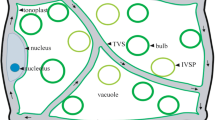
Abstract
Plants have evolved various means for controlled and organized cell destruction, known as programmed cell death (PCD). In plant immune responses against microbial infection, hypersensitive cell death as a form of PCD is a crucial event to prevent the spread of biotrophic pathogens. Recent live cell imaging techniques have revealed dynamic features and significant roles of cytoskeletons and the vacuole during defense responses and the PCD. Actin microfilaments (MFs) focus on the infection sites and function as tracks for the polar transport of antimicrobial materials. To accomplish hypersensitive cell death, further dynamic changes in cytoskeletons are induced. MFs play a role in the structural and functional regulation of the vacuole, leading to execution of the PCD. We here overview spatiotemporal dynamic changes in the cytoskeletons and the vacuoles triggered by signals from pathogens, and propose a hypothetical model for MF-regulated vacuole-mediated PCD in plant immunity.


Similar content being viewed by others
References
Aist JR (1976) Papillae and related wound plugs of plant cells. Annu Rev Phytopathol 14:145–163
Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck SC, Edwards H, Ramonell K, Somerville CR, Thordal-Christensen H (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 15:5118–5129
Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323:101–106
Binet MN, Humbert C, Lecourieux D, Vantard M, Pugin A (2001) Disruption of microtubular cytoskeleton induced by cryptogein, an elicitor of hypersensitive response in tobacco cells. Plant Physiol 125:564–572
Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15:441–447
Cahill D, Rookes J, Michalczyk A, McDonald K, Drake A (2002) Microtubule dynamics in compatible and incompatible interactions of soybean hypocotyls cells with Phytophthora sojae. Plant Pathol 51:629–640
Choi Y, Lee Y, Jeon BW, Staiger CJ, Lee Y (2008) Phosphatidylinositol 3- and 4-phosphate modulate actin filament reorganization in guard cells of day flower. Plant Cell Environ 31:366–377
Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323:95–101
Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, Schulze-Lefert P (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425:973–977
Escobar NM, Haupt S, Thow G, Boevink P, Chapman S, Oparka K (2003) High-throughput vital expression of cDNA-green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell 15:1507–1523
Franklin-Tong VE, Gourlay CW (2008) A role for actin in regulating apoptosis/programmed cell death: evidence spanning yeast, plants and animals. Biochem J 413:389–404
Gross P, Julius C, Schmelzer E, Hahlbrock K (1993) Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J 12:1735–1744
Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z (2005) A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol 169:127–138
Gunawardena AH, Greenwood JS, Dengler NG (2004) Programmed cell death remodels lace plant leaf shape during development. Plant Cell 16:60–73
Guo WJ, Ho TH (2008) An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells. Plant Physiol 147:1710–1722
Hardham AR, Takemoto D, White RG (2008) Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC Plant Biol 8:63
Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305:855–858
Hatsugai N, Kuroyanagi M, Nishimura M, Hara-Nishimura I (2006) A cellular suicide strategy of plants: vacuole-mediated cell death. Apoptosis 11:905–911
Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K, Nishimura M, Hara-Nishimura I (2009) A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev 23:2496–2506
Hazen BE, Bushnell WR (1983) Inhibition of the hypersensitive reaction in barley to powdery mildew by heat shock and cytochalasin B. Physiol Plant Pathol 23:421–438
Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Higaki T, Kutsuna N, Okubo E, Sano T, Hasezawa S (2006) Actin microfilaments regulate vacuolar structures and dynamics: dual observation of actin microfilaments and vacuolar membrane in living tobacco BY-2 cells. Plant Cell Physiol 47:839–852
Higaki T, Goh T, Hayashi T, Kutsuna N, Kadota Y, Hasezawa S, Sano T, Kuchitsu K (2007) Elicitor-induced cytoskeletal rearrangement relates to vacuolar dynamics and execution of cell death: in vivo imaging of hypersensitive cell death in tobacco BY-2 cells. Plant Cell Physiol 48:1414–1425
Higaki T, Kadota Y, Goh T, Hayashi T, Kutsuna N, Sano T, Hasezawa S, Kuchitsu K (2008) Vacuolar and cytoskeletal dynamics during elicitor-induced programmed cell death in tobacco BY-2 cells. Plant Signal Behav 3:700–703
Jones AM (2001) Programmed cell death in development and defense. Plant Physiol 125:94–97
Kadota Y, Kuchitsu K (2006) Regulation of elicitor-induced defense responses by Ca2+ channels and cell cycle in tobacco BY-2 cells. In: Nagata T, Matsuoka K, Inze D (eds) Biotechnology in agriculture and forestry 58 Tobacco BY-2 cells: from cellular dynamics to omics. Springer, Berlin, pp 207–221
Kadota Y, Furuichi T, Ogasawara Y, Goh T, Higashi K, Muto S, Kuchitsu K (2004a) Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem Biophys Res Commun 317:823–830
Kadota Y, Goh T, Tomatsu H, Tamauchi R, Higashi K, Muto S, Kuchitsu K (2004b) Cryptogein-induced initial events in tobacco BY-2 cells: pharmacological characterization of molecular relationship among cytosolic Ca2+ transients, anion efflux and production of reactive oxygen species. Plant Cell Physiol 45:160–170
Kadota Y, Watanabe T, Fujii S, Higashi K, Sano T, Nagata T, Hasezawa S, Kuchitsu K (2004c) Crosstalk between elicitor-induced cell death and cell cycle regulation in tobacco BY-2 cells. Plant J 40:131–142
Kadota Y, Watanabe T, Fujii S, Maeda Y, Ohno R, Higashi K, Sano T, Muto S, Hasezawa S, Kuchitsu K (2005) Cell cycle dependence of elicitor-induced signal transduction in tobacco BY-2 cells. Plant Cell Physiol 46:156–165
Kadota Y, Fujii S, Ogasawara Y, Maeda Y, Higashi K, Kuchitsu K (2006) Continuous recognition of the elicitor signal for several hours is prerequisite for induction of cell death and prolonged activation of signaling events in tobacco BY-2 cells. Plant Cell Physiol 47:1337–1342
Kobayashi I, Hakuno H (2003) Actin-related defense mechanism to reject penetration attempt by a non-pathogen is maintained in tobacco BY-2 cells. Planta 217:340–345
Kobayashi I, Kobayashi Y, Yamaoka N, Kunoh H (1992) Recognition of a pathogen and a nonpathogen by barley coleoptile cells. III. Response of microtubules and actin filaments in barley coleoptile cells to penetration attempts. Can J Bot 70:1815–1823
Kobayashi I, Kobayashi Y, Hardham AR (1994) Dynamic reorganization of microtubules and microfilaments in flax cells during the resistance response to flax rust infection. Planta 195:237–247
Kobayashi I, Kobayashi Y, Hardham AR (1997a) Inhibition of rust-induced hypersensitive response in flax cells by the microtubule inhibitor oryzalin. Aust J Plant Physiol 24:733–740
Kobayashi Y, Kobayashi I, Funaki Y, Fujimoto S, Takemoto T, Kunoh H (1997b) Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. Plant J 11:525–537
Koh S, Andre A, Edwards H, Ehrhardt D, Someville S (2005) Arabidopsis thaliana subcellular responses to compatible Erysiphe cichoracearum infections. Plant J 44:516–529
Kumagai F, Yoneda A, Tomida T, Sano T, Nagata T, Hasezawa S (2001) Fate of nascent microtubules organized at the M/G1 interface, as visualized by synchronized tobacco BY-2 cells stably expressing GFP-tubulin: time-sequence observations of the reorganization of cortical microtubules in living plant cells. Plant Cell Physiol 42:723–732
Kuriyama H (1999) Loss of tonoplast integrity programmed in tracheary element differentiation. Plant Physiol 121:763–774
