Abstract
The Chenopodiaceae is one of the families including C4 species among eudicots. In this family, the genus Chenopodium is considered to include only C3 species. However, we report here a transition from C3 photosynthesis to proto-Kranz to C3–C4 intermediate type in Chenopodium. We investigated leaf anatomical and photosynthetic traits of 15 species, of which 8 species showed non-Kranz anatomy and a CO2 compensation point (Γ) typical of C3 plants. However, 5 species showed proto-Kranz anatomy and a C3-like Γ, whereas C. strictum showed leaf anatomy and a Γ typical of C3–C4 intermediates. Chenopodium album accessions examined included both proto-Kranz and C3–C4 intermediate types, depending on locality. Glycine decarboxylase, a key photorespiratory enzyme that is involved in the decarboxylation of glycine, was located predominantly in the mesophyll (M) cells of C3 species, in both M and bundle-sheath (BS) cells in proto-Kranz species, and exclusively in BS cells in C3–C4 intermediate species. The M/BS tissue area ratio, number of chloroplasts and mitochondria per BS cell, distribution of these organelles to the centripetal region of BS cells, the degree of inner positioning (vacuolar side of chloroplasts) of mitochondria in M cells, and the size of BS mitochondria also changed with the change in glycine decarboxylase localization. All Chenopodium species examined were C3-like regarding activities and amounts of C3 and C4 photosynthetic enzymes and δ13C values, suggesting that these species perform photosynthesis without contribution of the C4 cycle. This study demonstrates that Chenopodium is not a C3 genus and is valuable for studying evolution of C3–C4 intermediates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photorespiration is an inevitable metabolic process in C3 plants that use ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) for primary fixation of CO2. In ordinary air, one-fourth of photosynthetically fixed CO2 was lost by photorespiration, resulting in decreased photosynthetic efficiency (Bauwe 2011; Sage et al. 2012). On the other hand, C4 plants have biochemical traits of photosynthesis associated with anatomical differentiation of leaves to reduce photorespiration (Hatch 1987). In general, C4 leaves exhibit Kranz-type anatomy, in which an external layer of mesophyll (M) and an internal layer of bundle sheath (BS) encircle vascular bundles (Edwards and Voznesenskaya 2011; Lundgren et al. 2014). In M cells, atmospheric CO2 is first fixed in C4 compounds, and they are moved to BS cells, where they are decarboxylated to supply CO2 for Rubisco. Increased CO2 concentration within BS cells suppresses photorespiration (Hatch 1987). Therefore, C4 plants have higher photosynthetic efficiency than C3 plants in environments that promote high rates of photorespiration. The C4 trait has evolved independently at least 66 times in flowering plants in response to multiple ecological drivers including decreasing atmospheric CO2 concentration (Sage et al. 2012). Much effort has recently focused on elucidating the evolution from C3 to C4 plants (reviewed in Christin and Osborne 2014; Sage et al. 2012, 2014). These works may provide a clue for engineering of C4 elements into C3 crops (Schlüter and Weber 2016).
An early study reported the existence of plants with traits intermediate between those of C3 and C4 plants (Kennedy and Laetsch 1974). These are called C3–C4 intermediate plants (Edwards and Ku 1987; Monson and Rawsthorne 2000). Leaves of most C3–C4 intermediate plants show Kranz-like anatomy, in which BS cells contain numerous chloroplasts and mitochondria (Edwards and Ku 1987; Sage et al. 2014). In these plants, the values of the CO2 compensation point (Г) and O2 inhibition of photosynthesis are intermediate between the values of C3 and C4 plants (Edwards and Ku 1987; Monson and Rawsthorne 2000). An apparent reduction in photorespiration in C3–C4 intermediates is accomplished by a particular biochemical system operating between the M and BS cells. This system is called the glycine shuttle (Monson and Rawsthorne 2000; Rawsthorne 1992). In C3–C4 intermediates, at least the P-protein, one of the 4 subunits constituting the glycine decarboxylase (GDC) multi-enzyme system, is absent in the M mitochondria, which renders GDC non-functional, and glycine generated in the M cells must be transported into the BS mitochondria to be decarboxylated by GDC (Rawsthorne 1992; Rawsthorne et al. 1988). In the BS cells of most C3–C4 intermediates, mitochondria are located between the centripetally located chloroplasts and the inner tangential walls (Brown and Hattersley 1989; Muhaidat et al. 2011; Rawsthorne 1992; Sage et al. 2013; Ueno et al. 2003; Ueno 2011). As a result, a large part of CO2 released from mitochondria by decarboxylation of glycine is captured by chloroplasts, resulting in suppression of CO2 loss from BS cells. Many C3–C4 intermediate species reduce photorespiratory CO2 loss only by using the glycine shuttle (type I intermediates), but in some intermediates a C4 cycle complements the glycine shuttle (type II intermediates; Edwards and Ku 1987).
Until now, 56 species with C3–C4 intermediate traits have been found in 2 monocot and 11 eudicot families (Lundgren and Christin 2017). Some genera, such as Flaveria (Ku et al. 1991; Sage et al. 2013), Heliotropium (Muhaidat et al. 2011; Vogan et al. 2007), Salsola (Voznesenskaya et al. 2013), Eleocharis (Roalson et al. 2010; Ueno et al. 1989), Alloteropsis (Bianconi et al. 2018), and Neurachne (Christin et al. 2012), include C3, C3–C4 intermediate, and C4 types and provide a unique opportunity to trace the evolution from C3 to C4 plants. Many of these studies suggest that the initial event in the evolution of C3–C4 and then C4 plants is the appearance of chloroplasts and mitochondria along the centripetal region of BS cells, with GDC activity present in both M and BS mitochondria. This phase is called the proto-Kranz type (Muhaidat et al. 2011; Sage et al. 2012, 2014). Type I intermediates would evolve from the proto-Kranz type with predominant accumulation of GDC in BS mitochondria and its decrease in M mitochondria. Complementation of the glycine shuttle by the increasing activity of the C4 cycle would then lead to type II intermediates. Finally, the C4 type would evolve from type II intermediates through the C4–like plants (Edwards and Ku 1987; Sage et al. 2012, 2014).
