Abstract
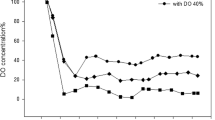
The impact of different levels of agitation speed, carbondioxide and dissolved oxygen concentration on the key parameters and production of rhG-CSF in Escherichia coli BL21(DE3)PLysS were studied. Lower carbondioxide concentrations as well as higher agitation speeds and dissolved oxygen concentrations led to reduction in the acetate concentrations, and enhanced the cell growth, but inhibited plasmid stability and rhG-CSF expression. Similarly, higher carbondioxide concentrations and lower agitation speeds as well as dissolved oxygen concentrations led to enhanced acetate concentrations, but inhibited the cell growth and protein expression. To address the bottlenecks, a two-stage agitation control strategy (strategy-1) and two-stage dissolved oxygen control strategy (strategy-2) were employed to establish the physiological and metabolic conditions, so as to improve the expression of rhG-CSF. By adopting strategy-1 the yields were improved 1.4-fold over constant speed of 550 rpm, 1.1-fold over constant dissolved oxygen of 45%, respectively. Similarly, using strategy-2 the yields were improved 1.6-fold over constant speed of 550 rpm, 1.3-fold over constant dissolved oxygen of 45%, respectively.







Similar content being viewed by others
References
Pavlou AK, Reichart JM (2004) Recombinant protein therapeutics-success rates, market trends and values to 2010. Nat Biotechnol 22:1513–1519, doi:10.1038/nbt1204-1513
Arbabi-Ghahroudi M et al (2005) Prokaryotic expression of antibodies. Cancer Metastasis Rev 24:501–519, doi:10.1007/s10555-005-6193-1
Morstyn G, Campbell L, Souza LM, Alton NK, Keech J, Green M, Sheridan W, Metcalf D, Fox R (1988) Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet 331:667–672, doi:10.1016/S0140-6736(88)91475-4
Hoglund M (1998) Glycosylated and non-glycosylated recombinant human granulocyte colony stimulating factor (rhG-CSF)—what is the difference? Med Oncol 15:229–233, doi:10.1007/BF02787205
Komatsu Y, Matsumoto T, Kuga T, Nishi T, Sekine S, Saito A, Okabe M, Morimoto M, Itoh S, Okabe T, Takaku F (1987) Cloning of granulocyte colony stimulating factor cDNA and its expression in Escherichia coli. Jpn J Cancer Res 78:1179–1181
Devlin PE, Drummond RJ, Toy P, Mark DF, Watt KW, Devlin JJ (1988) Alteration of amino-terminal codons of human granulocyte-colony-stimulating factor increases expression levels and allows efficient processing by methionine aminopeptidase in Escherichia coli. Gene 65:13–22, doi:10.1016/0378-1119(88)90412-X
Fallah MJ, Akbari B, Saeedinia AR, Karimi M, Zeinoddini M, Soleimani M, Maghsoudi M (2003) Over expression of recombinant human granulocyte colony stimulating factor in E. coli. IJMS 28:131–134
Scott A (2004) Biologics, coming back into balance. Chem Week 2:21–25
Sørensen HP, Mortensen KK (2005) Advanced strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115:113–128, doi:10.1016/j.jbiotec.2004.08.004
Vollbrecht D (1982) Restricted oxygen supply and excretion of metabolites. Eur J Appl Microbiol Biotechnol 15:111–116, doi:10.1007/BF00499516
Bylund F, Collet E, Enfors S-O, Larson G (1998) Substrate gradient formation in the large scale bioreactor lowers cell yield and increases by-product formation. Bioproc Eng 18:171–180, doi:10.1007/s004490050427
Akesson M, Karlsson EN, Hagander P, Axelsson JP, Tocaj A (1999) On-line detection of acetate formation in Escherichia coli cultures using dissolved oxygen responses to feed transients. Biotechnol Bioeng 64:590–598, doi:10.1002/(SICI)1097-0290(19990905)64:5<590::AID-BIT9>3.0.CO;2-T
