Abstract
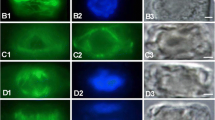
The cytoskeleton in plant cells is a dynamic structure that can rapidly respond to extracellular stimuli. Alteration of the organization of microtubules and actin microfilaments was examined in mesophyll cells of flax, Linum usitatissimum L., during attempted infection by the flax rust fungus, Melampsora lini (Ehrenb.) Lev. Flax leaves that had been inoculated with either a compatible (yielding a susceptible reaction) or an incompatible (yielding a resistant reaction) strain of M. lini were embedded in butyl-methylmethacrylate resin; sections of this material were immunofluorescently labelled with anti-tubulin or anti-actin and examined using confocal laser scanning microscopy. In uninfected leaves, microtubules in the mesophyll cells formed a transverse array in the cell cortex. Microfilaments radiated through the cytoplasm from the nucleus. In an incompatible interaction, microtubules and microfilaments were extensively reorganized in mesophyll cells that were in contact with fungal infection hyphae or haustorial mother cells before penetration of the cell by the infection peg. After the initiation of haustorium development, microtubules disappeared from the infected cells, and growth of the haustoria ceased. In an incompatible interaction, hypersensitive cell death occurred in more than 70% of infected cells but occurred in less than 20% of cells in compatible interactions. After the infected cell had undergone hypersensitive cell death, the cytoskeleton in neighbouring cells became focused on the walls shared with the necrotic cell. In compatible interactions, reorganization of the cytoskeleton was either not observed at all or was observed much less frequently up to 48 h after inoculation.
Similar content being viewed by others
Abbreviations
- FITC:
-
fluorescein isothiocyanate
- WGA:
-
wheatgerm agglutinin
References
Aist, J.R. (1983) Structural responses as resistance mechanisms. In: The dynamics of host defence, pp. 33–70, Bailey, J.A., Deverall, B.J., eds Academic Press, Sydney
Baskin, T.I., Busby, C.H., Fowke, L.C., Sammut, M., Gubler, F. (1992) Improvements in immunostaining samples embedded in methacrylate: localization of microtubules and other antigens throughout developing organs in plants of diverse taxa. Planta 187, 405–413
Bell, A.A. (1981) Biochemical mechanisms of disease resistance. Annu. Rev. Plant Physiol. 32, 21–81
Bourett, T., Hoch, H.C., Staples, R.C. (1987) Association of the microtubule cytoskeleton with the thigmotropic signal for appressorium formation in Uromyces. Mycologia 79, 540–545
Bruzzese, E., Hasan, S. (1983) A whole leaf clearing and staining technique for host specificity studies of rust fungi. Plant Pathol. 32, 335–338
Clancy, F.G., Coffey, M.D. (1980) Patterns of translocation, changes in invertase activity, and polyol formation in susceptible and resistant flax infected with the rust fungus Melampsora lini. Physiol. Plant Pathol. 17, 41–52
Coffey, M.D. (1976) Flax rust resistance involving the K gene: an ultrastructural survey. Can. J. Bot. 54, 1443–1457
Coffey, M.D., Allen, F.H.E. (1983) A quantitative histological and ultrastructural analysis of interactions between the flax rust and near-isogenic host lines varying in their degree of incompatibility. Can. J. Bot. 61, 1831–1850
Dixon, R.A., Lamb, C.J. (1990) Molecular communication in interactions between plants and microbial pathogens. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 339–367
Flor, H.H. (1956) The complementary genic systems in flax and flax rust. Adv. Genet. 8, 29–54
Gross, P., Julius, C., Schmelzer, E., Hahlbrock, K. (1993) Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J. 12, 1735–1744
