Abstract
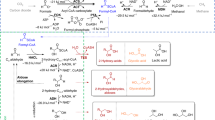
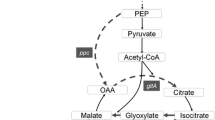
The review describes efforts toward metabolic engineering of production of organic acids. One aspect of the strategy involves the generation of an appropriate amount and type of reduced cofactor needed for the designed pathway. The ability to capture reducing power in the proper form, NADH or NADPH for the biosynthetic reactions leading to the organic acid, requires specific attention in designing the host and also depends on the feedstock used and cell energetic requirements for efficient metabolism during production. Recent work on the formation and commercial uses of a number of small mono- and diacids is discussed with redox differences, major biosynthetic precursors and engineering strategies outlined. Specific attention is given to those acids that are used in balancing cell redox or providing reduction equivalents for the cell, such as formate, which can be used in conjunction with metabolic engineering of other products to improve yields. Since a number of widely studied acids derived from oxaloacetate as an important precursor, several of these acids are covered with the general strategies and particular components summarized, including succinate, fumarate and malate. Since malate and fumarate are less reduced than succinate, the availability of reduction equivalents and level of aerobiosis are important parameters in optimizing production of these compounds in various hosts. Several other more oxidized acids are also discussed as in some cases, they may be desired products or their formation is minimized to afford higher yields of more reduced products. The placement and connections among acids in the typical central metabolic network are presented along with the use of a number of specific non-native enzymes to enhance routes to high production, where available alternative pathways and strategies are discussed. While many organic acids are derived from a few precursors within central metabolism, each organic acid has its own special requirements for high production and best compatibility with host physiology.






Similar content being viewed by others
References
Abbott DA, Zelle RM, Pronk JT, van Maris AJ (2009) Metabolic engineering of Saccharomyces cerevisiae for production of carboxylic acids: current status and challenges. FEMS Yeast Res 9(8):1123–1136
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2010) Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J Biotechnol 156(4):286–301. doi:10.1016/j.jbiotec.2011.06.017
el Aboulnaga H, Pinkenburg O, Schiffels J, El-Refai A, Buckel W, Selmer T (2013) Effect of an oxygen-tolerant bifurcating butyryl coenzyme A dehydrogenase/electron-transferring flavoprotein complex from Clostridium difficile on butyrate production in Escherichia coli. J Bacteriol 195(16):3704–3713. doi:10.1128/JB.00321-13
Akhtar J, Idris A, Abd Aziz R (2014) Recent advances in production of succinic acid from lignocellulosic biomass. Appl Microbiol Biotechnol 98(3):987–1000. doi:10.1007/s00253-013-5319-6
Alissandratos A, Kim HK, Easton CJ (2013) Formate production through biocatalysis. Bioengineered 4(5):348–350. doi:10.4161/bioe.25360
Amao Y, Shuto N, Furuno K, Obata A, Fuchino Y, Uemura K, Kajino T, Sekito T, Iwai S, Miyamoto Y, Matsuda M (2012) Artificial leaf device for solar fuel production. Faraday Discuss 155:289–296
Ammar EM, Jin Y, Wang Z, Yang ST (2014) Metabolic engineering of Propionibacterium freudenreichii: effect of expressing phosphoenolpyruvate carboxylase on propionic acid production. Appl Microbiol Biotechnol 98(18):7761–7772. doi:10.1007/s00253-014-5836-y
Auriol C, Bestel-Corre G, Claude JB, Soucaille P, Meynial-Salles I (2011) Stress-induced evolution of Escherichia coli points to original concepts in respiratory cofactor selectivity. P Natl Acad Sci USA 108(4):1278–1283. doi:10.1073/pnas.1010431108
Baek JM, Mazumdar S, Lee SW, Jung MY, Lim JH, Seo SW, Jung GY, Oh MK (2013) Butyrate production in engineered Escherichia coli with synthetic scaffolds. Biotechnol Bioeng 110(10):2790–2794. doi:10.1002/bit.24925
Balzer GJ, Thakker C, Bennett GN, San KY (2013) Metabolic engineering of Escherichia coli to minimize byproduct formate and improving succinate productivity through increasing NADH availability by heterologous expression of NAD(+)-dependent formate dehydrogenase. Metab Eng 20:1–8. doi:10.1016/j.ymben.2013.07.005
Bausch C, Peekhaus N, Utz C, Blais T, Murray E, Lowary T, Conway T (1998) Sequence analysis of the GntII (subsidiary) system for gluconate metabolism reveals a novel pathway for l-idonic acid catabolism in Escherichia coli. J Bacteriol 180(14):3704–3710
Bausch C, Ramsey M, Conway T (2004) Transcriptional organization and regulation of the l-idonic acid pathway (GntII system) in Escherichia coli. J Bacteriol 186(5):1388–1397
Beinert H, Kennedy MC (1993) Aconitase, a two-faced protein: enzyme and iron regulatory factor. Faseb J 7(15):1442–1449
Bengelsdorf FR, Straub M, Durre P (2013) Bacterial synthesis gas (syngas) fermentation. Environ Technol 34(13–16):1639–1651
Bentley R, Thiessen CP (1955) Cisaconitic decarboxylase. Science 122(3164):330
Bergler H, Fuchsbichler S, Hogenauer G, Turnowsky F (1996) The enoyl-[acyl-carrier-protein] reductase (FabI) of Escherichia coli, which catalyzes a key regulatory step in fatty acid biosynthesis, accepts NADH and NADPH as cofactors and is inhibited by palmitoyl-CoA. Eur J Biochem 242(3):689–694
Berrios-Rivera SJ, Bennett GN, San KY (2002) The effect of increasing NADH availability on the redistribution of metabolic fluxes in Escherichia coli chemostat cultures. Metab Eng 4(3):230–237
Berrios-Rivera SJ, Bennett GN, San KY (2002) Metabolic engineering of Escherichia coli: increase of NADH availability by overexpressing an NAD(+)-dependent formate dehydrogenase. Metab Eng 4(3):217–229
Berrios-Rivera SJ, Sanchez AM, Bennett GN, San KY (2004) Effect of different levels of NADH availability on metabolite distribution in Escherichia coli fermentation in minimal and complex media. Appl Microbiol Biotechnol 65(4):426–432. doi:10.1007/s00253-004-1609-3