Enfors SO, Jahic M, Rozkov A, Xu B, Hecker M, Jurgen B, Kruger E (2001) Physiological responses to mixing in large-scale bioreactors. J Biotechnol 85:175–185, doi:10.1016/S0168-1656(00)00365-5
Matsui T, Yokota H, Sato S, Mukutaka S, Takahasi J (1989) Pressurised culture of Escherichia for a high concentration. Agric Biol Chem 53:2115–2120
Pan JG, Rhee JS, Lebeault JM (1987) Physiological constraints in increasing biomass concentration of Escherichia coli B in fed-batch culture. Biotechnol Lett 9:89–94, doi:10.1007/BF01032744
Noronha SB, Yeh HJC, Spande TF, Shiloach J (2000) Investigation of the TCA cycle and the glyoylate shunt in Escherichia coli BL21 and JM109 using 13C-NMR/MS. Biotechnol Bioeng 68:316–327, doi:10.1002/(SICI)1097-0290(20000505)68:3<316::AID-BIT10>3.0.CO;2-2
Ko YF, Bentley WE, Weigand WA (1995) The effects of cellular energetics on foreign protein production. Appl Biochem Biotechnol 50:145–159, doi:10.1007/BF02783451
Kim JYH, Cha HJ (2003) Down-regulation of acetate pathway through antisense strategy in Escherichia coli: improved foreign protein production. Biotechnol Bioeng 83:841–853, doi:10.1002/bit.10735
Krebs A, Bridger WA (1980) The kinetic properties of phosphoenol pyruvate carboxykinase of Escherichia coli. Can J Biochem 58:309–318
Phue J, Noronha SB, Hattacharyya R, Wolfe AJ, Shiloach J (2005) Glucose metabolism at high density growth of Escherichia coli B and Escherichia coli K: differences in metabolic pathways are responsible for efficient glucose utilization in Escherichia coli B as determined by microoarrys and northern blot analyses. Biotechnol Bioeng 90:805–820, doi:10.1002/bit.20478
Xu B, Jahic M, Blomsten G, Enfors SO (1999) Glucose o[verflow metabolism and mixed-acid fermentation in aerobic large-scale fed-batch processed with Escherichia coli. Appl Microbiol Biotechnol 51:564–571, doi:10.1007/s002530051433
Bock A, Sawers G (1996) Fermentation. In: Neidhardt FC, Curtiss RIII, Ingraham J, Lin ECC, Low KB, Maagasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella. Cellular and molecular biology. ASM, Washington, DC, pp 262–282
Kessler D, Knappe J (1996) Anaerobic dissimilation of pyruvate in Escherichia coli and Salmonella. Cellular and molecular biology. ASM, Washington, DC, pp 199–205
Castan A, Näsman A, Enfors S-O (2002) Oxygen enriched air supply in Escherichia coli processes: production of biomass and recombinant human growth hormone. Enzyme Microb Tech 30:847–854
Jeong KJ, Lee SY (2001) Secretory production of human granulocyte colony stimulating factor in Escherichia coli. Protein expression and purif 23:311–318, doi:10.1006/prep.2001.1508
Krishna Rao DV, Venkateswara Rao J, Narasu ML, Bhujanga Rao AKS (2008) Optimization of the AT-content of codons immediately downstream of the initiation codon and evaluation of culture conditions for high-level expression of rhG-CSF in E. coli. Mol Biotechnol 38:221–232
Fass R, Clem TR, Shiloach J (1989) Use of a novel air separation system in a Fed-batch fermentation culture of Escherichia coli. Appl Environ Microbiol 55:305–307
García-Ochoa F, Castro EG, Santos VE (2000) Oxygen transfer and uptake rates during xanthan gum production. Enzyme Microb Technol 27:680–690, doi:10.1016/S0141-0229(00)00272-6
Phue J-N, Shiloach J (2005) Impact of dissolved oxygen concentration on acetate accumulation and physiology of E.coli BL21, evaluating transcription levels of key genes at different dissolved oxygen conditions. Metab Eng 7:353–363, doi:10.1016/j.ymben.2005.06.003