Among eudicots, the goosefoot family Chenopodiaceae (Caryophyllales) includes the greatest number of C4 species (about 40% of 1400 species; Sage et al. 1999). C4 species have been detected in four Chenopodiaceae subfamilies: Chenopodioideae, Salicornioideae, Salsoloideae, and Suaedoideae (Carolin et al. 1975; Jacobs 2001; Kadereit et al. 2003; Pyankov et al. 2001; Voznesenskaya et al. 2001b, 2002, 2007; Wen and Zhang 2011). In the Chenopodioideae, C4 species occur in the tribes Atripliceae (e.g., Atriplex and Axyris) and Camphorosmeae (e.g., Bassia and Kochia), but they have not been found in the tribe Chenopodieae (Freitag and Kadereit 2014; Kadereit et al. 2010; Sage 2004). In this family, C3–C4 intermediate species have been recorded in the genera Salsola, Rhaphidophyton, and Sedobassia (Freitag and Kadereit 2014; Schüssler et al. 2017; Voznesenskaya et al. 2001a, 2013; Wen and Zhang 2015).
The genus Chenopodium (Chenopodieae) is considered to include only C3 species (Jacobs 2001; Kadereit et al. 2010). This genus is cosmopolitan and includes about 150 species, most of which are annual herbs growing in arid and semi-arid regions and also on salt-rich soils (Fuentes-Bazan et al. 2012) and weeds of disturbed habitats and cultivated fields (Judd and Ferguson 1999). The seeds of some Chenopodium species such as C. quinoa, C. berlandieri, and C. formosanum are used as cereals, and the leaves and young shoots of C. album are eaten as vegetables (Judd and Ferguson 1999). Chenopodium album has been often used as a model plant to study the physiology of C3 photosynthesis (e.g., Haraguchi et al. 2009).
In our preliminary study on leaf anatomy of eudicot species in Japan, we have recently found that C. album has leaf structural traits of the proto-Kranz type, which clearly differ from those of the typical non-Kranz (C3) type. This finding motivated us to re-examine leaf anatomy and photosynthetic traits of Chenopodium species from various regions of the world. This study reports, for the first time, that Chenopodium includes proto-Kranz and C3–C4 intermediate types as well as C3 types and is therefore a valuable eudicot group to study the evolutionary and genetic transition from C3 to proto-Kranz to C3–C4 intermediate plants.
Materials and methods
Plant materials and growth conditions
The species and accessions of Chenopodium examined in this study are listed in Table 1. Seeds of two accessions of C. album were collected in upland fields of the National Institute of Agro-Environmental Sciences, Tsukuba, Ibaraki, Japan and along the roadside of Fukuoka City, Fukuoka, Japan. Seeds of C. ficifolium were also collected in upland fields of the National Institute of Agro-Environmental Sciences. Seeds of C. quinoa were provided by the NARO Genebank, Tsukuba, Japan. Seeds of five other accessions of C. album and 12 other species of Chenopodium were provided by the USDA Germplasm Resources, USA (Table 1). The seeds of C4 species of Amaranthus (A. cruentus, A. dubius, and A. hybridus), which were used as controls, were also a gift from the USDA Germplasm Resources (Table 1). All seeds were germinated in perforated multiwell nursery boxes filled with loam soil granules. Seedlings were grown for 3 weeks in a greenhouse at the experimental field of Kyushu University in July. The seedlings were then transplanted to 5-L pots (one plant per pot) with sandy loam soil containing nitrogen (ammonium nitrate), phosphorus (calcium superphosphate), and potassium (potassium chloride) fertilizers (1.0 g each). Plants were grown in a greenhouse [natural sunlight, wherein photosynthetic photon flux density (PPFD) at midday exceeded 1500 μmol m−2 s−1; 30–34 °C during the day and 24–27 °C during the night] for 1.5–2 months. Plants were watered daily. Fully expanded upper mature leaves taken from 3 plants per species (per accession for C. album) were used for analysis.
Anatomical and ultrastructural studies
Samples taken from the midsections of leaves (one leaf per plant) were fixed and embedded in Quetol resin (Nisshin EM, Shinjuku, Tokyo, Japan) as reported previously (Tsutsumi et al. 2017). Semithin sections (1 µm thickness) were cut with glass knives on an ultramicrotome (Reichert Ultracut S, Leica, Wien, Austria), mounted on glass slides, stained with 1% toluidine blue O, and observed under a light microscope (Eclipse Ci-L, Nikon Instech Co. Ltd., Tokyo, Japan). The profile areas of M and BS tissues between adjacent small vascular bundles were measured using the Image J software (National Institutes of Health, Bethesda, MD, USA), and the area ratio of M and BS tissues (M/BS tissue area ratio) was calculated. Simultaneously, the sizes (profile areas) of 8 M cells and 8 BS cells per plant were measured, and the size ratio of M and BS cells (M/BS cell size ratio) was calculated.
Ultrathin sections were cut with a diamond knife on the same ultramicrotome, picked up on Formvar-coated grids, stained with lead citrate, and viewed under a transmission electron microscope (JEM-100CX II K, JEOL Ltd., Tokyo, Japan) at 75 kV. The numbers and intracellular positions of chloroplasts and mitochondria were recorded for 5 M cells and 5 BS cells per plant as described by Hatakeyama and Ueno (2016). In BS cells, we counted chloroplasts and mitochondria in the inner halves of the cells (i.e., along the inner tangential wall and the inner half of the radial wall) and in the outer halves (i.e., along the outer tangential wall and the outer half of the radial wall). In M cells, we counted mitochondria on the vacuolar side of chloroplasts (inner position) and on the cell-wall side of chloroplasts, including isolated mitochondria not associated with chloroplasts but adjacent to the cell wall (outer position), as described in Hatakeyama and Ueno (2016). In M cells, chloroplasts in the intracellular position were not counted because all chloroplasts were adjacent to the cell wall. The numbers of chloroplasts and mitochondria per unit area were calculated in each of the 5 M and 5 BS cells using Image J software. On some sections, the sizes (profile areas) of 10 chloroplasts and 10 mitochondria per plant were measured.
To measure vein density, samples taken from the midsections of leaves (one leaf per plant) were fixed in a formalin–acetic acid–alcohol mixture and cleared in 80% lactic acid and chloral hydrate-saturated ethanol as described by Tsutsumi et al. (2017). The vein density (vein length per unit leaf area) was measured using Image J software.
Immunohistochemistry
Intercellular immunolocalization of photorespiratory and photosynthetic enzymes in M and BS cells was investigated under a light microscope. Small leaf segments (one leaf per plant) were fixed and embedded in paraffin, as described by Hatakeyama and Ueno (2016). Sections (10 µm thick) were cut on a rotary microtome (PR-50, Yamato Kohki Industrial Co. Ltd., Saitama, Japan), mounted on slides coated with poly-l-lysine (Sigma-Aldrich Inc., St Louis, MO, USA), and dried overnight. Immunostaining for the P-protein of GDC (GDC-P) and the large subunit of Rubisco (Rubisco LSU) was performed as described by Hatakeyama and Ueno (2016) with antisera against GDC-P and Rubisco LSU from pea leaves. The antisera were provided by Dr. D. J. Oliver (University of Idaho, Moscow, ID, USA) and the late Dr. S. Muto (Nagoya University, Nagoya, Japan), respectively.