Betancourt T, Pardo J, Soo K, Peppas NA (2010) Characterization of pH-responsive hydrogels of poly(itaconic acid-g-ethylene glycol) prepared by UV-initiated free radical polymerization as biomaterials for oral delivery of bioactive agents. J Biomed Mater Res A 93(1):175–188
Blazeck J, Miller J, Pan A, Gengler J, Holden C, Jamoussi M, Alper HS (2014) Metabolic engineering of Saccharomyces cerevisiae for itaconic acid production. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-5895-0
Blumhoff ML, Steiger MG, Mattanovich D, Sauer M (2013) Targeting enzymes to the right compartment: metabolic engineering for itaconic acid production by Aspergillus niger. Metab Eng 19:26–32. doi:10.1016/j.ymben.2013.05.003
Boston DJ, Xu C, Armstrong DW, MacDonnell FM (2013) Photochemical reduction of carbon dioxide to methanol and formate in a homogeneous system with pyridinium catalysts. J Am Chem Soc 135(44):16252–16255. doi:10.1021/ja406074w
Brown SH, Bashkirova L, Berka R, Chandler T, Doty T, McCall K, McCulloch M, McFarland S, Thompson S, Yaver D, Berry A (2013) Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of l-malic acid. Appl Microbiol Biotechnol 97(20):8903–8912. doi:10.1007/s00253-013-5132-2
Buckel W (1827) Thauer RK (2013) Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation. Biochim Biophys Acta 2:94–113. doi:10.1016/j.bbabio.2012.07.002
Cai X, Servinsky M, Kiel J, Sund C, Bennett GN (2013) Analysis of redox responses during TNT transformation by Clostridium acetobutylicum ATCC 824 and mutants exhibiting altered metabolism. Appl Microbiol Biotechnol 97(10):4651–4663. doi:10.1007/s00253-012-4253-3
Cao Y, Lin X (2011) Metabolically engineered Escherichia coli for biotechnological production of four-carbon 1,4-dicarboxylic acids. J Ind Microbiol Biotechnol 38(6):649–656. doi:10.1007/s10295-010-0913-4
Cao Y, Zhang R, Sun C, Cheng T, Liu Y, Xian M (2013) Fermentative succinate production: an emerging technology to replace the traditional petrochemical processes. BioMed Res Int 2013:723412. doi:10.1155/2013/723412
Causey TB, Shanmugam KT, Yomano LP, Ingram LO (2004) Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci USA 101(8):2235–2240
Causey TB, Zhou S, Shanmugam KT, Ingram LO (2003) Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. P Natl Acad Sci USA 100(3):825–832. doi:10.1073/pnas.0337684100
Centeno-Leija S, Utrilla J, Flores N, Rodriguez A, Gosset G, Martinez A (2013) Metabolic and transcriptional response of Escherichia coli with a NADP(+)-dependent glyceraldehyde 3-phosphate dehydrogenase from Streptococcus mutans. Antonie Van Leeuwenhoek 104(6):913–924. doi:10.1007/s10482-013-0010-6
Chatterjee R, Millard CS, Champion K, Clark DP, Donnelly MI (2001) Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl Environ Microbiol 67(1):148–154
Chen F, Feng XH, Liang JF, Xu H, Ouyang PK (2013) An oxidoreduction potential shift control strategy for high purity propionic acid production by Propionibacterium freudenreichii CCTCC M207015 with glycerol as sole carbon source. Bioprocess Biosyst Eng 36(9):1165–1176. doi:10.1007/s00449-012-0843-9
Chen T, Zhu N, Xia H (2014) Aerobic production of succinate from arabinose by metabolically engineered Corynebacterium glutamicum. Bioresour Technol 151:411–414. doi:10.1016/j.biortech.2013.10.017
Chen WN, Tan KY (2013) Malonate uptake and metabolism in Saccharomyces cerevisiae. Appl Biochem Biotechnol 171(1):44–62. doi:10.1007/s12010-013-0334-8
Chen X, Xu G, Xu N, Zou W, Zhu P, Liu L, Chen J (2013) Metabolic engineering of Torulopsis glabrata for malate production. Metab Eng 19:10–16. doi:10.1016/j.ymben.2013.05.002
Cheng KK, Wang GY, Zeng J, Zhang JA (2013) Improved succinate production by metabolic engineering. Biomed Res Int 2013:538790. doi:10.1155/2013/538790
Cheng KK, Zhao XB, Zeng J, Wu RC, Xu YZ, Liu DH, Zhang JA (2012) Downstream processing of biotechnological produced succinic acid. Appl Microbiol Biotechnol 95(4):841–850. doi:10.1007/s00253-012-4214-x
Chia DW, Yoder TJ, Reiter WD, Gibson SI (2000) Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211(5):743–751
Choi O, Um Y, Sang BI (2012) Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol Bioeng 109(10):2494–2502. doi:10.1002/bit.24520
Chowdhury NP, Mowafy AM, Demmer JK, Upadhyay V, Koelzer S, Jayamani E, Kahnt J, Hornung M, Demmer U, Ermler U, Buckel W (2014) Studies on the mechanism of electron bifurcation catalyzed by electron transferring flavoprotein (Etf) and butyryl-CoA dehydrogenase (Bcd) of Acidaminococcus fermentans. J Biol Chem 289(8):5145–5157. doi:10.1074/jbc.M113.521013
Clomburg JM, Vick JE, Blankschien MD, Rodriguez-Moya M, Gonzalez R (2012) A synthetic biology approach to engineer a functional reversal of the beta-oxidation cycle. ACS Synth Biol 1(11):541–554. doi:10.1021/sb3000782
Comalada M, Bailon E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Galvez J (2006) The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol 132(8):487–497. doi:10.1007/s00432-006-0092-x
Crable BR, Plugge CM, McInerney MJ, Stams AJ (2011) Formate formation and formate conversion in biological fuels production. Enzyme Res 2011:532536. doi:10.4061/2011/532536
De Renobales M, Rogers L, Kolattukudy PE (1980) Involvement of a thioesterase in the production of short-chain fatty acids in the uropygial glands of mallard ducks (Anas platyrhynchos). Arch Biochem Biophys 205(2):464–477
DeBolt S, Cook DR, Ford CM (2006) l-tartaric acid synthesis from vitamin C in higher plants. Proc Natl Acad Sci USA 103(14):5608–5613
Debolt S, Melino V, Ford CM (2007) Ascorbate as a biosynthetic precursor in plants. Ann Bot 99(1):3–8
Dekishima Y, Lan EI, Shen CR, Cho KM, Liao JC (2011) Extending carbon chain length of 1-butanol pathway for 1-hexanol synthesis from glucose by engineered Escherichia coli. J Am Chem Soc 133(30):11399–11401. doi:10.1021/ja203814d
Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature 476(7360):355–359. doi:10.1038/nature10333
Ding Y, Li S, Dou C, Yu Y, Huang H (2011) Production of fumaric acid by Rhizopus oryzae: role of carbon–nitrogen ratio. Appl Biochem Biotechnol 164(8):1461–1467. doi:10.1007/s12010-011-9226-y
