Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose. Microb Cell Factories 14:1–10. doi:10.1186/s12934-015-0364-8
Zhang J, Kang Z, Chen J, Du G (2015) Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli. Sci Rep UK 5:1440–1444. doi:10.1038/srep08584
Zhang J, Kang Z, Ding W, Chen J, Du G (2016) Integrated optimization of the in vivo heme biosynthesis pathway and the in vitro iron concentration for 5-aminolevulinate production. Appl Biochem Biotechnol 178:1252–1262. doi:10.1007/s12010-015-1942-2
Zhang J, Weng H, Ding W, Kang Z (2016) N-terminal engineering of glutamyl-tRNA reductase with positive charge arginine to increase 5-aminolevulinic acid biosynthesis. Bioengineered. doi:10.1080/21655979.2016.1230572
Zhang L, Chen J, Chen N, Sun J, Zheng P, Ma Y (2013) Cloning of two 5-aminolevulinic acid synthase isozymes HemA and HemO from Rhodopseudomonas palustris with favorable characteristics for 5-aminolevulinic acid production. Biotechnol Lett 35:763–768. doi:10.1007/s10529-013-1143-4
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31670092), the Natural Science Foundation of Jiangsu Province (BK20141107), a grant from the Key Technologies R&D Program of Jiangsu Province, China (BE2014607) and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT_15R26).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, W., Weng, H., Du, G. et al. 5-Aminolevulinic acid production from inexpensive glucose by engineering the C4 pathway in Escherichia coli . J Ind Microbiol Biotechnol 44, 1127–1135 (2017). https://doi.org/10.1007/s10295-017-1940-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1940-1