Gubler, F. (1989) Immunofluorescence localization of microtubules in plant root tips embedded in butyl-methyl methacrylate. Cell Biol. Int. Rep. 13, 137–145
Gunning, B.E.S., Hardham, A.R. (1982) Microtubules. Annu. Rev. Plant Physiol. 33, 651–698
Hardham, A.R., Green, P.B., Lang, J.M. (1980) Reorganization of cortical microtubules and cellulose deposition during leaf formation in Graptopetalum paraguayense. Planta 149, 181–195
Hazen, B.E., Bushnell, W.R. (1983) Inhibition of the hypersensitive reaction in barley to powdery mildew by heat shock and cytochalasin B. Physiol. Plant Pathol. 23, 421–438
Heath, M.C. (1972) Ultrastructure of host and nonhost reactions to cowpea rust. Phytopathology 62, 27–38
Heslop-Harrison, J., Heslop-Harrison, Y. (1989) Myosin associated with the surfaces of organelles, vegetative nuclei and generative cells in angiosperm pollen grains and tubes. J. Cell Sci. 94, 319–325
Hush, J.M., Overall, R.L. (1992) Re-orientation of cortical F-actin is not necessary for wound-induced microtubule re-orientation and cell polarity establishment. Protoplasma 169, 97–106
Ishida, K., Katsumi, M. (1991) Immunofluorescence microscopical observation of cortical microtubule arrangement as affected by gibberellin in d5 mutant of Zea mays L. Plant Cell Physiol. 32, 409–417
Kadowaki, T., Koyasu, S., Nishida, E., Sakai, H., Takaku, F., Yahara, I., Kasuga, M. (1986) Insulin-like growth factors, insulin, and epidermal growth factor cause rapid cytoskeletal reorganization in KB cells. J. Biol. Chem. 34, 16141–16147
Keen, N.T., Littlefield, L.J. (1979) The possible association of phytoalexins with resistance gene expression in flax to Melampsora lini. Physiol. Plant Pathol. 14, 265–280
Kobayashi, H., Fukuda, H., Shibaoka, H. (1987) Reorganization of actin filaments associated with the differentiation of tracheary elements in Zinnia mesophyll cells. Protoplasma 138, 69–71
Kobayashi, I., Kobayashi, Y., Yamaoka, N., Kunoh, H. (1991) An immunofluorescent cytochemical technique applying micromanipulation to detect microtubules in plant tissues inoculated with fungal spores. Can. J. Bot. 69, 2634–2636
Kobayashi, I., Kobayashi, Y., Yamaoka, N., Kunoh, H. (1992) Recognition of a pathogen and a nonpathogen by barley coleoptile cells. (III). Responses of microtubules and actin filaments in barley coleoptile cells to penetration attempts. Can. J. Bot. 70, 1815–1823
Köhle, H., Jeblick, W., Poten, F., Blaschek, W., Kauss, H. (1985) Chitosan-elicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol. 77, 544–551
La Claire, J.W., II. (1989) Actin cytoskeleton in intact and wounded coenocytic green algae. Planta 177, 47–57
Lawrence, G.J. (1988) Melampsora lini, rust of flax and linseed. In: Advances in plant pathology, vol 6: Genetics of plant pathogenic fungi, pp. 313–331, Williams, D.S., Ingram P.H., eds. Academic Press, London
Lawrence, G.J., Mayo, G.M.E., Shepherd, K.W. (1981) Interactions between genes controlling pathogenicity in the flax rust fungus. Phytopathology 71, 12–19
Littlefield, L.J. (1972) Development of haustoria of Melampsora lini. Can. J. Bot. 50, 1701–1703
Littlefield, L.J. (1973) Histological evidence for diverse mechanisms of resistance to flax rust, Melampsora lini (Ehrenb.) Lev. Physiol. Plant Pathol. 3, 241–247
Littlefield, L.J., Aronson, S.J. (1969) Histological studies of Melampsora lini resistance in flax. Can. J. Bot. 47, 1713–1717
Lloyd, C.W. (1987) The plant cytoskeleton: the impact of fluorescence microscopy. Annu. Rev. Plant Physiol. 38, 119–139
Mendgen, K., Lange, M., Bretschneider, K. (1985) Quantitative estimation of the surface carbohydrates on the infection structures of rust fungi with enzymes and lectins. Arch. Microbiol. 140, 307–311