Dishisha T, Stahl A, Lundmark S, Hatti-Kaul R (2013) An economical biorefinery process for propionic acid production from glycerol and potato juice using high cell density fermentation. Bioresour Technol 135:504–512. doi:10.1016/j.biortech.2012.08.098
Dwiarti L, Yamane K, Yamatani H, Kahar P, Okabe M (2002) Purification and characterization of cis-aconitic acid decarboxylase from Aspergillus terreus TN484-M1. J Biosci Bioeng 94:29–33
Dwidar M, Park JY, Mitchell RJ, Sang BI (2012) The future of butyric acid in industry. Sci World J 2012:471417. doi:10.1100/2012/471417
Eggeman T, Verser D (2005) Recovery of organic acids from fermentation broths. Appl Biochem Biotechnol 121–124:605–618
Elfari M, Ha SW, Bremus C, Merfort M, Khodaverdi V, Herrmann U, Sahm H, Gorisch H (2005) A Gluconobacter oxydans mutant converting glucose almost quantitatively to 5-keto-d-gluconic acid. Appl Microbiol Biotechnol 66(6):668–674
Felthouse TR, Burnett JC, Horrell B, Mummey MJ, Kuo Y-J (2001) Maleic anhydride, maleic acid, and fumaric acid. Huntsman Petrochemical Corp., Austin, TX
Feng X, Chen F, Xu H, Wu B, Li H, Li S, Ouyang P (2011) Green and economical production of propionic acid by Propionibacterium freudenreichii CCTCC M207015 in plant fibrous-bed bioreactor. Bioresour Technol 102(10):6141–6146. doi:10.1016/j.biortech.2011.02.087
Fernandez CE, Mancera M, Holler E, Bou JJ, Galbis JA, Munoz-Guerra S (2005) Low-molecular-weight poly(alpha-methyl beta, l-malate) of microbial origin: synthesis and crystallization. Macromol Biosci 5(2):172–176
Flint DH (1993) Escherichia coli fumarase A catalyzes the isomerization of enol and keto oxalacetic acid. Biochemistry 32(3):799–805
Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol 56:41–71. doi:10.1146/annurev.arplant.56.032604.144106
Fujita E, Muckerman JT, Himeda Y (2013) Interconversion of CO2 and formic acid by bio-inspired Ir complexes with pendent bases. Biochim Biophys Acta 1827(8–9):1031–1038. doi:10.1016/j.bbabio.2012.11.004
Gadd GM (1999) Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. Adv Microb Physiol 41:47–92
Girbal L, Soucaille P (1994) Regulation of Clostridium acetobutylicum metabolism as revealed by mixed-substrate steady-state continuous cultures: role of NADH/NAD ratio and ATP pool. J Bacteriol 176(21):6433–6438
Green MA, Fry SC (2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-o-oxalyl-l-threonate. Nature 433(7021):83–87
Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F (2010) From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev 23(2):366–384. doi:10.1017/S0954422410000247
Haluska A (2010) Increasing fermentative butanol production in Clostridium beijerinckii using oxidized extracellular electron shuttling molecules. University of Illinois, Illinois
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27(2):104–119. doi:10.1111/j.1365-2036.2007.03562.x
Handke P, Lynch SA, Gill RT (2011) Application and engineering of fatty acid biosynthesis in Escherichia coli for advanced fuels and chemicals. Metab Eng 13(1):28–37. doi:10.1016/j.ymben.2010.10.007
Hatch JL, Finneran KT (2008) Influence of reduced electron shuttling compounds on biological H2 production in the fermentative pure culture Clostridium beijerinckii. Curr Microbiol 56(3):268–273. doi:10.1007/s00284-007-9073-9
Herrmann G, Jayamani E, Mai G, Buckel W (2008) Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J Bacteriol 190(3):784–791. doi:10.1128/JB.01422-07
Herrmann U, Merfort M, Jeude M, Bringer-Meyer S, Sahm H (2004) Biotransformation of glucose to 5-keto-d-gluconic acid by recombinant Gluconobacter oxydans DSM 2343. Appl Microbiol Biotechnol 64(1):86–90
Hetzel M, Brock M, Selmer T, Pierik AJ, Golding BT, Buckel W (2003) Acryloyl-CoA reductase from Clostridium propionicum. An enzyme complex of propionyl-CoA dehydrogenase and electron-transferring flavoprotein. Eur J Biochem 270(5): 902–910
Holdom K, Winskill N (1988) Fermentation process and microorganism for producing aconitic acid. USA Patent 4740464
Holscher T, Schleyer U, Merfort M, Bringer-Meyer S, Gorisch H, Sahm H (2009) Glucose oxidation and PQQ-dependent dehydrogenases in Gluconobacter oxydans. J Mol Microbiol Biotechnol 16(1–2):6–13
Hong SH, Lee SY (2001) Metabolic flux analysis for succinic acid production by recombinant Escherichia coli with amplified malic enzyme activity. Biotechnol Bioeng 74(2):89–95
Hong SH, Lee SY (2002) Importance of redox balance on the production of succinic acid by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 58(3):286–290
Huang X, Lu X, Li Y, Li X, Li JJ (2014) Improving itaconic acid production through genetic engineering of an industrial Aspergillus terreus strain. Microb Cell Fact 13(1):119. doi:10.1186/s12934-014-0119-y
Iddar A, Valverde F, Serrano A, Soukri A (2003) Purification of recombinant non-phosphorylating NADP-dependent glyceraldehyde-3-phosphate dehydrogenase from Streptococcus pyogenes expressed in E. coli. Mol Cell Biochem 247(1–2):195–203
Ihara M, Kawano Y, Urano M, Okabe A (2013) Light driven CO2 fixation by using cyanobacterial photosystem I and NADPH-dependent formate dehydrogenase. PLoS ONE 8(8):e71581. doi:10.1371/journal.pone.0071581
Ilmen M, Koivuranta K, Ruohonen L, Rajgarhia V, Suominen P, Penttila M (2013) Production of l-lactic acid by the yeast Candida sonorensis expressing heterologous bacterial and fungal lactate dehydrogenases. Microb Cell Fact 12:53. doi: 10.1186/1475-2859-12-53
Jan J, Martinez I, Wang Y, Bennett GN, San KY (2013) Metabolic engineering and transhydrogenase effects on NADPH availability in Escherichia coli. Biotechnol Prog 29(5):1124–1130. doi:10.1002/btpr.1765
Jang YS, Im JA, Choi SY, Lee JI, Lee SY (2014) Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab Eng 23:165–174. doi:10.1016/j.ymben.2014.03.004
Jansen ML, van Gulik WM (2014) Towards large scale fermentative production of succinic acid. Curr Opin Biotechnol 30:190–197
Janssen PH (1991) Fermentation of l-tartrate by a newly isolated gram-negative glycolytic bacterium. Antonie Van Leeuwenhoek 59(3):191–198
