Abstract
Soft tissues are characterized by a nonlinear mechanical response, highly affected by the multiscale structure of collagen fibers. The effectiveness and the calibration of constitutive models play a major role on the reliability and the applicability of computational models in biomechanics. This paper presents a procedure for the identification of the relationship between collagen-related structural features in soft tissues with model parameters of classical polynomial- and exponential-based constitutive models. Histological features at microscale, as well as biochemical and biophysical properties at nanoscale, are addressed by employing a multiscale structural description of soft tissue mechanics as benchmark data set. Both the direct (from structure to parameters) and the inverse (from parameters to structure) problem are addressed. Suitable optimization problems are introduced for accurate numerical and approximated analytical direct relationships. The inverse identification has been addressed by providing also a measure of the reliability of the computed estimates. Results show the effectiveness of the proposed strategies and allow to discuss the fitting capabilities of classical constitutive approaches in terms of parameters identification.















Similar content being viewed by others
References
Andriotis OG, Chang SW, Vanleene M, Howarth PH, Davies DE, Shefelbine SJ, Buehler MJ, Thurner PJ (2015) Structure–mechanics relationships of collagen fibrils in the osteogenesis imperfecta mouse model. J R Soc Interface 12:20150701
Angel G, Gheorghe V (1985) Interferometric evaluation of collagen concentration in tendon fibers. Connect Tissue Res 13:323–337
Auricchio F, Ferrara A, Morganti S (2012) Comparison and critical analysis of invariant-based models with respect to their ability in fitting human aortic valve data. Ann Solid Struct Mech 4:1–14
Auricchio F, Conti M, Ferrara A (2014) How constitutive model complexity can affect the capability to fit experimental data: a focus on human carotid arteries and extension/inflation data. Arch Comput Methods E 21:273–292
Balzani D, Schröder J, Neff P, Holzapfel GA (2006) A polyconvex framework for soft biological tissues. Adjustment to experimental data. Int J Solids Struct 43:6052–6070
Bianchi D, Marino M, Vairo G (2016) An integrated computational approach for aortic mechanics including geometric, histological and chemico-physical data. J Biomech 49:2331–2340
Biehler J, Kehl S, Gee MW, Schmies F, Pelisek J, Maier A, Reeps C, Eckstein HH, Wall WA (2016) Probabilistic noninvasive prediction of wall properties of abdominal aortic aneurysms using Bayesian regression. Biomech Model Mechanobiol 16:45–61
Bozec L, Horton M (2005) Topography and mechanical properties of single molecules of type I collagen using atomic force microscopy. Biophys J 88:4223–4231
Brüel A, Ørtoft G, Oxlund H (1998) Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis 140:135–145
Buehler MJ, Wong SY (2007) Entropic elasticity controls nanomechanics of single tropocollagen molecules. Biophys J 93:37–43
Carmo M, Colombo L, Bruno A, Corsi FRM, Roncoroni L, Cuttin MS, Radice F, Mussini E, Settembrini PG (2002) Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 23:543–549
Cortes DH, Elliott DM (2014) Accurate prediction of stress in fibers with distributed orientations using generalized high-order structure tensors. Mech Mater 75:73–83
Cyron CJ, Humphrey JD (2017) Growth and remodeling of load-bearing biological soft tissues. Meccanica 52:645–664
Diamant J, Keller A, Baer E, Litt M, Arridge R (1972) Collagen: ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci 180:293–315
Ferrara A, Pandolfi A (2008) Numerical modelling of fracture in human arteries. Comput Methods Appl Mech Eng 11:553–567
Fratzl P (2008) Collagen: structure and mechanics. Springer, New York
Freed AD, Einstein DR, Vesely I (2005) Invariant formulation for dispersed transverse isotropy in aortic heart valves. Biomech Model Mechanobiol 4:100–117
Gao H, Li WG, Cai L, Berry C, Luo XY (2015) Parameter estimation of the Holzapfel–Ogden law for healthy myocardium. J Eng Math 95:231–248
Gao H, Aderhold A, Mangion K, Luo XY, Husmeier D, Berry C (2017) Changes and classification in myocardial contractile function in left ventricle following acute myocardial infarction. J R Soc Interface 14:20170203
Gasser TC, Ogden RW, Holzapfel GA (2006) Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface 3:15–35