Nick, P., Schäfer, E., Hertel, R., Furuya, M. (1991) On the putative role of microtubules in gravitropism of maize coleoptiles. Plant Cell Physiol. 32, 873–880
Nishida, E., Gotoh, Y. (1992) Mitogen-activated protein kinase and cytoskeleton in mitogenic signal transduction. Int. Rev. Cytol. 138, 211–238
Parthasarathy, M.V. (1985) F-actin architecture in coleoptile epidermal cells. Eur. J. Cell Biol. 39, 1–12
Parthasarathy, M.V., Perdue, T.D., Witztum, A., Alvernaz, J. (1985) Actin network as a normal component of the cytoskeleton in many vascular plant cells. Am. J. Bot. 72, 1318–1323
Quader, H., Hofmann, A., Schnepf, E. (1989) Reorganization of the endoplasmic reticulum in epidermal cells of onion bulb scales after cold stress: involvement of cytoskeletal elements. Planta 177, 273–280
Roberts, I.N., Lloyd, C.W., Roberts, K. (1985) Ethylene-induced microtubule reorientations: mediation by helical arrays. Planta 164, 439–447
Seagull, R.W. (1992) A quantitative electron microscopic study of changes in microtubule arrays and wall microfibril orientation during in vitro cotton fibre development. J. Cell Sci. 101, 561–577
Shain, L., Järlfors, U. (1987) Ultrastructure of eastern cottonwood clones susceptible or resistant to leaf rust. Can. J. Bot. 65, 1586–1598
Smart, M.G. (1991) The plant cell wall as a barrier to fungal invasion. In: The fungus spore and disease initiation in plants and animals, pp. 47–66, Cole, G.T., H.C. Hoch, eds Plenum Press, New York
Staiger, C.J., Schliwa, M. (1987) Actin localization and function in higher plants. Protoplasma 141, 1–12
Staples, R.C., Hoch, H.C. (1987) Infection structures — form and function. Exp. Mycol. 11, 163–169
Taylor, J., Mims, C.W. (1991) Fungal development and host cell responses to the rust fungus Puccinia substriata var. indica in seedling and mature leaves of susceptible and resistant pearl millet. Can. J. Bot. 69, 1207–1219
Timmis, J.N., Whisson, D.L., Binns, A.M., Mayo, M.J., Mayo, G.M.E. (1990) Deletion mutation as a means of isolating avirulence genes in flax rust. Theor. Appl. Genet. 79, 411–416
Tomiyama, K., Sato, K., Doke, N. (1982) Effect of cytochalasin B and colchicine on hypersensitive death of potato cells infected by incompatible race of Phytophthora infestans. Ann. Phytopathol. Soc. Japan 48, 228–230
Traas, J.A., Doonan, J.H., Rawlins, D.J., Shaw, P.J., Watts, J., Lloyd, C.W. (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J. Cell Biol. 105, 387–395
Tu, J.C. (1978) Effect of calcium, magnesium and cytochalasin B on the formation of local lesions by alfalfa mosaic virus in Phaseolus vulgaris. Physiol. Plant Pathol. 12, 167–172
Vaughan, M.A., Vaughn, K.C. (1987) Effects of microfilament disrupters on microfilament distribution and morphology in maize root cells. Histochemistry 87, 129–137
White R.G., Badelt, K., Overall, R.L., Vesk, M. (1994) Actin associated with plasmodesmata. Protoplasma, in press
Williamson, R.E. (1986) Organelle movements along actin filaments and microtubules. Plant Physiol. 82, 631–634
Williamson, R.E. (1993) Organelle movements. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 181–202
Xu, P., Lloyd, C.W., Staiger, C.J., Drøbak, B.K. (1992) Association of phosphatidylinositol 4-kinase with the plant cytoskeleton. Plant Cell 4, 941–951
Author information
Authors and Affiliations
Corresponding author
Additional information
We thank Dr. G.J. Lawrence for providing valuable discussions and materials.
Rights and permissions
About this article
Cite this article
Kobayashi, I., Kobayashi, Y. & Hardham, A.R. Dynamic reorganization of microtubules and microfilaments in flax cells during the resistance response to flax rust infection. Planta 195, 237–247 (1994). https://doi.org/10.1007/BF00199684
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00199684