Jantama K, Haupt MJ, Svoronos SA, Zhang X, Moore JC, Shanmugam KT, Ingram LO (2008) Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol Bioeng 99(5):1140–1153
Jarboe LR, Zhang X, Wang X, Moore JC, Shanmugam KT, Ingram LO (2010) Metabolic engineering for production of biorenewable fuels and chemicals: contributions of synthetic biology. J Biomed Biotechnol 2010:761042. doi:10.1155/2010/761042
Javidpour P, Pereira JH, Goh EB, McAndrew RP, Ma SM, Friedland GD, Keasling JD, Chhabra SR, Adams PD, Beller HR (2014) Biochemical and structural studies of NADH-dependent FabG used to increase the bacterial production of fatty acids under anaerobic conditions. Appl Environ Microbiol 80(2):497–505. doi:10.1128/AEM.03194-13
Jeon B, Yi J, Park D (2014) Effects of H2 and electrochemical reducing power on metabolite production by Clostridium acetobutylicum KCTC1037. Biosci Biotechnol Biochem 78(3):503–509. doi:10.1080/09168451.2014.882743
Jiang L, Wang J, Liang S, Cai J, Xu Z, Cen P, Yang S, Li S (2011) Enhanced butyric acid tolerance and bioproduction by Clostridium tyrobutyricum immobilized in a fibrous bed bioreactor. Biotechnol Bioeng 108(1):31–40. doi:10.1002/bit.22927
Kaup B, Bringer-Meyer S, Sahm H (2004) Metabolic engineering of Escherichia coli: construction of an efficient biocatalyst for d-mannitol formation in a whole-cell biotransformation. Appl Microbiol Biotechnol 64(3):333–339. doi:10.1007/s00253-003-1470-9
Kawai S, Mori S, Mukai T, Hashimoto W, Murata K (2001) Molecular characterization of Escherichia coli NAD kinase. Eur J Biochem 268(15):4359–4365
Killestein J, Rudick RA, Polman CH (2011) Oral treatment for multiple sclerosis. Lancet Neurol 10(11):1026–1034. doi:10.1016/S1474-4422(11)70228-9
Kim BH, Bellows P, Datta R, Zeikus JG (1984) Control of carbon and electron flow in Clostridium acetobutylicum fermentations: utilization of carbon monoxide to inhibit hydrogen production and to enhance butanol yields. Appl Environ Microb 48(4):764–770
Kim OB, Reimann J, Lukas H, Schumacher U, Grimpo J, Dunnwald P, Unden G (2009) Regulation of tartrate metabolism by TtdR and relation to the DcuS–DcuR-regulated C4-dicarboxylate metabolism of Escherichia coli. Microbiology 155(Pt 11):3632–3640
Kim OB, Unden G (2007) The l-tartrate/succinate antiporter TtdT (YgjE) of l-tartrate fermentation in Escherichia coli. J Bacteriol 189(5):1597–1603
Kim P, Laivenieks M, Vieille C, Zeikus JG (2004) Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinate production in Escherichia coli. Appl Environ Microbiol 70(2):1238–1241
Kim YB, Lenz RW (2001) Polyesters from microorganisms. Adv Biochem Eng Biotechnol 71:51–79
Kim YS (2002) Malonate metabolism: biochemistry, molecular biology, physiology, and industrial application. J Biochem Mol Biol 35(5):443–451
Klasen R, Bringer-Meyer S, Sahm H (1992) Incapability of Gluconobacter oxydans to produce tartaric acid. Biotechnol Bioeng 40(1):183–186
Klement T, Buchs J (2013) Itaconic acid—a biotechnological process in change. Bioresour Technol 135:422–431. doi:10.1016/j.biortech.2012.11.141
Kurzrock T, Weuster-Botz D (2010) Recovery of succinic acid from fermentation broth. Biotechnol Lett 32(3):331–339. doi:10.1007/s10529-009-0163-6
Kwon YD, Kwon OH, Lee HS, Kim P (2007) The effect of NADP-dependent malic enzyme expression and anaerobic C4 metabolism in Escherichia coli compared with other anaplerotic enzymes. J Appl Microbiol 103(6):2340–2345
Laivenieks M, Vieille C, Zeikus JG (1997) Cloning, sequencing, and overexpression of the Anaerobiospirillum succiniciproducens phosphoenolpyruvate carboxykinase (pckA) gene. Appl Environ Microbiol 63(6):2273–2280
Lan EI, Liao JC (2012) ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. P Natl Acad Sci USA 109(16):6018–6023. doi:10.1073/pnas.1200074109
Lauble H, Kennedy MC, Beinert H, Stout CD (1994) Crystal structures of aconitase with trans-aconitate and nitrocitrate bound. J Mol Biol 237(4):437–451
Le Notre J, Witte-van Dijk SC, van Haveren J, Scott EL, Sanders JP (2014) Synthesis of bio-based methacrylic acid by decarboxylation of itaconic acid and citric acid catalyzed by solid transition-metal catalysts. Chem Sus Chem. doi:10.1002/cssc.201402117
Leduc YA, Prasad L, Laivenieks M, Zeikus JG, Delbaere LT (2005) Structure of PEP carboxykinase from the succinate-producing Actinobacillus succinogenes: a new conserved active-site motif. Acta Crystallogr D Biol Crystallogr 61(Pt 7):903–912
Lee SK, Chou H, Ham TS, Lee TS, Keasling JD (2008) Metabolic engineering of microorganisms for biofuels production: from bugs to synthetic biology to fuels. Curr Opin Biotechnol 19(6):556–563
Lee WH, Kim MD, Jin YS, Seo JH (2013) Engineering of NADPH regenerators in Escherichia coli for enhanced biotransformation. Appl Microbiol Biotechnol 97(7):2761–2772. doi:10.1007/s00253-013-4750-z
Lehmann D, Honicke D, Ehrenreich A, Schmidt M, Weuster-Botz D, Bahl H, Lutke-Eversloh T (2012) Modifying the product pattern of Clostridium acetobutylicum: physiological effects of disrupting the acetate and acetone formation pathways. Appl Microbiol Biotechnol 94(3):743–754. doi:10.1007/s00253-011-3852-8
Li H, Opgenorth PH, Wernick DG, Rogers S, Wu TY, Higashide W, Malati P, Huo YX, Cho KM, Liao JC (2012) Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335(6076):1596. doi:10.1126/science.1217643
Li M, San KY (2013) Improved fatty acid productivity
Li M, Zhang X, Agrawal A, San KY (2012) Effect of acetate formation pathway and long chain fatty acid CoA-ligase on the free fatty acid production in E. coli expressing acy-ACP thioesterase from Ricinus communis. Metab Eng 14(4):380–387. doi:10.1016/j.ymben.2012.03.007
Lian J, Zhao H (2014) Reversal of the beta-oxidation cycle in Saccharomyces cerevisiae for production of fuels and chemicals. ACS Synth Biol. doi:10.1021/sb500243c
Liang L, Liu R, Wang G, Gou D, Ma J, Chen K, Jiang M, Wei P, Ouyang P (2012) Regulation of NAD(H) pool and NADH/NAD(+) ratio by overexpression of nicotinic acid phosphoribosyltransferase for succinic acid production in Escherichia coli NZN111. Enzyme Microb Technol 51(5):286–293. doi:10.1016/j.enzmictec.2012.07.011