Gautieri A, Buehler MJ, Redaelli A (2009) Deformation rate controls elasticity and unfolding pathway of single tropocollagen molecules. J Mech Behav Biomed Mater 2:130–137
Hansen KA, Weiss JA, Barton JK (2002) Recruitment of tendon crimp with applied tensile strain. J Biomech Eng 124:72–77
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61:1–48
Lanir Y (1979) A structural theory for the homogeneous biaxial stress-strain relationships in flat collageneous tissues. J Biomech 12:423–436
Liu T, Hall TJ, Barbone PE, Oberai AA (2017) Inferring spatial variations of microstructural properties from macroscopic mechanical response. Biomech Model Mechanobiol 16:479–496
Maceri F, Marino M, Vairo G (2010) A unified multiscale mechanical model for soft collagenous tissues with regular fiber arrangement. J Biomech 43:355–363
Maceri F, Marino M, Vairo G (2012) An insight on multiscale tendon modeling in muscle-tendon integrated behavior. Biomech Model Mechanobiol 11:505–517
Maceri F, Marino M, Vairo G (2013) Age-dependent arterial mechanics via a multiscale elastic approach. Int J Comput Methods Eng Sci Mech 14:141–151
Marino M (2016) Molecular and intermolecular effects in collagen fibril mechanics: a multiscale analytical model compared with atomistic and experimental studies. Biomech Model Mechanobiol 15:133–154
Marino M, Wriggers P (2017) Finite strain response of crimped fibers under uniaxial traction: an analytical approach applied to collagen. J Mech Phys Solids 98:429–453
Marino M, Pontrelli G, Vairo G, Wriggers P (2017a) A chemo-mechano-biological formulation for the effects of biochemical alterations on arterial mechanics: the role of molecular transport and multiscale tissue remodelling. J R Soc Interface 14:20170615
Marino M, Pontrelli G, Vairo G, Wriggers P (2017b) Coupling microscale transport and tissue mechanics: modelling strategies for arterial multiphysics. In: Becker S (ed) Microscale Transport Modelling in Biological Processes. Elsevier, London, pp 77–112
Marino M, Vairo G (2014) Computational modelling of soft tissues and ligaments. In: Jin Z (ed) Computational modelling of biomechanics and biotribology in the musculoskeletal system. Woodhead publishing series in biomaterials 81. Woodhead Publishing Limited, Cambridge, pp 141–172
Marko JF, Siggia ED (1995) Stretching DNA. Macromolecules 28:8759–8770
Niestrawska JA, Viertler C, Regitnig P, Cohnert TU, Sommer G, Holzapfel GA (2016) Microstructure and mechanics of healthy and aneurysmatic abdominal aortas: experimental analysis and modelling. J R Soc Interface 13:20160620
Pandolfi A, Vasta M (2012) Fiber distributed hyperelastic modeling of biological tissues. Mech Mater 44:151–162
Panwar P, Lamour G, Mackenzie NCW, Yang H, Ko F, Li H, Brömme D (2015) Changes in structural–mechanical properties and degradability of collagen during ageing-associated modifications. J Biol Chem 290:23291–23306
Perotti LE, Ponnaluri AV, Krishnamoorthi S, Balzani D, Ennis DB, Klug WS (2017) Method for the unique identification of hyperelastic material properties using full field measures. Application to the passive myocardium material response. Int J Numer Method Biomed Eng 33:e2866
Puglisi G, Saccomandi G (2016) Multi-scale modelling of rubber-like materials and soft tissues: an appraisal. Proc Math Phys Eng Sci 472:20160060
Robertson AM, Duan X, Aziz KM, Hill MR, Watkins SC, Cebral JR (2015) Diversity in the strength and structure of unruptured cerebral aneurysms. Ann Biomed Eng 43:1502–1515
Saito M, Marumo K, Fujii K, Ishioka N (1997) Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem 253:26–32
Sasaki N, Odajima S (1996) Elongation mechanism of collagen fibrils and force-strain relations of tendon at each level of structural hierarchy. J Biomech 29:1131–1136
Schriefl AJ, Zeindlinger G, Pierce DM, Regitnig P, Holzapfel GA (2012) Determination of the layer-specific distributed collagen fibre orientations in human thoracic and abdominal aortas and common iliac arteries. J R Soc Interface 9:1275–1286
Schröder J, von Hoegen M (2016) An engineering tool to estimate eigenstresses in three-dimensional patient-specific arteries. Comput Methods Appl Mech Eng 306:364–381
Schröder J, Neff P (2003) Invariant formulation of hyperelastic transverse isotropy based on polyconvex free energy functions. Int J Solids Struct 40:401–445
Strutz T (2011) Data fitting and uncertainty—a practical introduction to weighted least squares and beyond. Springer, Berlin
Svensson RB, Mulder H, Kovanen V, Magnusson SP (2013) Fracture mechanics of collagen fibrils: influence of natural cross-links. Biophys J 104:2476–2484
