Abstract
Charrs (Salvelinus) reach their southernmost distribution in Japan, and are uniquely adapted to the short, steep streams of this island archipelago. Southern Asian Dolly Varden (Salvelinus curilus) occur only in Hokkaido Island, whereas white-spotted charr (Salvelinus leucomaenis) range to southern Honshu. Both species diverged from an ancestral lineage during the late Pliocene/early Pleistocene, when lowered sea levels created semi-enclosed water bodies in the seas of Japan and Okhotsk. Genetic analyses showed S. curilus represents the most ancient divergence from the Dolly Varden (Salvelinus malma) - Arctic charr (Salvelinus alpinus) group, and revealed five lineages of S. leucomaenis which align differently than traditional subspecies. Japanese charr display diverse and flexible life histories including anadromous fish with partial migration, and fluvial, adfluvial, and resident forms. In Hokkaido, Dolly Varden are distributed upstream and white-spotted charr downstream. They coexist in narrow sympatric zones through adaptive shifts by Dolly Varden in behavior and morphology that facilitate benthic foraging. Both species hybridize with native and nonnative salmonids, and are displaced from microhabitats and decline in abundance when rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta) invade. Japan streams contain over 95,000 erosion control dams which create short stream fragments (medians ~200 m). This has increased extirpation of charr populations via lower genetic diversity and stochastic and demographic factors. Tributaries provide complex rearing habitats, afford refuges from floods, and supply recruits that sustain populations in mainstem fragments and create metapopulations in connected riverscapes. Charr play central roles in linked stream-riparian food webs, and cause direct and indirect effects that cascade to streambed algae and riparian predators when linkages are disrupted by anthropogenic effects or altered by native parasites. Many charr populations are threatened by habitat fragmentation and introgression or invasion by nonnative forms, but efforts to conserve charr are growing. These include restoring connectivity among pure populations above barriers that prevent invasions, protecting tributary nurseries, and instituting angling regulations to protect headwater populations. Key steps include inventorying pure populations, identifying conservation units, selecting appropriate management based on connectivity and biotic interactions, and engaging stakeholders and youth to engender an ethic for conserving irreplaceable charr lineages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The charrs native to Japan are both beautiful and unique. In autumn 1988, when the senior author attended the International Symposium on Charrs and Masu Salmon in Sapporo, Japan (Noakes 1989), relatively little was known about these fishes. Several of the 85 papers published in the symposium volume concerned the complexities of charr systematics in Japan, but only six other papers and five abstracts addressed the biology, ecology, evolution, or management of Japanese charr (Kawanabe et al. 1989). In contrast, 26 papers and abstracts concerned specifically Arctic charr (Salvelinus alpinus).
Charrs of the genus Salvelinus reach their southernmost distribution in Japan, and are uniquely adapted to aquatic ecosystems of the island archipelago. The region is geologically young and tectonically active, so streams on the relatively narrow island chain are short and steep (Yoshimura et al. 2005), and habitats for charr are often fragmented by natural waterfalls. Charr populations must also contend with wide seasonal variation in flow owing to high annual precipitation (ca. 1–2 m) and flooding from monsoons and typhoons, although other populations occupy more benign environments in lakes or are anadromous in coastal watersheds. This broad array of habitats occupied has led to wide variation in appearance and life history (Morita 2019).
Why is a review of Japanese charr needed now? The 1988 symposium catalyzed much research on charrs in Japan, and collaborations among Japanese and international researchers who became fascinated with these fishes. This led to a proliferation of publications on their evolution, ecology, and conservation during the intervening 35 years, including more than 140 papers reviewed here. This research provides important information relevant to the conservation and management of charrs in Japan and other regions, and yet key challenges remain. The purpose of this paper is to briefly review this literature, and provide an overview of the past and present for native charr in Japan. We then compare them to two charr in North America that span the range of habitat connectivity and biotic interactions found on that continent, and suggest conservation actions that could promote a better future for the unique and beautiful charr in Japan.