Lim JH, Seo SW, Kim SY, Jung GY (2013) Refactoring redox cofactor regeneration for high-yield biocatalysis of glucose to butyric acid in Escherichia coli. Bioresour Technol 135:568–573. doi:10.1016/j.biortech.2012.09.091
Lin H, Bennett GN, San KY (2005) Genetic reconstruction of the aerobic central metabolism in Escherichia coli for the absolute aerobic production of succinate. Biotechnol Bioeng 89(2):148–156. doi:10.1002/bit.20298
Lin H, Bennett GN, San KY (2005) Metabolic engineering of aerobic succinate production systems in Escherichia coli to improve process productivity and achieve the maximum theoretical succinate yield. Metab Eng 7(2):116–127. doi:10.1016/j.ymben.2004.10.003
Litsanov B, Brocker M, Bott M (2012) Toward homosuccinate fermentation: metabolic engineering of Corynebacterium glutamicum for anaerobic production of succinate from glucose and formate. Appl Environ Microb 78(9):3325–3337. doi:10.1128/AEM.07790-11
Liu J, Gao Q, Xu N, Liu L (2013) Genome-scale reconstruction and in silico analysis of Aspergillus terreus metabolism. Mol BioSyst 9(7):1939–1948. doi:10.1039/c3mb70090a
Liu L, Zhu Y, Li J, Wang M, Lee P, Du G, Chen J (2012) Microbial production of propionic acid from propionibacteria: current state, challenges and perspectives. Crit Rev Biotechnol 32(4):374–381. doi:10.3109/07388551.2011.651428
Liu P, Jarboe LR (2012) Metabolic engineering of biocatalysts for carboxylic acids production. Comput Struct Biotechnol J 3:e201210011. doi:10.5936/csbj.201210011
Liu X, Zhu Y, Yang ST (2006) Construction and characterization of ack deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid and hydrogen production. Biotechnol Prog 22(5):1265–1275. doi:10.1021/bp060082g
Liu Y, Zhang YG, Zhang RB, Zhang F, Zhu J (2011) Glycerol/glucose co-fermentation: one more proficient process to produce propionic acid by Propionibacterium acidipropionici. Curr Microbiol 62(1):152–158. doi:10.1007/s00284-010-9683-5
Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, Black KL, Holler E (2008) Nanoconjugate based on polymalic acid for tumor targeting. Chem Biol Interact 171(2):195–203
Lovitt RW, Kell DB, Morris JG (1987) The physiology of Clostridium sporogenes NCIB 8053 growing in defined media. J Appl Bacteriol 62(1):81–92
Lovley DR, Nevin KP (2013) Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr Opin Biotechnol 24(3):385–390. doi:10.1016/j.copbio.2013.02.012
Ma J, Gou D, Liang L, Liu R, Chen X, Zhang C, Zhang J, Chen K, Jiang M (2013) Enhancement of succinate production by metabolically engineered Escherichia coli with co-expression of nicotinic acid phosphoribosyltransferase and pyruvate carboxylase. Appl Microbiol Biotechnol 97(15):6739–6747. doi:10.1007/s00253-013-4910-1
Makela MR, Hilden K, Lundell TK (2010) Oxalate decarboxylase: biotechnological update and prevalence of the enzyme in filamentous fungi. Appl Microbiol Biotechnol 87(3):801–814. doi:10.1007/s00253-010-2650-z
Martinez I, Zhu J, Lin H, Bennett GN, San KY (2008) Replacing Escherichia coli NAD-dependent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with a NADP-dependent enzyme from Clostridium acetobutylicum facilitates NADPH dependent pathways. Metab Eng 10(6):352–359. doi:10.1016/j.ymben.2008.09.001
Matzerath I, Klaui W, Klasen R, H. S (1995) Vanadate catalysed oxidation of 5-keto-Image-gluconic acid to tartaric acid: the unexpected effect of phosphate and carbonate on rate and selectivity. Inorganica Chimica Acta pp 203–205
McGinn SM, Beauchemin KA, Coates T, Colombatto D (2004) Methane emissions from beef cattle: effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J Anim Sci 82(11):3346–3356
Merfort M, Herrmann U, Bringer-Meyer S, Sahm H (2006) High-yield 5-keto-d-gluconic acid formation is mediated by soluble and membrane-bound gluconate-5-dehydrogenases of Gluconobacter oxydans. Appl Microbiol Biotechnol 73(2):443–451
Merfort M, Herrmann U, Ha SW, Elfari M, Bringer-Meyer S, Gorisch H, Sahm H (2006) Modification of the membrane-bound glucose oxidation system in Gluconobacter oxydans significantly increases gluconate and 5-keto-d-gluconic acid accumulation. Biotechnol J 1(5):556–563
Meussen BJ, de Graaff LH, Sanders JP, Weusthuis RA (2012) Metabolic engineering of Rhizopus oryzae for the production of platform chemicals. Appl Microbiol Biotechnol 94(4):875–886. doi:10.1007/s00253-012-4033-0
Mohanraj S, Kodhaiyolii S, Rengasamy M, Pugalenthi V (2014) Green synthesized iron oxide nanoparticles effect on fermentative hydrogen production by Clostridium acetobutylicum. Appl Biochem Biotechnol 173(1):318–331. doi:10.1007/s12010-014-0843-0
Moret S, Dyson PJ, Laurenczy G (2014) Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat Commun 5:4017. doi:10.1038/ncomms5017
Moshaverinia A, Roohpour N, Ansari S, Moshaverinia M, Schricker S, Darr JA, Rehman IU (2009) Effects of N-vinylpyrrolidone (NVP) containing polyelectrolytes on surface properties of conventional glass-ionomer cements (GIC). Dent Mater 25(10):1240–1247
Ni Y, Sun Z (2009) Recent progress on industrial fermentative production of acetone–butanol–ethanol by Clostridium acetobutylicum in China. Appl Microbiol Biotechnol 83(3):415–423. doi:10.1007/s00253-009-2003-y
Nicolas C, Kiefer P, Letisse F, Kromer J, Massou S, Soucaille P, Wittmann C, Lindley ND, Portais JC (2007) Response of the central metabolism of Escherichia coli to modified expression of the gene encoding the glucose-6-phosphate dehydrogenase. FEBS Lett 581(20):3771–3776. doi:10.1016/j.febslet.2007.06.066
Nikolau BJ, Perera MA, Brachova L, Shanks B (2008) Platform biochemicals for a biorenewable chemical industry. Plant J 54(4):536–545. doi:10.1111/j.1365-313X.2008.03484.x
Okabe M, Lies D, Kanamasa S, Park EY (2009) Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol 84(4):597–606. doi:10.1007/s00253-009-2132-3
Okamura E, Tomita T, Sawa R, Nishiyama M, Kuzuyama T (2010) Unprecedented acetoacetyl-coenzyme A synthesizing enzyme of the thiolase superfamily involved in the mevalonate pathway. P Natl Acad Sci USA 107(25):11265–11270. doi:10.1073/pnas.1000532107