Kurusu T, Yagala T, Miyao A, Hirochika H, Kuchitsu K (2005) Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J 42:798–809
Kurusu T, Hamada H, Sugiyama Y, Yagala T, Kadota Y, Furuichi T, Hayashi T, Umemura K, Komatsu S, Miyao A, Hirochika H, Kuchitsu K (2010) Negative feedback regulation of microbe-associated molecular pattern-induced cytosolic Ca2+ transients by protein phosphorylation. J Plant Res. doi:10.1007/s10265-010-0388-4
Kutsuna N, Hasezawa S (2002) Dynamic organization of vacuolar and microtubule structures during cell cycle progression in synchronized tobacco BY-2 cells. Plant Cell Physiol 43:965–973
Kutsuna N, Hasezawa S (2005) Morphometrical study of plant vacuolar dynamics in single cells using three-dimensional reconstruction form optical sections. Microsc Res Tech 68:296–306
Kwon C, Neu C, Pajonk S, Yun HS, Lipka U, Humphry M, Bau S, Straus M, Kwaaitaal M, Rampelt H, El Kasmi F, Jürgens G, Parker J, Panstruga R, Lipka V, Schulze-Lefert P (2008) Co-option of a default secretory pathway for plant immune responses. Nature 451:835–840
Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14:2627–2641
Lee YJ, Szumlanski A, Nielsen E, Yang Z (2008) Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol 181:1155–1168
Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, Llorente F, Molina A, Parker J, Somerville S, Schulze-Lefert P (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310:1180–1183
Marty F (1999) Plant vacuoles. Plant Cell 11:587–600
Meyer D, Pajonk S, Micali C, O’Connell R, Schulze-Lefert P (2009) Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J 57:986–999
Miklis M, Consonni C, Bhat RA, Lipka V, Schulze-Lefert P, Panstruga R (2007) Barley MLO modulates actin-dependent and actin-independent antifungal defense pathways at the cell periphery. Plant Physiol 144:1132–1143
Mongrand S, Stanislas T, Bayer EM, Lherminier J, Simon-Plas F (2010) Membrane rafts in plant cells. Trends Plant Sci 15:656–663
Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E (2008) The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot 59:501–520
Nakaune S, Yamada K, Kondo M, Kato T, Tabata S, Nishimura M, Hara-Nishimura I (2005) A vacuolar processing enzyme, δVPE, is involved in seed coat formation at the early stage of seed development. Plant Cell 17:876–887
Obara K, Kuriyama H, Fukuda H (2001) Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in Zinnia. Plant Physiol 125:615–626
Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, Nara M, Suzuki K, Tanokura M, Kuchitsu K (2008) Synergistic activation of Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 283:8885–8892
Pajerowska-Mukhtar K, Dong X (2009) A kiss of death-proteasome-mediated membrane fusion and programmed cell death in plant defense against bacterial infection. Genes Dev 23:2449–2454
Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9:1157–1168
Poulter NS, Vatovec S, Franklin-Tong VE (2008) Microtubules are a target for self-incompatibility signaling in Papaver pollen. Plant Physiol 146:1358–1367
Reape TJ, McCabe PF (2010) Apoptotic-like regulation of programmed cell death in plants. Apoptosis 15:249–256
Reisen D, Marty F, Leborgne-Castel N (2005) New insights into the tonoplast architecture of plant vacuoles and vacuolar dynamics during osmotic stress. BMC Plant Biol 5:13
Saito SY, Watabe S, Ozaki H, Kobayashi M, Suzuki T, Kobayashi H, Fusetani N, Karaki H (1998) Actin-depolymerizing effect of dimeric macrolides, bistheonellide A and swinholide A. J Biochem 123:571–578
Saito C, Ueda T, Abe H, Wada Y, Kuroiwa T, Hisada A, Furuya M, Nakano A (2002) A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J 29:245–255
Samaj J, Ovecka M, Hlavacka A, Lecourieux F, Meskiene I, Lichtscheidl I, Lenart P, Salaj J, Volkmann D, Bögre L, Baluska F, Hirt H (2002) Involvement of the mitogen-activated protein kinase SIMK in regulation of root hair tip growth. EMBO J 21:3296–3306