The MathWorks, Inc. MATLAB, Release R2014a, Global Optimization Toolbox User’s Guide (2014)
Tsamis A, Krawiec JT, Vorp DA (2013) Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface 10:20121004(22)
Ugray Z, Lasdon L, Plummer J, Glover F, Kelly J, Martí R (2007) Scatter search and local NLP solvers: a multistart framework for global optimization. INFORMS J Comput 19:328–340
Vaishnav RN, Young JT, Patel DJ (1973) Distribution of stresses and of strain-energy density through the wall thickness in a canine aortic segment. Circ Res 32:577–583
van der Slot AJ, van Dura EA, de Wit EC, Degroot J, Huizinga TWJ, Bank RA, Zuurmoond AM (2005) Elevated formation of pyridinoline cross-links by profibrotic cytokines is associated with enhanced lysyl hydroxylase 2b levels. BBA Mol Basis Dis 1741:95–102
von Hoegen M, Marino M, Schröder J, Wriggers P (2018) Direct and inverse identification of constitutive parameters from the structure of soft tissues. Part 2: arrangement of collagen fibers (to be submitted)
Wittek A, Karatolios K, Bihari P, Schmitz-Rixen T, Moosdorf R, Vogt S, Blase C (2013) In vivo determination of elastic properties of the human aorta based on 4D ultrasound data. J Mech Behav Biomed Mater 27:167–183
Acknowledgements
M. Marino acknowledges that this work has been carried out within the framework of the SMART BIOTECS alliance between the Technical University of Braunschweig and the Leibniz University of Hannover. This initiative is financially supported by the Ministry of Economy and Culture (MWK) of Lower Saxony, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Michele Marino and Markus von Hoegen have contributed equally to this work.
Appendices
A Multiscale structural approach: collagen mechanics
The benchmark data set \(\sigma = \sigma (\lambda ,\mathcal {S})\) is obtained by employing a multiscale structural approach for the description of collagen nonlinearities, in the formulation presented by Marino and Wriggers (2017) and Marino et al. (2017a), whose governing equations are here reported for the sake of completeness. This approach allows to introduce the explicit dependence of the stress function on the set \(\mathcal {S}\) of structural features in Eq. (10). For the sake of compactness, other parameters governing the multiscale structural approach are briefly summarized in Table 7, where the values employed in all numerical simulations (in agreement with experimental measurements) are also reported.
Since present work focuses on collagen contribution, the matrix-related isotropic contribution \({\varPsi }_{iso}\) is introduced by following a phenomenological Mooney–Rivlin approach as in Eq. (3a). The strain-energy term \({\varPsi }_{ani}\) in Eq. (2) associated with collagen fibers is defined as:
where \(\lambda _4 = \sqrt{I_4}\) [see Eq. (4)] and \(E_F=E_F(\lambda _4)\) is the equivalent tangent modulus of crimped collagen fibers. The latter is defined as \(E_F(\lambda _4) =C_F(\lambda _4)/\lambda _4\), with \(C_F\) being the along-the-chord tangent modulus of Euler–Bernoulli curvilinear beams whose geometry corresponds to the one of fibers, defined as (dependences omitted):
Here, \(\ell _F = \ell _F(\lambda _4)\) and \(H_F=H_F(\lambda _4)\) are fiber quarter-of-period and amplitude in the current configuration. As a modeling choice, it is defined \(\ell _F(\lambda _4)=\lambda _4 \ell _{F,o}\) with \(\ell _{F,o}=L_{F,o}/4\) being fiber reference quarter-of-period, while \(H_F(\lambda _4)\) is obtained from the solution of the geometry evolution Eq.:
with \(H_F(1) = H_{F,o}\), which gives the evolution of crimp amplitude upon fiber deformation. Moreover, \(E_f\) in Eq. (A.2) represents the tangent modulus of fiber material, that is of collagen fibrils, depending on entropy-related \(\lambda _m^s\) and energy-related \(\lambda _m^h\) molecular stretches. Hence, it results \(E_f=E_f(\lambda _m^s,\lambda _m^h)\) where:
with molecular tangent modulus \(E_m\) equal to:
depending on entropy-related \(E_m^s\) and energetic-related \(E_m^h\) stiffnesses:
In agreement with integrations in Eq. (A.1), the functional dependences \(\lambda _m^s =\lambda _m^s(\lambda _4)\) and \(\lambda _m^h =\lambda _m^h(\lambda _4)\) are obtained via the interscale compatibility relationships:
with \(\lambda _m^s(1) = \lambda _m^h(1) = 1\), depending on the following interscale relationships derived from equilibrium and compatibility conditions:
where \(dH_F/d\lambda _4\) in Eq. (A.6a) can be found in Eq. (A.3).