Protein A—immunogold electron microscopy
To evaluate exactly the accumulation level of GDC-P in mitochondria of M and BS cells, a quantitative immunogold labeling study was made under an electron microscope. Small leaf segments (one leaf per plant) were fixed and embedded in Lowicryl K4 M resin (Chemische Werke Lowi GmbH, Waldkraiburg, Germany) as described by Ueno (1992). Ultrathin sections on Formvar-coated grids were immunolabeled with the antiserum against GDC-P and InnovaCoat Gold − 20 nm protein A nanoparticle conjugate (Innova Biosciences, Cambridge, England, UK), stained with lead citrate, and viewed under a transmission electron microscope as described by Ueno (1992). As a negative control, the antiserum was replaced by non-immune serum.
The density of GDC-P labeling was determined for mitochondria and other intracellular locations by counting the gold particles on electron micrographs at 20,000× magnification and calculating the number of particles per unit area (μm−2) with Image J software. We examined 13–18 mitochondria of palisade M cells and 20 mitochondria of BS cells in several sections per leaf. The density of labeling was calculated as the mean of 3 plants.
Western blots
Leaves were frozen in liquid nitrogen and stored in a deep freezer (− 80 °C). Extraction of soluble proteins, SDS-PAGE, and Western blotting were performed as described by Ueno (1992) with antisera against phosphoenolpyruvate carboxylase (PEPC) and pyruvate, Pi dikinase (PPDK) from maize leaves (provided by Dr. T. Sugiyama, RIKEN, Yokohama, Japan). For GDC-P and Rubisco LSU, we used the same antisera for immunohistochemistry.
Enzyme assays
Parts of frozen leaves were used to measure the activities of Rubisco, PEPC, NADP-malic enzyme (NADP-ME), and NAD-malic enzyme (NAD-ME) as described by Ueno (1992), except that all enzymes were assayed at 30 °C.
Gas exchange measurements
Net CO2 assimilation rate (A) was measured using an LI-6400 portable photosynthesis system (Li-Cor Inc., Lincoln, NE, USA) at a PPFD of 1000 μmol m−2 s−1, a leaf temperature of 30 °C, a relative humidity of 60%, and a CO2 concentration of 380 μL L−1, as described in Ueno et al. (2003). Light within the chamber was provided by a 6400-02 LED Light Source (Li-Cor Inc.). The Γ value was determined by extrapolating the initial slope of A versus the intercellular CO2 concentration through the x-axis, where A equals zero.
Carbon isotope ratio
One leaf from three plants was air-dried at 80 °C and ground in a mortar with a pestle. Two mg of leaf powder was used to measure 12C and 13C contents. Carbon isotope ratios were measured at SI Science, Kita-katsushika, Saitama, Japan by using the elemental analyzer–isotope ratio mass spectrometer (EA-IRMS) system (Thermo Fisher Scientific, Waltham, MA), as described by Sato and Suzuki (2010). The isotope ratio was expressed in δ notation as parts per million (‰) with respect to the Pee Dee belemnite standard.
Statistical analysis
Data were presented as mean ± SD (n = 3 plants), except carbon isotope ratios. These data were analyzed using Statcel4 software (OMS Publisher, Tokorozawa, Saitama, Japan). We tested the significance (P < 0.05) of the differences in GDC-P labeling density between M and BS mitochondria using Student’s t test and that of the differences in structural, biochemical, and physiological traits among species and among photosynthetic types of Chenopodium by the Tukey–Kramer test as a post hoc test, associated with ANOVA. For enzyme activities, data of Amaranthus C4 species were added to statistical analysis. Pearson’s correlation coefficients between Γ values and quantitative parameters of cells and organelles were calculated.
Results
Leaf anatomy
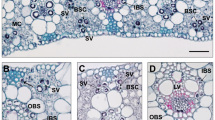
Light microscopy revealed a large variation in chloroplast numbers and arrangement in BS cells among Chenopodium species examined (Fig. 1, S1–S3). We classified leaf anatomy of Chenopodium into 3 types: non-Kranz, proto-Kranz, and Kranz-like types (Fig. 1; Table 2). In non-Kranz anatomy (Fig. 1a, b, S1), BS cells contained few chloroplasts in the inner half (centripetal region) along the vascular bundle, but many chloroplasts occurred in the outer half adjacent to intercellular spaces. Chenopodium atrovirens, C. hians, C. incanum, C. leptophyllum, C. pallidicaule, C. quinoa, C. standleyanum, and C. vulvaria showed non-Kranz anatomy (Table 2). In proto-Kranz anatomy (Fig. 1c, d, S2, S3a–c), BS cells contained more chloroplasts in the centripetal region than did non-Kranz-type BS cells, and those chloroplasts surrounded the vascular bundle. Six accessions of C. album, C. berlandieri, C. ficifolium, C. formosanum, C. giganteum, and C. nevadense showed proto-Kranz anatomy (Table 2). In Kranz-like anatomy (Fig. 1e, f), BS cells contained many more chloroplasts in the centripetal region than did proto-Kranz-type BS cells, but fewer than did BS cells of a C4 species of Amaranthus (Fig. S3d). Chenopodium strictum and a C. album accession from Arizona showed Kranz-like anatomy (Table 2). In all 3 anatomical types, M was differentiated into palisade tissue on the adaxial side and spongy tissue on the abaxial side (Fig. 1, S1–S3).
Immunohistochemical localization of GDC-P and Rubisco LSU
In the non-Kranz type, GDC-P was detected in M cells and to a lesser extent in BS cells (Fig. 2a, b, S4; Table 2). In the proto-Kranz type, GDC-P was detected in both M and BS cells (Fig. 2c, d, S5, S6a–c), but the degree of staining varied among species and C. album accessions (Table 2). In C. giganteum, C. nevadense, and four accessions of C. album (from Finland, France, India and Poland), the staining was stronger in BS cells than in M cells, and a distinct brown ring surrounded the vascular bundle; this ring represented a dense accumulation of GDC-P in mitochondria, as shown later by ultrastructural observation. In the C. album accession from Arizona and C. strictum, which had Kranz-like anatomy, GDC-P staining was detected exclusively in BS cells (Fig. 2e, f; Table 2). In a C4 species of Amaranthus also, GDC-P staining occurred exclusively in BS cells (Fig. S6d), as known in many C4 species (Yoshimura et al. 2004). In Chenopodium species, regardless of the anatomical type, Rubisco LSU was detected in chloroplasts of both M and BS cells (Fig. S7).