Sano T, Higaki T, Oda Y, Hayashi T, Hasezawa S (2005) Appearance of actin microfilament ‘twin peaks’ in mitosis and their function in cell plate formation, as visualized in tobacco BY-2 cells expressing GFP-fimbrin. Plant J 44:595–605
Schreiber K, Ckurshumova W, Peek J, Desveaux D (2008) A high-throughput chemical screen for resistance to Pseudomonas syringae in Arabidopsis. Plant J 54:522–531
Schütz I, Gus-Mayer S, Schmelzer E (2006) Profilin and Rop GTPases are localized at infection sites of plant cells. Protoplasma 227:229–235
Shimada C, Lipka V, O’Connell R, Okuno T, Schulze-Lefert P, Takano Y (2006) Nonhost resistance in Arabidopsis–Colletotrichum interactions acts at the cell periphery and requires actin filament function. Mol Plant Microbe Interact 19:270–279
Skalamera D, Heath MC (1995) Changes in the plant endomembrane system associated with callose synthesis during the interaction between cowpea (Vigna unguiculata) and the cowpea fungus (Uromyces vignae). Can J Bot 73:1731–1738
Skalamera D, Heath MC (1996) Cellular mechanisms of callose deposition in response to fungal infection or chemical damage. Can J Bot 74:1236–1242
Skalamera D, Heath MC (1998) Changes in the cytoskeleton accompanying infection-induced nuclear movements and the hypersensitive response in plant cells invaded by rust fungi. Plant J 16:191–200
Skalamera D, Jibodh S, Heath MC (1997) Callose deposition during the interaction between cowpea (Vigna unguiculata) and the monokaryotic stage of the cowpea fungus (Uromyces vignae). New Phytol 136:511–524
Smertenko AP, Bozhkov PV, Filonova LH, von Arnold S, Hussey PJ (2003) Re-organisation of the cytoskeleton during developmental programmed cell death in Picea abies embryos. Plant J 33:813–824
Stein M, Dittgen J, Sánchez-Rodríguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18:731–746
Takemoto D, Maeda H, Yoshioka H, Doke N, Kawakita K (1999) Effect of cytochalasin D on defense responses of potato tuber discs treated with hyphal wall components of Phytophthora infestans. Plant Sci 141:219–226
Takemoto D, Jones DA, Hardham AR (2003) GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 33:775–792
Takemoto D, Jones DA, Hardham AR (2006) Re-organization of the cytoskeleton and endoplasmic reticulum in the Arabidopsis pen1-1 mutant inoculated with the non-adapted powdery mildew pathogen, Blumeria graminis f. sp. hordei. Mol Plant Pathol 7:553–563
Tang X, Lancelle SA, Hepler PK (1989) Fluorescence microscopic localization of actin in pollen tubes: comparison of actin antibody and phalloidin staining. Cell Motil Cytoskelet 12:216–224
Thomas SG, Huang S, Li S, Staiger CJ, Franklin-Tong VE (2006) Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. J Cell Biol 174:221–229
Tian M, Chaudhry F, Ruzicka DR, Meagher RB, Staiger CJ, Day B (2009) Arabidopsis actin-depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol 150:815–824
Tomiyama K, Sato K, Doke N (1982) Effect of cytochalasin B and colchicines on hypersensitive death of potato cells infected by incompatible race of Phytophthora infestans. Ann Phytopathol Soc Jpn 48:228–230
Uemura T, Yoshimura SH, Takeyasu K, Sato MH (2002) Vacuolar membrane dynamics revealed by GFP–AtVam3 fusion protein. Genes Cells 7:743–753
Wright H, van Doorn WG, Gunawardena AH (2009) In vivo study of developmental programmed cell death using the lace plant (Aponogeton madagascariensis; Aponogetonaceae) leaf model system. Am J Bot 96:865–876
Yun BW, Atkinson HA, Gaborit C, Greenland A, Read ND, Pallas JA, Loake GJ (2003) Loss of actin cytoskeletal function and EDS1 activity, in combination, severely compromises non-host resistance in Arabidopsis against wheat powdery mildew. Plant J 34:768–777
Acknowledgments