Orjuela A, Orjuela A, Lira CT, Miller DJ (2013) A novel process for recovery of fermentation-derived succinic acid: process design and economic analysis. Bioresour Technol 139:235–241. doi:10.1016/j.biortech.2013.03.174
Oshima T, Biville F (2006) Functional identification of ygiP as a positive regulator of the ttdA–ttdB–ygjE operon. Microbiology 152(Pt 7):2129–2135
Peguin S, Soucaille P (1995) Modulation of carbon and electron flow in Clostridium acetobutylicum addition. Appl Environ Microb 61(1):403–405
Peguin S, Soucaille P (1996) Modulation of metabolism of Clostridium acetobutylicum grown in chemostat culture in a three-electrode potentiostatic system with methyl viologen as electron carrier. Biotechnol Bioeng 51(3):342–348. doi:10.1002/(SICI)1097-0290(19960805)51:3<342:AID-BIT9>3.0.CO;2-D
Pohl NL, Hans M, Lee HY, Kim YS, Cane DE, Khosla C (2001) Remarkably broad substrate tolerance of malonyl-CoA synthetase, an enzyme capable of intracellular synthesis of polyketide precursors. J Am Chem Soc 123(24):5822–5823
Portnoy VA, Scott DA, Lewis NE, Tarasova Y, Osterman AL, Palsson BO (2010) Deletion of genes encoding cytochrome oxidases and quinol monooxygenase blocks the aerobic–anaerobic shift in Escherichia coli K-12 MG1655. Appl Environ Microb 76(19):6529–6540. doi:10.1128/AEM.01178-10
Pracharoenwattana I, Zhou W, Keech O, Francisco PB, Udomchalothorn T, Tschoep H, Stitt M, Gibon Y, Smith SM (2010) Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J 62(5):785–795. doi:10.1111/j.1365-313X.2010.04189.x
Rao G, Mutharasan R (1987) Altered Electron Flow in Continuous Cultures of Clostridium acetobutylicum Induced by Viologen Dyes. Appl Environ Microb 53(6):1232–1235
Reaney SK, Begg C, Bungard SJ, Guest JR (1993) Identification of the L-tartrate dehydratase genes (ttdA and ttdB) of Escherichia coli and evolutionary relationship with the class I fumarase genes. J Gen Microbiol 139(7):1523–1530
Reda T, Plugge CM, Abram NJ, Hirst J (2008) Reversible interconversion of carbon dioxide and formate by an electroactive enzyme. P Natl Acad Sci USA 105(31):10654–10658. doi:10.1073/pnas.0801290105
Reich K, Thaci D, Mrowietz U, Kamps A, Neureither M, Luger T (2009) Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis—a retrospective study (FUTURE). Journal der Deutschen Dermatologischen Gesellschaft 7(7):603–611. doi:10.1111/j.1610-0387.2009.07120.x
Roa Engel CA, Straathof AJ, Zijlmans TW, van Gulik WM, van der Wielen LA (2008) Fumaric acid production by fermentation. Appl Microbiol Biotechnol 78(3):379–389. doi:10.1007/s00253-007-1341-x
Rode H, Giffhorn F (1982) Ferrous- or cobalt ion-dependent d-(-)-tartrate dehydratase of pseudomonads: purification and properties. J Bacteriol 151(3):1602–1604
Rode H, Giffhorn F (1983) Adaptation of Rhodopseudomonas sphaeroides to Growth on d-(-)-Tartrate and Large-Scale Production of a Constitutive d-(-)-Tartrate Dehydratase During Growth on dl-Malate. Appl Environ Microbiol 45(2):716–719
Rude MA, Schirmer A (2009) New microbial fuels: a biotech perspective. Curr Opin Microbiol 12(3):274–281. doi:10.1016/j.mib.2009.04.004
Rush B (2012) Turning a novel yeast into a platform host for industrial production of fuels and chemicals. In: Metabolic Engineering IX, Biarritz, France, Metabolic Engineering IX. Engineering Conferences International
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403(1):25–34. doi:10.1016/S0003-9861(02)00017-6
Salusjarvi T, Povelainen M, Hvorslev N, Eneyskaya EV, Kulminskaya AA, Shabalin KA, Neustroev KN, Kalkkinen N, Miasnikov AN (2004) Cloning of a gluconate/polyol dehydrogenase gene from Gluconobacter suboxydans IFO 12528, characterisation of the enzyme and its use for the production of 5-ketogluconate in a recombinant Escherichia coli strain. Appl Microbiol Biotechnol 65(3):306–314
San KY, Bennett GN, Berrios-Rivera SJ, Vadali RV, Yang YT, Horton E, Rudolph FB, Sariyar B, Blackwood K (2002) Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng 4(2):182–192. doi:10.1006/mben.2001.0220
San KY, Li M (2013) Bacteria and method for synthesizing fatty acids. USA Patent WO/2013/059218
Sanchez AM, Andrews J, Hussein I, Bennett GN, San KY (2006) Effect of overexpression of a soluble pyridine nucleotide transhydrogenase (UdhA) on the production of poly(3-hydroxybutyrate) in Escherichia coli. Biotechnol Prog 22(2):420–425. doi:10.1021/bp050375u
Sauer M, Porro D, Mattanovich D, Branduardi P (2010) 16 years research on lactic acid production with yeast—ready for the market? Biotechnol Genet Eng Rev 27:229–256
Sauer U, Canonaco F, Heri S, Perrenoud A, Fischer E (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem 279(8):6613–6619. doi:10.1074/jbc.M311657200
Sauer U, Eikmanns BJ (2005) The PEP–pyruvate–oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev 29(4):765–794
Sawers G, Watson G (1998) A glycyl radical solution: oxygen-dependent interconversion of pyruvate formate-lyase. Mol Microbiol 29(4):945–954
Selmer T, Pierik AJ, Heider J (2005) New glycyl radical enzymes catalysing key metabolic steps in anaerobic bacteria. Biol Chem 386(10):981–988. doi:10.1515/BC.2005.114
Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC (2011) Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Appl Environ Microb 77(9):2905–2915. doi:10.1128/AEM.03034-10
Shine WE, Mancha M, Stumpf PK (1976) Fat metabolism in higher plants. The function of acyl thioesterases in the metabolism of acyl-coenzymes A and acyl-acyl carrier proteins. Arch Biochem Biophys 172(1):110–116
Shisler KA, Broderick JB (2014) Glycyl radical activating enzymes: structure, mechanism, and substrate interactions. Arch Biochem Biophys 546:64–71. doi:10.1016/j.abb.2014.01.020
Song CW, Kim DI, Choi S, Jang JW, Lee SY (2013) Metabolic engineering of Escherichia coli for the production of fumaric acid. Biotechnol Bioeng 110(7):2025–2034. doi:10.1002/bit.24868
Sparling R, Islam R, Cicek N, Carere C, Chow H, Levin DB (2006) Formate synthesis by Clostridium thermocellum during anaerobic fermentation. Can J Microbiol 52(7):681–688. doi:10.1139/w06-021
Srikanth S, Maesen M, Dominguez-Benetton X, Vanbroekhoven K, Pant D (2014) Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES). Bioresour Technol 165:350–354. doi:10.1016/j.biortech.2014.01.129