CO2 gas exchange and carbon isotope ratio
As expected from leaf anatomy and GDC-P localization, Chenopodium species showed a large variation in Г (20–66 µL L−1; Table 2). These Г values were higher than that in a control C4 species, Amaranthus dubius (Table 2). In the Arizona accession of C. album and C. strictum, which have Kranz-like anatomy, the Г values were 20 and 26 µL L−1, respectively, which are typical for C3–C4 intermediates (Edwards and Ku 1987; Monson and Rawsthorne 2000). The Г values of non-Kranz species ranged from 45 to 66 µL L−1, whereas those of proto-Kranz species ranged from 35 to 51 µL L−1 (Table 2). The average Г values of the 3 anatomical types in Chenopodium significantly differed from each other. Relative to the average Г value of the non-Kranz type, the average Г value of the proto-Kranz type was 23% lower, and that of the Kranz-like type was 57% lower. The A values of Chenopodium species ranged from 10 to 25 µmol m−2 s−1, and that of A. dubius was 17.6 µmol m−2 s−1 (Table 2). The δ13C values of Chenopodium species ranged from − 32.6‰ to − 26.7‰, whereas that of A. dubius was − 13.1‰ (Table 2). These values were within C3 and C4 range, respectively (Ehleringer and Osmond 1991). The average A and δ13C values did not differ significantly among the anatomical types of Chenopodium.
Activities and amounts of photosynthetic and photorespiratory enzymes
Activities of C3 and C4 photosynthetic enzymes were measured for five non-Kranz species, six proto-Kranz species (including 4 C. album accessions), the Arizona accession of C. album and C. strictum (both Kranz-like type), and two control C4 species of Amaranthus (Table 3). The average activities of Rubisco were higher in all anatomical types of Chenopodium than in the C4 species, whereas there were no significant differences among the three anatomical types of Chenopodium. In contrast, activities of PEPC were much lower in Chenopodium species than in the C4 species; there were no significant differences among the three anatomical types. Similar trends were found for NADP-ME and NAD-ME (Table 3). In several species, there were large differences in the enzyme activities among three plants examined, resulting in large standard deviation. It was considered that these differences were probably caused by those in growth rate of plants.
Western blot analyses of photosynthetic and photorespiratory enzymes were done for five species of Chenopodium (three accessions from C. album) representing the three anatomical types and a control C4 species, A. dubius (Fig. 3). The levels of Rubisco LSU and GDC-P were higher, and those of PEPC and PPDK were much lower, in Chenopodium than in A. dubius.
Western blots of leaf extracts of Chenopodium species. Total soluble protein (20 µg for GDC-P, PEPC, and PPDK and 2.5 µg for Rubisco LSU) was subjected to SDS-PAGE, blotting on nitrocellulose membranes, and identification with antisera against the indicated photorespiratory and photosynthetic enzymes
Quantification of leaf inner structure
We investigated the leaf inner structure of the representatives of the three anatomical types in more detail (Table 4). There were no significant differences in vein density among the three anatomical types. The sectional area of M tissue was highest in the non-Kranz type and lowest in the Kranz-like type, whereas that of BS tissue showed a reverse tendency. As a result, the M/BS tissue area ratio was higher in the non-Kranz type than in the proto-Kranz and Kranz-like types (Table 4). The size of M and BS cells varied greatly among Chenopodium species. For example, the size of M cells in C. atrovirens was more than five times that in the Indian accession of C. album. Nevertheless, the M/BS cell size ratio was almost constant among species of the same anatomical type but was lowest in the proto-Kranz type and highest in the non-Kranz type (Table 4). There was a high positive correlation between Г and the M/BS tissue area ratio (Fig. 4a) and a weaker positive correlation between Г and the M/BS cell size ratio (Fig. 4b).
Relationships between CO2 compensation point (Г) and M/BS tissue area ratio (a), M/BS cell size ratio (b), the ratio of chloroplasts located in the inner half of BS cells (c), the ratio of mitochondria located in the inner half of BS cells (d), the ratio of mitochondria located in the inner position of M cells (e), size of BS mitochondria (f), number of chloroplasts per BS cell (g), and number of mitochondria per BS cell (h) in Chenopodium species. Filled circles, non-Kranz type; white circles, proto-Kranz type; triangles, Kranz-like type. Significant at P: * < 0.05; ** < 0.01; *** < 0.001
Ultrastructure and quantification of organelles
In the non-Kranz type, only a few chloroplasts and mitochondria were located in the centripetal region of BS cells (Fig. 5b, c, S8b, c, e, f). In the proto-Kranz type, more chloroplasts and mitochondria were located in the centripetal region, where many mitochondria were located between chloroplasts and vascular tissues (Fig. 5e, f, S8 h, i, k, l). In the Kranz-like type, the preferential localization of these organelles in the centripetal region was most pronounced (Fig. 5h, i, S8n, o). The ratio of chloroplasts and mitochondria in the inner half of BS cells was higher in both proto-Kranz and Kranz-like types than in the non-Kranz type (Table 5). In the M cells of the non-Kranz type, most mitochondria were located on the vacuolar side of chloroplasts (in the inner position) (Fig. 5a; Table 5; see Fig. 5d, g for the proto-Kranz and Kranz-like types). The ratio of mitochondria in the inner position decreased from the non-Kranz to the proto-Kranz to the Kranz-like type (Table 5). There were significant correlations between Г and the centripetal positioning of chloroplasts and mitochondria in BS cells (Fig. 4c, d) and between Г and the inner positioning of M mitochondria (Fig. 4e).
Ultrastructure of mesophyll cells (a, d, g) and bundle-sheath cells at a low (b, e, f) and at a high (c, f, i) magnification in Chenopodium species. (a–c) C. vulvaria; (d–f) C. album (India); (g–i) C. album (Arizona, USA). BSC bundle-sheath cell, ICS intercellular space, MC mesophyll cell, V vascular bundle, c chloroplast, mt mitochondrion, n nucleus. Bars = 3 µm
In M cells, the size of chloroplasts and mitochondria and the number of chloroplasts and mitochondria per cell did not differ among the three types, and their numbers per unit area were higher in the proto-Kranz type than in the other two types (Table 6). In BS cells, the number of chloroplasts per cell was highest in the Kranz-like type, and the number of mitochondria was lowest in the non-Kranz type (Table 6). The numbers of these organelles per unit area tended to be lowest in the non-Kranz type (Table 6). In BS cells, the size of chloroplasts did not differ significantly among the three types, and the mitochondria were smallest in the non-Kranz and largest in the Kranz-like type (Table 6). There were significant negative correlations between Г and the size of BS mitochondria (Fig. 4f), the number of chloroplasts and mitochondria per BS cell (Fig. 4g, h).