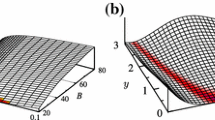
B Additional plots for functions \(\kappa _P(\mathcal {S})\)
Functions \(\kappa _P(\mathcal {S})\), as well as the corresponding objective functions at optimum \(f_{obj}^{P}(\kappa _{P}(\mathcal {S}),\mathcal {S},0.5)\), are reported here for the polynomial and exponential strain-energy terms in Eq. (10), i.e., with \(P=pol\) and exp. Employing indexes i, j in \(\{1, \ldots , n_s\}\) with \(i \ne j\), planes \(\mathbb {S}^{(ij)}\) are introduced in the space of structural features \(\mathcal {S}\). Within \(\mathbb {S}^{(ij)}\), couples \(s_i,s_j \in \mathcal {S}\) vary, while other features \(s_v \ne s_i,s_j\) are kept equal to reference values \(\bar{\mathcal {S}}\) in Table 1, namely
Addressing the polynomial law in Eq. (5a), function \(\kappa _{pol}(\mathcal {S})\) is reported in Figs. 16, 17 and 18 for \(k_1\), \(k_2\) and \(k_3\), respectively. Fitting capabilities are evaluated in Fig. 19 where the value of the objective function at optimum \(f_{obj}^{pol}(\kappa _{pol}(\mathcal {S}),\mathcal {S},0.5)\) is shown.
Function \(\kappa _{exp}(\mathcal {S})\) for the exponential strain-energy term in Eq. (5b) is shown in Figs. 20, 21 and 22 for \(k_1\), \(k_2\) and \(k_3\), respectively. Figure 23 reports the value of the corresponding objective function at optimum \(f_{obj}^{exp}(\kappa _{exp}(\mathcal {S}),\mathcal {S},0.5)\).
Polynomial strain-energy term [see Eq. (5a)]. Trace of function \(k_1 \in \kappa _{pol}(\mathcal {S})\), see Sect. 2.3, on planes \(\mathbb {S}^{(12)}\) (top left), \(\mathbb {S}^{(13)}\) (top right) \(\mathbb {S}^{(14)}\), (middle left), \(\mathbb {S}^{(23)}\) (middle right), \(\mathbb {S}^{(24)}\) (bottom left), and \(\mathbb {S}^{(34)}\) (bottom right) in the space of structural features [see Eq. (B.1)]. Scatter data represent the computed discrete values \(\kappa _P(\mathcal {S}_g)\) for \(\mathcal {S}_g \in \mathbb {S}^{(ij)}\)
Polynomial strain-energy term [see Eq. (5a)]. Trace of function \(k_2 \in \kappa _{pol}(\mathcal {S})\), see Sect. 2.3, on planes \(\mathbb {S}^{(12)}\) (top left), \(\mathbb {S}^{(13)}\) (top right) \(\mathbb {S}^{(14)}\), (middle left), \(\mathbb {S}^{(23)}\) (middle right), \(\mathbb {S}^{(24)}\) (bottom left), and \(\mathbb {S}^{(34)}\) (bottom right) in the space of structural features [see Eq. (B.1)]. Scatter data represent the computed discrete values \(\kappa _P(\mathcal {S}_g)\) for \(\mathcal {S}_g \in \mathbb {S}^{(ij)}\)
Polynomial strain-energy term [see Eq. (5a)]. Trace of function \(k_3 \in \kappa _{pol}(\mathcal {S})\), see Sect. 2.3, on planes \(\mathbb {S}^{(12)}\) (top left), \(\mathbb {S}^{(13)}\) (top right) \(\mathbb {S}^{(14)}\), (middle left), \(\mathbb {S}^{(23)}\) (middle right), \(\mathbb {S}^{(24)}\) (bottom left), and \(\mathbb {S}^{(34)}\) (bottom right) in the space of structural features [see Eq. (B.1)]. Scatter data represent the computed discrete values \(\kappa _P(\mathcal {S}_g)\) for \(\mathcal {S}_g \in \mathbb {S}^{(ij)}\)