The authors thank to Drs. Tatsuaki Goh (Kobe University), Yasuhiro Kadota (The Sainsbury Laboratory), Teruyuki Hayashi (National Institute of Agrobiological Sciences), Natsumaro Kutsuna (The University of Tokyo), and Toshio Sano (Hosei University) for helpful discussions. This work was supported in part by Grants-in-Aid Scientific Research on Priority Areas (17051027 to K.K., 19039008 and 20061008 to S.H.), for Scientific Research on Innovative Areas (21200067 to T.K.), for Exploratory Research (21658118 to K.K.) and for Young Scientists (B) (21780041 to T.K.) from the Ministry of Education, Science, Culture, Sports and Technology of Japan, and by grants from Japan Science and Technology Agency, for Adaptable and Seamless Technology transfer Program through target-driven R&D (AS221Z03504E) to T.K.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was contributed at the invitation of the Editorial Committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Reorganization of actin microfilaments (MFs) in S-phase-synchronized tobacco (Nicotiana tabacum) BY-2 cells treated with cryptogein. Cell cortex (a and b) and mid-plane images (c and d) were obtained in synchronized BY-2 cells expressing GFP-ABD2 just after (S phase; a and c) and 12 h after (b and d) cryptogein treatment. Fine cortical meshworks of MFs at S phase (a) change into transversely oriented heavy bundles after cryptogein treatment (b). Endoplasmic MFs that connect the nuclear periphery to the cell cortex (c) decrease, which accompanies nuclear translocation from the cell center to the cell periphery (d). Scale bars: 20 μm (TIFF 5460 kb)
Supplemental Fig. 2
Reorganization of microtubules (MTs) in S-phase-synchronized tobacco (Nicotiana tabacum) BY-2 cells treated with cryptogein. Cell cortex (a and b) and mid-plane images (c and d) were obtained in synchronized BY-2 cells expressing GFP-tubulin just after (S phase; a and c) and 12 h after (b and d) cryptogein treatment. Most of the cortical MTs at S phase (a) were disrupted at 12 h after cryptogein treatment (b). Endoplasmic MTs that connect the nuclear periphery to the cell cortex (c) are decreased accompanying nuclear translocation from the cell center to the cell periphery (d). Scale bars: 20 μm (TIFF 6571 kb)
Supplemental Fig. 3
Changes in vacuolar morphology and permeability in S-phase-synchronized tobacco (Nicotiana tabacum) BY-2 cells treated with cryptogein. Vacuoles are prestained with a fluorescent probe, BCECF. DIC (a-c) and BCECF fluorescence images (d-f) in representative cells at 0 (S phase; a and d), 12 (b and e), and 24 h (c and f) after cryptogein treatment are shown. The complicated morphology of vacuoles that are passed through by transvacuolar strands at S phase (a and d) changes into simple structures around 12 h after cryptogein treatment (b and e). Thereafter, cells without BCECF fluorescence appear (c and f; an arrowed cell). Double staining of Evans blue and BCECF (g) shows the cells without BCECF fluorescence are dead. Yellow dashed lines indicate the dead cells stained with Evans blue. Scale bars: 20 μm (TIFF 7302 kb)
Supplemental Fig. 4
Reorganization of the vacuolar membranes (VMs) in S-phase-synchronized tobacco (Nicotiana tabacum) BY-2 cells treated with cryptogein. Optical sections at mid-plane were obtained in synchronized BY-2 cells expressing GFP-AtVAM3 just after (S phase; a), 12 h after (b), and 18 h after (c) cryptogein treatment. Most of the TVSs at S phase (a) were converted into bulb-like structures by 12 h after cryptogein treatment (b). Thereafter, the bulb-like structures disappeared and the large vacuole became simple-shaped structures (c). Scale bars: 20 μm (TIFF 2856 kb)
Rights and permissions
About this article
Cite this article
Higaki, T., Kurusu, T., Hasezawa, S. et al. Dynamic intracellular reorganization of cytoskeletons and the vacuole in defense responses and hypersensitive cell death in plants. J Plant Res 124, 315–324 (2011). https://doi.org/10.1007/s10265-011-0408-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-011-0408-z