Immunogold localization of GDC-P
In the non-Kranz and proto-Kranz types, GDC-P was detected in the mitochondria of both M and BS cells (Fig. S9a–d). In both types, the labeling density did not differ significantly between M and BS mitochondria (Fig. 6). In the Kranz-like type, GDC-P was detected almost exclusively in BS mitochondria (Fig. 6, S9e, f).
Quantification of immunogold labeling of GDC-P in the mitochondria of mesophyll cells (MC) and bundle-sheath cells (BSC) of Chenopodium species. Numbers of gold particles per unit area of mitochondria are given as mean ± SD of three plants. * significant differences between MC and BSC at P < 0.05. NS not significant. Labeling densities in the cell area excluding mitochondria were between 0.05 and 0.15 µm−2
Discussion
Photosynthetic types in Chenopodium
Although Chenopodium is considered to be a C3 genus, our study revealed that Chenopodium species show great variation in leaf anatomy, and some species have biochemical and physiological traits characteristic of C3–C4 intermediates. Light microscopy showed a difference in chloroplast number in the centripetal region of BS cells, an indication of the existence of non-Kranz, proto-Kranz, and Kranz-like species in this genus (Table 2). Gas exchange measurements showed that the non-Kranz type had the highest Г values, typical of C3 plants, whereas the Kranz-like type had the lowest Г values, typical of C3–C4 intermediate plants (Edwards and Ku 1987). GDC-P was found mainly in M cells in the non-Kranz type and exclusively in BS cells in the Kranz-like type. The latter GDC-P distribution is responsible for the operation of the glycine shuttle (Monson and Rawsthorne 2000; Rawsthorne 1992; Rawsthorne et al. 1988). These data suggest that the Arizona accession of C. album and C. strictum (Kranz-like type) are C3–C4 intermediates.
We found numerous proto-Kranz species, intermediate between the non-Kranz and Kranz-like types (Table 2). The BS cells of proto-Kranz species also contained a considerable number of chloroplasts in the centripetal region, but the number was somewhat lower than in the BS cells of the Kranz-like type. In the proto-Kranz type, GDC-P immunostaining was detected in both M and BS cells, but the relative intensity of staining between these cells varied among species (Table 2). These Chenopodium species resemble the proto-Kranz plants previously found in some genera, such as Heliotropium (Muhaidat et al. 2011; Vogan et al. 2007), Flaveria (Sage et al. 2013), Salsola (Schüssler et al. 2017; Voznesenskaya et al. 2013), and Steinchisma (Brown et al. 1983; Khoshravesh et al. 2016).
In comparison with the C4Amaranthus species, Chenopodium species had higher activities of the C3 enzyme Rubisco and lower activities of the C4 enzymes PEPC, NADP-ME and NAD-ME (Table 3). Western blot analysis showed that Chenopodium species accumulated smaller amounts of PEPC and PPDK but greater amounts of Rubisco and GDC than did Amaranthus species. These data suggest that Chenopodium species perform photosynthesis without contribution of the C4 cycle. Rubisco accumulated in all chloroplasts of M and BS cells. We concluded that the Arizona accession of C. album and C. strictum (Kranz-like anatomy) are type I C3–C4 intermediates, Chenopodium species with non-Kranz anatomy are C3, and other species of Chenopodium and the remaining accessions of C. album are of the proto-Kranz type. This conclusion is also supported by δ13C values of Chenopodium species (Table 2). Previous studies reported that type I C3–C4 intermediates and proto-Kranz type have C3-like δ13C values (Edwards and Ku 1987; Vogan et al. 2007), as in Chenopodium species. This is due to these plants originally fixing CO2 via Rubisco, not via the C4 cycle (von Caemmerer and Hubick 1989).
The genus Chenopodium is polyphyletic and combines species from three clades of Chenopodioideae (Kadereit et al. 2003). Our study investigated 15 species, which is only about 10% of the known Chenopodium species. From these limited data, it would be difficult to reliably deduce on the phylogenetic relationships among photosynthetic types in Chenopodium, and a more extensive survey would be required. In general, most C3–C4 intermediate species have been found in genera that include C4 species (Sage et al. 2014), but as far as we know no C4 species has been identified in Chenopodium. A few genera include C3–C4 intermediate species with C3 species (e.g., Moricandia, Diplotaxis, and Brassica in Brassicaceae, Schlüter et al. 2017; Ueno 2011; Parthenium in Compositae, Moore et al. 1987).
Transition of leaf structural and photosynthetic traits in Chenopodium
Our study revealed a gradation of structural and photosynthetic traits from C3 to proto-Kranz to C3–C4 intermediate type in Chenopodium. Muhaidat et al. (2007) found no significant difference in vein density between closely related C3 and C4 species of eudicots. In Chenopodium, we also found no significant differences in vein density among the three types, but the great variation in size of M and BS cells among Chenopodium species might affect the tendency of changes in vein density (Table 4). The M/BS tissue area ratio is higher in C3 species than in C4 species (Hattersley 1984; Muhaidat et al. 2011). In Chenopodium, the M/BS tissue area ratio decreased from C3 (non-Kranz) to proto-Kranz to C3–C4 intermediate (Kranz-like) type (Table 4; Fig. 4a). The M/BS cell size ratio showed a similar trend (Table 4; Fig. 4b). Therefore, volume changes at the tissue and cell levels appear to occur during the transition from C3 to proto-Kranz to C3–C4 intermediate species in Chenopodium, although the differences between the proto-Kranz and C3–C4 intermediates were somewhat indistinct.
In the BS cells of C3–C4 intermediate plants, the amount and positioning of chloroplasts and mitochondria are critical structural traits involved in photosynthesis (Brown and Hattersley 1989; Edwards and Ku 1987; Muhaidat et al. 2011; Rawsthorne 1992; Sage et al. 2013; Ueno et al. 2003; Ueno 2011; Voznesenskaya et al. 2013). In the BS cells of Chenopodium, the size of mitochondria increased from C3 to proto-Kranz to C3–C4 intermediate species (Fig. 4f), but there was no significant difference in chloroplast size (Table 6). The numbers of chloroplasts and mitochondria per cell and per unit cell area tended to be lowest in C3 species (Table 6; Fig. 4g, h). The distribution of chloroplasts and mitochondria to the inner half of BS cells (centripetal positioning) was also lowest in C3 species (Table 5; Fig. 4c, d), as reported for other genera (Brown et al. 1983; Khoshravesh et al. 2016; Muhaidat et al. 2011; Rawsthorne 1992; Sage et al. 2013; Ueno 2011; Ueno et al. 2003; Voznesenskaya et al. 2013). The mitochondria in BS cells were located between the centripetally located chloroplasts and the inner tangential walls. These structural features would help to capture photorespiratory CO2 released from mitochondria and to suppress the escape of CO2 from BS cells (Rawsthorne 1992; Sage et al. 2014).