Polynomial strain-energy term [see Eq. (5a)]. Trace of the objective function at optimum \(f_{obj}^{pol}(\kappa _{pol}(\mathcal {S}),\mathcal {S},0.5)\) on planes \(\mathbb {S}^{(12)}\) (top left), \(\mathbb {S}^{(13)}\) (top right) \(\mathbb {S}^{(14)}\), (middle left), \(\mathbb {S}^{(23)}\) (middle right), \(\mathbb {S}^{(24)}\) (bottom left), and \(\mathbb {P}^{(34)}\) (bottom right) in the space of structural features [see Eq. (B.1)]. Scatter data represent the computed discrete values corresponding to \(\kappa _P(\mathcal {S}_g)\) for \(\mathcal {S}_g \in \mathbb {S}^{(ij)}\)
Exponential strain-energy term [see Eq. (5b)]. Trace of function \(k_1 \in \kappa _{exp}(\mathcal {S})\), see Sect. 2.3, on planes \(\mathbb {S}^{(12)}\) (top left), \(\mathbb {S}^{(13)}\) (top right) \(\mathbb {S}^{(14)}\), (middle left), \(\mathbb {S}^{(23)}\) (middle right), \(\mathbb {S}^{(24)}\) (bottom left), and \(\mathbb {S}^{(34)}\) (bottom right) in the space of structural features [see Eq. (B.1)]. Scatter data represent the computed discrete values \(\kappa _P(\mathcal {S}_g)\) for \(\mathcal {S}_g \in \mathbb {S}^{(ij)}\)
Exponential strain-energy term [see Eq. (5b)]. Trace of function \(k_2 \in \kappa _{exp}(\mathcal {S})\), see Sect. 2.3, on planes \(\mathbb {S}^{(12)}\) (top left), \(\mathbb {S}^{(13)}\) (top right) \(\mathbb {S}^{(14)}\), (middle left), \(\mathbb {S}^{(23)}\) (middle right), \(\mathbb {S}^{(24)}\) (bottom left), and \(\mathbb {S}^{(34)}\) (bottom right) in the space of structural features [see Eq. (B.1)]. Scatter data represent the computed discrete values \(\kappa _P(\mathcal {S}_g)\) for \(\mathcal {S}_g \in \mathbb {S}^{(ij)}\)
Exponential strain-energy term [see Eq. (5b)]. Trace of function \(k_3 \in \kappa _{exp}(\mathcal {S})\), see Sect. 2.3, on planes \(\mathbb {S}^{(12)}\) (top left), \(\mathbb {S}^{(13)}\) (top right) \(\mathbb {S}^{(14)}\), (middle left), \(\mathbb {S}^{(23)}\) (middle right), \(\mathbb {S}^{(24)}\) (bottom left), and \(\mathbb {S}^{(34)}\) (bottom right) in the space of structural features [see Eq. (B.1)]. Scatter data represent the computed discrete values \(\kappa _P(\mathcal {S}_g)\) for \(\mathcal {S}_g \in \mathbb {S}^{(ij)}\)
Exponential strain-energy term [see Eq. (5b)]. Trace of the objective function at optimum \(f_{obj}^{exp}(\kappa _{exp}(\mathcal {S}),\mathcal {S},0.5)\) on planes \(\mathbb {S}^{(12)}\) (top left), \(\mathbb {S}^{(13)}\) (top right) \(\mathbb {S}^{(14)}\), (middle left), \(\mathbb {S}^{(23)}\) (middle right), \(\mathbb {S}^{(24)}\) (bottom left), and \(\mathbb {S}^{(34)}\) (bottom right) in the space of structural features [see Eq. (B.1)]. Scatter data represent the computed discrete values corresponding to \(\kappa _P(\mathcal {S}_g)\) for \(\mathcal {S}_g \in \mathbb {S}^{(ij)}\)
Rights and permissions
About this article
Cite this article
Marino, M., von Hoegen, M., Schröder, J. et al. Direct and inverse identification of constitutive parameters from the structure of soft tissues. Part 1: micro- and nanostructure of collagen fibers. Biomech Model Mechanobiol 17, 1011–1036 (2018). https://doi.org/10.1007/s10237-018-1009-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-018-1009-8