Khalilzadeh R, Shojaosadati SA, Bahrami A, Maghsoudi N (2004) Fed-batch cultivation of recombinant Escherichia coli producing human interferon-γ under controlled specific growth rate. Iran J Biotehnol 2:113–121
Panda AK, Khan RH, Appa Rao KBC, Totey SM (1999) Kinetic of inclusion body production in batch and high cell density fed-batch culture of Escherichia coli expressing ovine growth hormone. J Biotechnol 75:161–172, doi:10.1016/S0168-1656(99)00157-1
Hesham EIE, Joachim K, Ursula R (2006) Agitation effects on morphology and protein productive fractions of filamentous and pelleted growth forms of recombinant Aspergillus niger. Process Biochem 41:2103–2112, doi:10.1016/j.procbio.2006.05.024
Wang F, Du Guo-Cheng, Li Yin, Chen Jian (2005) Effects of dissolved oxygen concentration and two-stage oxygen supply strategy on the production of γ-CGTase by Bacillus macorous. Process Biochem 40:3468–3473, doi:10.1016/j.procbio.2005.02.019
Sabbagh NE, Linda MH, McNeil B (2008) Effects of carbon dioxide on growth, nutrient consumption, cephalosporin C synthesis and morphology of Acremonium chrysogenum in batch culture. Enzyme Microb Technol 42:315–324, doi:10.1016/j.enzmictec.2007.10.012
Osman E (2001) Effects of high-pressure carbondioxide on Escherichia coli in nutrient broth and milk. Int J Food Microbiol 65:131–135, doi:10.1016/S0168-1605(00)00499-2
Holm H (1996) Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol Rev 59(8):2465–2473
Antonio DL, Vanessa H, Enrique G, Octavio TR (2003) Effects of dissolved oxygen tension on the production of recombinant pencillin acylase in Escherichia coli. Enzyme Micob Technol 33:689–697
Jingle L, Zhinan X, Tongfeng L, Jianping L, Peilin C (2008) Effects of cultivation conditions on the prodiction of natamycin with streptomyces gilvosporeus LK-196. Enzyme Microb Technol 42:145–150, doi:10.1016/j.enzmictec.2007.08.012
Guo-Bin L, Guo-Chang D, Jain C (2008) A novel strategy of enhanced glutathione production in high cell density cultivation of candida utilis—Cysteine addition combined with dissolved oxygen controlling. Enzyme Microb Technol 42:284–289, doi:10.1016/j.enzmictec.2007.10.008
Mao XB, Zhong JJ (2004) Hyper production of cardycepin by two stage dissolved oxygen control in submerged cultivation of medicinal mushroom Cordyceps militaris in bioreactors. Biotechnol Prog 20:1408–1413, doi:10.1021/bp049765r
Li Y, Hugenholtz J, Chen J, Lun SY (2002) Enhancement of pyruvate productionby Torulopsis glabrata using a two stage oxygen supply control strategy. Appl Microb Biotechnol 60:101–106, doi:10.1007/s00253-002-1064-y
Lee CY, Lee SJ, Jung KH, Katosh S, Lee EK (2003) High dissolved oxygen tension enhances heterologous protein expression by recombinant pichia pastoris. Process Biochem 38:1147–1154, doi:10.1016/S0032-9592(02)00280-7
Acknowledgments
The authors are highly thankful to the authorities of Natco Pharma Limited, Hyderabad for providing support in all aspects of this work. The technical support from the Analytical Development Division of Natco Research Centre is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna Rao, D.V., Ramu, C.T., Rao, J.V. et al. Impact of dissolved oxygen concentration on some key parameters and production of rhG-CSF in batch fermentation. J Ind Microbiol Biotechnol 35, 991–1000 (2008). https://doi.org/10.1007/s10295-008-0374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0374-1