In M cells, the size of chloroplasts and mitochondria and number of chloroplasts and mitochondria per cell did not differ among the three types, but the degree of inner positioning of mitochondria gradually decreased from C3 to proto-Kranz to C3–C4 intermediate species (Table 6; Fig. 4e). In contrast, the immunogold labeling density of GDC did not differ significantly between the M and BS mitochondria of C3 and proto-Kranz species, but in C3–C4 intermediates, GDC accumulated exclusively in BS mitochondria (Fig. 6). Most mitochondria in M cells are located on the vacuolar side of chloroplasts (inner position) in C3 grasses (Busch et al. 2013; Hatakeyama and Ueno 2016; Sage and Sage 2009) but are adjacent to the cell wall (outer position) in C4 grasses (Hatakeyama and Ueno 2017). This difference is associated with the difference in localization of GDC and Rubisco (these enzymes are present in C3 M cells but absent in C4 M cells) and thereby the difference in the requirement for scavenging of photorespiratory CO2 released from mitochondria (Hatakeyama and Ueno 2017). This relationship between mitochondria positioning and photosynthetic types in grasses appears to be also applicable to Chenopodium species.
Our data suggest that the main structural and biochemical events during the transition from C3 to proto-Kranz to C3–C4 intermediate type in Chenopodium are (1) the increase in BS relative to M tissue area (volume); (2) the increase in the number of chloroplasts and mitochondria in BS cells; (3) the increase in the distribution of these organelles to the centripetal region of BS cells; (4) the enlargement of BS mitochondria; and (5) the increase in the level of GDC in BS relative to M tissue. These changes allow limited operation of the glycine shuttle in the proto-Kranz type and full operation of the shuttle in the C3–C4 intermediate type, resulting in decreasing Г values (Fig. 4). Our data on the proto-Kranz type also clearly indicate that a complete suppression of GDC-P expression in M cells is not required for the reduction in Г, consistent with our previous studies on artificial hybrids with different genome constitution between C3–C4 intermediate and C3 species of Brassicaceae (Ueno et al. 2003). The average Г value in the proto-Kranz type of Chenopodium was reduced by 23% relative to that of the C3 species. In proto-Kranz species from other genera, a 5–15% reduction in Г has been reported (Sage et al. 2012). Because Chenopodium includes numerous proto-Kranz species together with C3–C4 intermediates, this eudicot genus provides a unique opportunity to elucidate the evolution from C3 to proto-Kranz to C3–C4 intermediate plants.
Photosynthetic types in C. album
We showed that, among C. album accessions examined, the accession from Arizona was of the C3–C4 intermediate type, whereas the remaining six accessions from different localities were of the proto-Kranz type (Table 2). These data suggest that C. album may include different photosynthetic types within a species. However, it is well known that C. album is a heterogenous assemblage of many taxonomic entities with cosmopolitan distribution, probably because many weedy and semi-domesticated forms have arisen by hybridization and polyploidization (Bhargava et al. 2006; Ohri 2015). It has been recently suggested that hybridization may also be involved in the occurrence of C3–C4 intermediates (Kadereit et al. 2017; Ueno et al. 2006). Strict genetic and taxonomic studies will be required to ascertain whether different photosynthetic types occur within C. album. On the other hand, it cannot be ruled out that environmental factors may influence the expression level of C3–C4 intermediate traits (Teese 1995). Oono et al. (2017) have recently reported that high growth temperature and low nitrogen level in soil induce a decrease in Г and stronger expression of GDC-P in BS cells relative to M cells in the Tsukuba accession of C. album. Further research on C. album would provide better understanding of the ecological and adaptive aspects and the expression of C3–C4 intermediate traits.
Change history
25 April 2020
The article Transition from C3 to Correspondence t
References
Bauwe H (2011) Photorespiration: the bridge to C4 photosynthesis. In: Raghavendra AS, Sage RF (eds) C4 photosynthesis and related CO2 concentrating mechanisms. Springer, Heidelberg-Berlin, pp 81–108
Bhargava A, Shukla S, Ohri D (2006) Karyotypic studies on some cultivated and wild species of Chenopodium (Chenopodiaceae). Genet Resour Crop Evol 53:1309–1320. https://doi.org/10.1007/s10722-005-3879-8
Bianconi ME, Dunning LT, Moreno-Villena JJ et al (2018) Gene duplication and dosage effects during the early emergence of C4 photosynthesis in the grass genus Alloteropsis. J Exp Bot 69:1967–1980. https://doi.org/10.1093/jxb/ery029
Brown RH, Hattersley PW (1989) Leaf anatomy of C3–C4 species as related to evolution of C4 photosynthesis. Plant Physiol 91:1543–1550. https://doi.org/10.1104/pp.91.4.1543
Brown RH, Bouton JH, Rigsby L, Rigler M (1983) Photosynthesis of grass species differing in carbon dioxide fixation pathways. VII. Ultrastructural characteristics of Panicum species in the Laxa group. Plant Physiol 71:425–431. https://doi.org/10.1104/pp.71.2.425
Busch FA, Sage TL, Cousins AB, Sage RF (2013) C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ 36:200–212. https://doi.org/10.1111/j.1365-3040.2012.02567.x
Carolin RC, Jacobs SWL, Vesk M (1975) Leaf structure in Chenopodiaceae. Bot Jahrb Syst 95:226–255
Christin PA, Osborne CP (2014) The evolutionary ecology of C4 plants. New Phytol 204:765–781. https://doi.org/10.1111/nph.13033
Christin PA, Wallace MJ, Clayton H et al (2012) Multiple photosynthetic transitions, polyploidy, and lateral gene transfer in the grass subtribe Neurachninae. J Exp Bot 63:6297–6308. https://doi.org/10.1093/jxb/ers282
Edwards GE, Ku MSB (1987) The biochemistry of C3–C4 intermediates. In: Hatch MD, Boardman NK (eds) The biochemistry of plants. Academic Press, New York, pp 275–325