Steiger MG, Blumhoff ML, Mattanovich D, Sauer M (2013) Biochemistry of microbial itaconic acid production. Front Microbiol. doi:10.3389/Fmicb.2013.00023
Stols L, Donnelly MI (1997) Production of succinic acid through overexpression of NAD(+)-dependent malic enzyme in an Escherichia coli mutant. Appl Environ Microbiol 63(7):2695–2701
Straathof AJ, van Gulik WM (2012) Production of fumaric acid by fermentation. Sub-cellular biochemistry 64:225–240. doi:10.1007/978-94-007-5055-5_11
Suwannakham S, Huang Y, Yang ST (2006) Construction and characterization of ack knock-out mutants of Propionibacterium acidipropionici for enhanced propionic acid fermentation. Biotechnol Bioeng 94(2):383–395. doi:10.1002/bit.20866
Suwannakham S, Yang ST (2005) Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnol Bioeng 91(3):325–337. doi:10.1002/bit.20473
Svedruzic D, Jonsson S, Toyota CG, Reinhardt LA, Ricagno S, Lindqvist Y, Richards NG (2005) The enzymes of oxalate metabolism: unexpected structures and mechanisms. Arch Biochem Biophys 433(1):176–192. doi:10.1016/j.abb.2004.08.032
Tevz G, Bencina M, Legisa M (2010) Enhancing itaconic acid production by Aspergillus terreus. Appl Microbiol Biotechnol 87(5):1657–1664. doi:10.1007/s00253-010-2642-z
Thakker C, Martinez I, San KY, Bennett GN (2012) Succinate production in Escherichia coli. Biotechnol J 7(2):213–224. doi:10.1002/biot.201100061
Thakker C, San KY, Bennett GN (2013) Production of succinic acid by engineered E. coli strains using soybean carbohydrates as feedstock under aerobic fermentation conditions. Bioresour Technol 130:398–405. doi:10.1016/j.biortech.2012.10.154
Thauer RK (1973) CO 2 reduction to formate in Clostridium acidiurici. J Bacteriol 114(1):443–444
Thauer RK, Fuchs G, Kaufer B (1975) Reduced ferredoxin: CO2 oxidoreductase from Clostridium pasteurianum. Effect of ligands to transition metals on the activity and the stability of the enzyme. Hoppe Seylers Z Physiol Chem 356(6):653–662
Thelen JJ, Ohlrogge JB (2002) Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng 4(1):12–21. doi:10.1006/mben.2001.0204
van der Straat L, de Graaff LH (2014) Pathway transfer in fungi: Transporters are the key to success. Bioengineered 5(5)
Van der Werf MJ, Guettler MV, Jain MK, Zeikus JG (1997) Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch Microbiol 167(6):332–342
Van Immerseel F, Boyen F, Gantois I, Timbermont L, Bohez L, Pasmans F, Haesebrouck F, Ducatelle R (2005) Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult Sci 84(12):1851–1856
Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ (2009) Butyrate-induced transcriptional changes in human colonic mucosa. PLoS ONE 4(8):e6759. doi:10.1371/journal.pone.0006759
Vasconcelos I, Girbal L, Soucaille P (1994) Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J Bacteriol 176(5):1443–1450
Versari A, Castellari M, Spinabelli U, Galassi S (2001) Recovery of tartaric acid from industrial enological wastes. J Chem Technol Biotechnol 76:485–488
Voyame P, Toghill KE, Mendez MA, Girault HH (2013) Photoreduction of CO2 using [Ru(bpy)2(CO)L]n+ catalysts in biphasic solution/supercritical CO2 systems. Inorg Chem 52(19):10949–10957. doi:10.1021/ic401031j
Wallace KK, Bao ZY, Dai H, Digate R, Schuler G, Speedie MK, Reynolds KA (1995) Purification of crotonyl-CoA reductase from Streptomyces collinus and cloning, sequencing and expression of the corresponding gene in Escherichia coli. European journal of biochemistry/FEBS 233(3):954–962
Wang S, Huang H, Kahnt J, Thauer RK (2013) Clostridium acidurici electron-bifurcating formate dehydrogenase. Appl Environ Microb 79(19):6176–6179. doi:10.1128/AEM.02015-13
Wang Y, San KY, Bennett GN (2013) Cofactor engineering for advancing chemical biotechnology. Curr Opin Biotechnol 24(6):994–999. doi:10.1016/j.copbio.2013.03.022
Wang Y, San KY, Bennett GN (2013) Improvement of NADPH bioavailability in Escherichia coli by replacing NAD(+)-dependent glyceraldehyde-3-phosphate dehydrogenase GapA with NADP (+)-dependent GapB from Bacillus subtilis and addition of NAD kinase. J Ind Microbiol Biotechnol 40(12):1449–1460. doi:10.1007/s10295-013-1335-x
Wang Y, San KY, Bennett GN (2013) Improvement of NADPH bioavailability in Escherichia coli through the use of phosphofructokinase deficient strains. Appl Microbiol Biotechnol 97(15):6883–6893. doi:10.1007/s00253-013-4859-0
Warnick TA, Methe BA, Leschine SB (2002) Clostridium phytofermentans sp. nov., a cellulolytic mesophile from forest soil. Int J Syst Evol Microbiol 52(Pt 4): 1155–1160
Wei D, Liu X, Yang ST (2013) Butyric acid production from sugarcane bagasse hydrolysate by Clostridium tyrobutyricum immobilized in a fibrous-bed bioreactor. Bioresour Technol 129:553–560. doi:10.1016/j.biortech.2012.11.065
Wendisch VF, Bott M, Eikmanns BJ (2006) Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for biotechnological production of organic acids and amino acids. Curr Opin Microbiol 9(3):268–274. doi:10.1016/j.mib.2006.03.001
Werpy T, Petersen G (eds) (2004) Top value added chemicals from biomass, vol 1. USDOE, Washington DC
Wieschalka S, Blombach B, Bott M, Eikmanns BJ (2013) Bio-based production of organic acids with Corynebacterium glutamicum. Microb Biotechnol 6(2):87–102. doi:10.1111/1751-7915.12013
Willke T, Vorlop KD (2001) Biotechnological production of itaconic acid. Appl Microbiol Biotechnol 56(3–4):289–295
Woehlke G, Dimroth P (1994) Anaerobic growth of Salmonella typhimurium on l(+)- and d(-)-tartrate involves an oxaloacetate decarboxylase Na+ pump. Arch Microbiol 162(4):233–237
Woskow SA, Glatz BA (1991) Propionic Acid Production by a Propionic Acid-Tolerant Strain of Propionibacterium acidipropionici in Batch and Semicontinuous Fermentation. Appl Environ Microb 57(10):2821–2828
Wu H, Karanjikar M, San KY (2014) Metabolic engineering of Escherichia coli for efficient free fatty acid production from glycerol. Metab Eng 25:82–91. doi:10.1016/j.ymben.2014.06.009
Xie D, Zhao J, Weng Y (2010) Synthesis and application of novel multi-arm poly(carboxylic acid)s for glass-ionomer restoratives. J Biomater Appl 24(5):419–436