Edwards GE, Voznesenskaya EV (2011) C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants. In: Raghavendra AS, Sage RF (eds) C4 photosynthesis and related CO2 concentrating mechanisms. Springer, Heidelberg-Berlin, pp 29–61
Ehleringer JR, Osmond CB (1991) Stable isotopes. In: Pearcy PW, Ehleringer JR, Mooney HA, Rundel PW (eds) Plant physiological ecology, field methods and instrumentation. Chapman and Hall, London, pp 281–300
Freitag H, Kadereit G (2014) C3 and C4 leaf anatomy types in Camphorosmeae (Camphorosmoideae, Chenopodiaceae). Plant Syst Evol 300:665–687. https://doi.org/10.1007/s00606-013-0912-9
Fuentes-Bazan S, Mansion G, Borsch T (2012) Towards a species level tree of the globally diverse genus Chenopodium (Chenopodiaceae). Mol Phylog Evol 62:359–374. https://doi.org/10.1016/j.ympev.2011.10.006
Haraguchi A, Li B, Matsuki S, Nagata O et al (2009) Variation and plasticity of photosynthesis and respiration in local populations of fat-hen Chenopodium album in northern Japan. Plant Species Biol 24:189–201. https://doi.org/10.1111/j.1442-1984.2009.00254.x
Hatakeyama Y, Ueno O (2016) Intracellular position of mitochondria and chloroplasts in bundle sheath and mesophyll cells of C3 grasses in relation to photorespiratory CO2 loss. Plant Prod Sci 19:540–551. https://doi.org/10.1080/1343943X.2016.1212667
Hatakeyama Y, Ueno O (2017) Intracellular position of mitochondria in mesophyll cells differs between C3 and C4 grasses. J Plant Res 130:885–892. https://doi.org/10.1007/s10265-017-0947-z
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895:81–106. https://doi.org/10.1016/S0304-4173(87)80009-5
Hattersley PW (1984) Characterization of C4 type leaf anatomy in grasses (Poaceae). Mesophyll: bundle sheath area ratios. Ann Bot 53:163–179. https://doi.org/10.1093/oxfordjournals.aob.a086678
Jacobs SWL (2001) Review of leaf anatomy and ultrastructure in the Chenopodiaceae (Caryophyllales). J Torrey Bot Soc 128:236–253. https://www.jstor.org/stable/3088716
Judd WS, Ferguson IK (1999) The genera of Chenopodiaceae in the Southeastern United States. Harvard Papers Bot 4:365–416
Kadereit G, Borsch T, Weising K, Freitag H (2003) Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. Int J Plant Sci 164:959–986. https://doi.org/10.1086/378649
Kadereit G, Mavrodiev EV, Zacharias EH, Sukhorukov AP (2010) Molecular phylogeny of Atripliceae (Chenopodioideae, Chenopodiaceae): implications for systematics, biogeography, flower and fruit evolution, and the origin of C4 photosynthesis. Amer J Bot 97:1664–1687. https://doi.org/10.3732/ajb.1000169
Kadereit G, Bohley K, Lauterbach M et al (2017) C3–C4 intermediates may be of hybrid origin—a reminder. New Phytol 215:70–76. https://doi.org/10.1111/nph.14567
Kennedy RA, Laetsch WM (1974) Plant species intermediate for C3, C4 photosynthesis. Science 184:1087–1089. https://doi.org/10.1126/science.184.4141.1087
Khoshravesh R, Stinson CR, Stata M et al (2016) C3–C4 intermediacy in grasses: organelle enrichment and distribution, glycine decarboxylase expression, and the rise of C2 photosynthesis. J Exp Bot 67:3065–3078. https://doi.org/10.1093/jxb/erw150
Ku MSB, Wu J, Dai Z et al (1991) Photosynthetic and photorespiratory characteristics of Flaveria species. Plant Physiol 96:518–528. https://doi.org/10.1104/pp.96.2.518
Lundgren MR, Christin PA (2017) Despite phylogenetic effects, C3–C4 lineages bridge the ecological gap to C4 photosynthesis. J Exp Bot 68:241–254. https://doi.org/10.1093/jxb/eru186
Lundgren MR, Osborne CP, Christin PA (2014) Deconstructing Kranz anatomy to understanding C4 evolution. J Exp Bot 65:3357–3369. https://doi.org/10.1093/jxb/eru186
Monson RK, Rawsthorne S (2000) CO2 assimilation in C3–C4 intermediate plants. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis: physiology and metabolism. Kluwer Academic Publishers, Dordrecht, pp 533–550
Moore BD, Franceschi VR, Cheng SH et al (1987) Photosynthetic characteristics of the C3–C4 intermediate Parthenium hysterophorus. Plant Physiol 85:984–989. https://doi.org/10.1104/pp.85.4.978
Muhaidat R, Sage RF, Dengler NG (2007) Diversity of Kranz anatomy and biochemistry in C4 eudicots. Amer J Bot 94:362–381. https://doi.org/10.3732/ajb.94.3.362
Muhaidat R, Sage TL, Frohlich MW et al (2011) Characterization of C3–C4 intermediate species in the genus Heliotropium L. (Boraginaceae): anatomy, ultrastructure and enzyme activity. Plant Cell Environ 34:1723–1736. https://doi.org/10.1111/j.1365-3040.2011.02367.x
Ohri D (2015) The taxonomic riddle of Chenopodium album L. complex (Amaranthaceae). Nucleus 58:131–134. https://doi.org/10.1007/s1323
Oono J, Hatakeyama Y, Yabiku T, Ueno O (2017) High temperature and low nitrogen level enhance the expression of C3–C4 intermediate traits in Chenopodium album. Abstracts of the 243 Meeting of the Crop Science Society of Japan, p 217
Pyankov VI, Artyusheva EG, Edwards GE et al (2001) Phylogenetic analysis of tribe Salsoleae (Chenopodiaceae) based on ribosomal ITS sequences: implications for the evolution of photosynthetic types. Amer J Bot 88:1189–1198. https://doi.org/10.2307/3558329
Rawsthorne S (1992) C3–C4 intermediate photosynthesis: linking physiology to gene expression. Plant J 2:267–274. https://doi.org/10.1111/j.1365-313X.1992.00267.x
Rawsthorne S, Hylton CM, Smith AM, Woolhouse HW (1988) Distribution of photorespiratory enzymes between bundle-sheath and mesophyll cells in leaves of the C3–C4 intermediate species Moricandia arvensis (L.) DC. Planta 176:527–532. https://doi.org/10.1007/BF00397660
Roalson EH, Hinchliff CE, Trevisan R, da Silva CRM (2010) Phylogenetic relationships in Eleocharis (Cyperaceae): C4 photosynthesis origins and patterns of diversification in the spikerushes. Syst Bot 35:257–271. https://doi.org/10.1600/036364410791638270
Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161:341–370. https://doi.org/10.1111/j.1469-8137.2004.00974.x
Sage TL, Sage RF (2009) The functional anatomy of rice leaves: implications for refixation of photorespiratory CO2 and effects to engineer C4 photosynthesis into rice. Plant Cell Physiol 50:756–772. https://doi.org/10.1093/pcp/pcp033
Sage RF, Li M, Monson RK (1999) The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK (eds) C4 plant biology. Academic Press, San Diego, pp 551–584
Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63:19–47. https://doi.org/10.1146/annurev-arplant-042811-105511
Sage TL, Busch FA, Johnson DC et al (2013) Initial events during the evolution of C4 photosynthesis in C3 species of Flaveria. Plant Physiol 163:1266–1276. https://doi.org/10.1104/pp.113.221119
Sage RF, Kocacinar F, Sage TL (2014) From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis. J Exp Bot 65:3341–3356. https://doi.org/10.1093/jxb/eru180
Sato R, Suzuki Y (2010) Carbon and nitrogen stable isotope analysis by EA/IRMS. Res Org Geochem 26:21–29
Schlüter U, Weber APM (2016) The road to C4 photosynthesis: evolution of a complex trait via intermediary states. Plant Cell Physiol 57:881–889. https://doi.org/10.1093/pcp/pcw009
Schlüter U, Bräutigam A, Gowik U et al (2017) Photosynthesis in C3–C4 intermediate Moricandia species. J Exp Bot 68:191–206. https://doi.org/10.1093/jxb/erw391
Schüssler C, Freitag H, Koteyeva N et al (2017) Molecular phylogeny and forms of photosynthesis in tribe Salsoleae (Chenopodiaceae). J Exp Bot 68:207–223. https://doi.org/10.1093/jxb/erw432
Teese P (1995) Intraspecific variation for CO2 compensation point and differential growth among variants in a C3–C4 intermediate plant. Oecologia 102:371–376
Tsutsumi N, Tohya M, Nakashima T, Ueno O (2017) Variations in structural, biochemical and physiological traits of photosynthesis and resource use efficiency in Amaranthus species (NAD-ME-type C4). Plant Prod Sci 20:300–312. https://doi.org/10.1080/1343943X.2017.1320948
Ueno O (1992) Immunogold localization of photosynthetic enzymes in leaves of Aristida latifolia, a unique C4 grass with a double chlorenchymatous bundle sheath. Physiol Plant 85:189–196. https://doi.org/10.1111/j.1399-3054.1992.tb04722.x
Ueno O (2011) Structural and biochemical characterization of the C3–C4 intermediate Brassica gravinae and relatives, with particular reference to cellular distribution of Rubisco. J Exp Bot 62:5347–5355. https://doi.org/10.1093/jxb/err187
Ueno O, Samejima M, Koyama T (1989) Distribution and evolution of C4 syndrome in Eleocharis, a sedge group inhabiting wet and aquatic environments, based on culm anatomy and carbon isotope ratios. Ann Bot 64:425–438. https://doi.org/10.1093/oxfordjournals.aob.a087861
Ueno O, Bang SW, Wada Y et al (2003) Structural and biochemical dissection of photorespiration in hybrids differing in genome constitution between Diplotaxis tenuifolia (C3–C4) and radish (C3). Plant Physiol 132:1550–1559. https://doi.org/10.1104/pp.103.021329
Ueno O, Wada Y, Wakai M, Bang SW (2006) Evidence from photosynthetic characteristics for the hybrid origin of Diplotaxis muralis from a C3–C4 intermediate and a C3 species. Plant Biol 8:253–259. https://doi.org/10.1055/s-2005-873050
Vogan PJ, Frohlich MW, Sage RF (2007) The functional significance of C3–C4 intermediate traits in Heliotropium L. (Boraginaceae): gas exchange perspectives. Plant Cell Environ 30:1337–1345. https://doi.org/10.1111/j.1365-3040.2007.01706.x
von Caemmerer S, Hubick KT (1989) Short-term carbon-isotope discrimination in C3–C4 intermediate species. Planta 178:475–481. https://doi.org/10.1007/BF00963817
Voznesenskaya EV, Artyusheva EG, Franceschi VR et al (2001a) Salsola arbusculiformis, a C3–C4 intermediate in Salsoleae (Chenopodiaceae). Ann Bot 88:337–348. https://doi.org/10.1006/anbo.2001.1457
Voznesenskaya EV, Franceschi VR, Kiirats O et al (2001b) Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414:543–546. https://doi.org/10.1038/35107073
Voznesenskaya EV, Franceschi VR, Kiirats O et al (2002) Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant J 31:649–662. https://doi.org/10.1046/j.1365-313X.2002.01385.x
Voznesenskaya EV, Chuong SDX, Koteyeva NK et al (2007) Structural, biochemical, and physiological characterization of C4 photosynthesis in species having two vastly different types of Kranz anatomy in genus Suaeda (Chenopodiaceae). Plant Biol 9:745–757. https://doi.org/10.1055/s-2007-965579
Voznesenskaya EV, Koteyeva NK, Akhani H et al (2013) Structural and physiological analyses in Salsoleae (Chenopodiaceae) indicate multiple transitions among C3, intermediate, and C4 photosynthesis. J Exp Bot 64:3583–3604. https://doi.org/10.1093/jxb/ert191
Wen Z, Zhang M (2011) Anatomical types of leaves and assimilating shoots and carbon 13C/12C isotope fractionation in Chinese representatives of Salsoleae s.l. (Chenopodiaceae). Flora 206:720–730. https://doi.org/10.1016/j.flora.2010.11.015
Wen Z, Zhang M (2015) Salsola laricifolia, another C3-C4 intermediate species in tribe Salsoleae s. l. (Chenopodiaceae). Photosynth Res 123:33–43. https://doi.org/10.1007/s11120-014-0037-1
Yoshimura Y, Kubota F, Ueno O (2004) Structural and biochemical bases of photorespiration in C4 plants: quantification of organelles and glycine decarboxylase. Planta 220:307–317. https://doi.org/10.1007/s00425-004-1335-1
Acknowledgements
We thank the Plant Introduction Station, ARS, USDA and the NARO Genebank, Tsukuba for their gifts of seeds and Prof. N. Furuya, Faculty of Agriculture, Kyushu University, for the use of an electron microscope. This study was supported by Japan Society for the Promotion of Science KAKENHI (Grant No. JP15K14638) to O.U.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yorimitsu, Y., Kadosono, A., Hatakeyama, Y. et al. Transition from C3 to proto-Kranz to C3–C4 intermediate type in the genus Chenopodium (Chenopodiaceae). J Plant Res 132, 839–855 (2019). https://doi.org/10.1007/s10265-019-01135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-019-01135-5