Xu G, Chen X, Liu L, Jiang L (2013) Fumaric acid production in Saccharomyces cerevisiae by simultaneous use of oxidative and reductive routes. Bioresour Technol 148:91–96. doi:10.1016/j.biortech.2013.08.115
Xu G, Liu L, Chen J (2012) Reconstruction of cytosolic fumaric acid biosynthetic pathways in Saccharomyces cerevisiae. Microb Cell Fact 11:24. doi:10.1186/1475-2859-11-24
Xu G, Zou W, Chen X, Xu N, Liu L, Chen J (2012) Fumaric acid production in Saccharomyces cerevisiae by in silico aided metabolic engineering. PLoS ONE 7(12):e52086. doi:10.1371/journal.pone.0052086
Xu P, Qiu J, Gao C, Ma C (2008) Biotechnological routes to pyruvate production. J Biosci Bioeng 105(3):169–175. doi:10.1263/jbb.105.169
Xu Q, Li S, Huang H, Wen J (2012) Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol Adv 30(6):1685–1696. doi:10.1016/j.biotechadv.2012.08.007
Xue J, Isern NG, Ewing RJ, Liyu AV, Sears JA, Knapp H, Iversen J, Sisk DR, Ahring BK, Majors PD (2014) New generation NMR bioreactor coupled with high-resolution NMR spectroscopy leads to novel discoveries in Moorella thermoacetica metabolic profiles. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-5847-8
Yamauchi Y, Hirasawa T, Nishii M, Furusawa C, Shimizu H (2014) Enhanced acetic acid and succinic acid production under microaerobic conditions by Corynebacterium glutamicum harboring Escherichia coli transhydrogenase gene pntAB. J Gener Appl Microbiol 60(3):112–118
Yarlagadda VN, Gupta A, Dodge CJ, Francis AJ (2012) Effect of exogenous electron shuttles on growth and fermentative metabolism in Clostridium sp. BC1. Bioresour Technol 108:295–299. doi:10.1016/j.biortech.2011.12.040
Ye X, Honda K, Morimoto Y, Okano K, Ohtake H (2013) Direct conversion of glucose to malate by synthetic metabolic engineering. J Biotechnol 164(1):34–40. doi:10.1016/j.jbiotec.2012.11.011
Ye X, Morgenroth E, Zhang X, Finneran KT (2011) Anthrahydroquinone-2,6,-disulfonate (AH2QDS) increases hydrogen molar yield and xylose utilization in growing cultures of Clostridium beijerinckii. Appl Microbiol Biotechnol 92(4):855–864. doi: 10.1007/s00253-011-3571-1
Yew WS, Fedorov AA, Fedorov EV, Wood BM, Almo SC, Gerlt JA (2006) Evolution of enzymatic activities in the enolase superfamily: d-tartrate dehydratase from Bradyrhizobium japonicum. Biochemistry 45(49):14598–14608
Yum DY, Lee BY, Pan JG (1999) Identification of the yqhE and yafB genes encoding two 2, 5-diketo-D-gluconate reductases in Escherichia coli. Appl Environ Microbiol 65(8):3341–3346
Zelic B, Bolf N, Vasic-Racki D (2006) Modeling of the pyruvate production with Escherichia coli: comparison of mechanistic and neural networks-based models. Bioprocess Biosyst Eng 29(1):39–47
Zelic B, Gerharz T, Bott M, Vasic-Racki D, Wandrey C, Takors R (2003) Fed-batch process for pyruvate production by recombinant Escherichia coli YYC 202 strain. Eng Life 3:299–305
Zelic B, Gostovic S, Vuorilehto K, Vasic-Racki D, Takors R (2004) Process strategies to enhance pyruvate production with recombinant Escherichia coli: from repetitive fed-batch to in situ product recovery with fully integrated electrodialysis. Biotechnol Bioeng 85(6):638–646
Zelle RM, de Hulster E, Kloezen W, Pronk JT, van Maris AJ (2010) Key process conditions for production of C(4) dicarboxylic acids in bioreactor batch cultures of an engineered Saccharomyces cerevisiae strain. Appl Environ Microb 76(3):744–750. doi:10.1128/AEM.02396-09
Zhang A, Yang ST (2009) Engineering Propionibacterium acidipropionici for enhanced propionic acid tolerance and fermentation. Biotechnol Bioeng 104(4):766–773. doi:10.1002/bit.22437
Zhang B, Skory CD, Yang ST (2012) Metabolic engineering of Rhizopus oryzae: effects of overexpressing pyc and pepc genes on fumaric acid biosynthesis from glucose. Metab Eng 14(5):512–520. doi:10.1016/j.ymben.2012.07.001
Zhang C, Yang H, Yang F, Ma Y (2009) Current progress on butyric acid production by fermentation. Curr Microbiol 59(6):656–663. doi:10.1007/s00284-009-9491-y
Zhang X, Jantama K, Moore JC, Jarboe LR, Shanmugam KT, Ingram LO (2009) Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc Natl Acad Sci USA 106(48):20180–20185
Zhang X, Li M, Agrawal A, San KY (2011) Efficient free fatty acid production in Escherichia coli using plant acyl-ACP thioesterases. Metab Eng 13(6):713–722. doi:10.1016/j.ymben.2011.09.007
Zhang X, Shanmugam KT, Ingram LO (2010) Fermentation of glycerol to succinate by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 76(8):2397–2401
Zhang X, Wang X, Shanmugam KT, Ingram LO (2011) l-malate production by metabolically engineered Escherichia coli. Appl Environ Microb 77(2):427–434. doi:10.1128/AEM.01971-10
Zhang Y, Yu M, Yang ST (2012) Effects of ptb knockout on butyric acid fermentation by Clostridium tyrobutyricum. Biotechnol Prog 28(1):52–59. doi:10.1002/btpr.730
Zhu J, Sanchez A, Bennett GN, San KY (2011) Manipulating respiratory levels in Escherichia coli for aerobic formation of reduced chemical products. Metab Eng 13(6):704–712. doi:10.1016/j.ymben.2011.09.006
Zhu J, Thakker C, San KY, Bennett G (2011) Effect of culture operating conditions on succinate production in a multiphase fed-batch bioreactor using an engineered Escherichia coli strain. Appl Microbiol Biotechnol 92(3):499–508. doi:10.1007/s00253-011-3314-3
Zhuge X, Liu L, Shin HD, Chen RR, Li J, Du G, Chen J (2013) Development of a Propionibacterium-Escherichia coli shuttle vector for metabolic engineering of Propionibacterium jensenii, an efficient producer of propionic acid. Appl Environ Microb 79(15):4595–4602. doi:10.1128/AEM.00737-13
Acknowledgments
This work was supported by NSF CBET-1033552. I.M. acknowledges the financial support by FONDECYT 11110411.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: Metabolic Engineering.
Rights and permissions
About this article
Cite this article
Thakker, C., Martínez, I., Li, W. et al. Metabolic engineering of carbon and redox flow in the production of small organic acids. J Ind Microbiol Biotechnol 42, 403–422 (2015). https://doi.org/10.1007/s10295-014-1560-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1560-y