Species and forms of charr in Japan
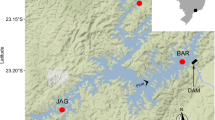
Two species of charr are native to the Japanese archipelago (Fig. 1), southern Asian Dolly Varden (Salvelinus curilus, syn. Salvelinus malma krascheninnikovi; Markevich and Esin 2019), called “Oshorokoma” in Japanese (a word from the original Ainu inhabitants of Hokkaido), and white-spotted charr (Salvelinus leucomaenis) or “Iwana” (meaning rock fish; Morita 2019). In Japan, southern Asian Dolly Varden are distributed only in Hokkaido, but they range north to nearly the tip of Kamchatka, on Sakhalin Island, and along the Russian Far East coast from Uda Bay south to the northern border of South Korea, including in northern tributaries of the Yellow Sea (Mori 1936; Sato 1980; Markevich and Esin 2019). They are now considered a separate species from sister lineages of Dolly Varden (Salvelinus malma) distributed east around the Pacific rim to Washington state in the USA (Yamamoto et al. 2014). Miyabe charr (Salvelinus curilus miyabei), a lacustrine-adapted subspecies consisting of subpopulations that home to separate tributaries (Maekawa 1985), is endemic to Lake Shikaribetsu, Hokkaido (Maekawa 1984).
Distribution of charr in Japan (after Morita 2019; K. Morita images). Southern Asian Dolly Varden (Salvelinus curilus) inhabit only Hokkaido and nearby islands. White-spotted charr (S. leucomaenis) co-occur with them in all these regions except on Shiretoko Peninsula where only Dolly Varden are present. Miyabe charr (S. c. miyabei) occur only in Lake Shikaribetsu, located at the green oval. Locations of introduced populations of each species are also shown
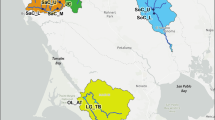
White-spotted charr are distributed throughout most of Honshu Island and all of Hokkaido except Shiretoko Peninsula (Fig. 1). They extend north throughout Sakhalin and to all watersheds of the Kamchatka Peninsula and Sea of Okhotsk, and south along the Sea of Japan coast to northern North Korea (Mori 1936; Markevich and Esin 2019). The traditional taxonomy recognized four subspecies, arrayed from north to south: resident “Ezo-iwana” and anadromous “Amemasu” (rain trout, Salvelinus leucomaenis leucomaenis) in Hokkaido, “Nikko-iwana” (Salvelinus leucomaenis pluvius) in northern Honshu, “Yamato-iwana” and “Kirikuchi” charr (Salvelinus leucomaenis japonicus) in southern Honshu, and “Gogi” (Salvelinus leucomaenis imbrius) in western Honshu (Fig. 2; Kawanabe 1989; Morita 2019). Kirikuchi charr are genetically distinct southernmost populations restricted to seven small, isolated headwater tributaries on the Kii Peninsula (Sato et al. 2010). Introductions have established populations of southern Asian Dolly Varden and Miyabe charr in rivers of southern Hokkaido (Morita 2019) and white-spotted charr farther south in Shikoku and Kyushu islands (Kondou et al. 1999; Inoue et al. 2023).
Distribution of five genetic groups of white-spotted charr determined by Bayesian population assignment of 394 individuals from 48 river systems throughout their range based on 588 single-nucleotide polymorphisms (modified from Yamamoto et al. 2023, with permission). Dashed curves and colored location markers show the distributions of the four traditional subspecies: Salvelinus leucomaenis leucomaenis (blue), S. l. pluvius (yellow), S. l. japonicus (red), and S. l. imbrius (green)
Evolution and zoogeography of charr in Japan
Japan is at or near the center of origin for southern Asian Dolly Varden and white-spotted charr, both of which diverged from an ancestral lineage in the late Pliocene through early Pleistocene epochs (Esin and Markevich 2018). The region was not ice covered during Pleistocene glaciation, but sea level declines of 120–140 m isolated the seas of Japan and Okhotsk as semi-enclosed bodies of brackish or fresh water, promoting genetic divergence. Analysis of mtDNA from throughout the range of Dolly Varden revealed three main lineages (Yamamoto et al. 2014). The western lineage, identified as southern Asian Dolly Varden, represents the most ancient divergence from the ancestral lineage, dated to 3.5–1.2 MYA (Million Years Ago; Esin and Markevich 2018) in a center of origin in the southern Sea of Okhotsk. This lineage is considered basal to the more derived sister clades of central Dolly Varden (Salvelinus malma malma), eastern Dolly Varden (Salvelinus malma lordi), and all Arctic charr.
White-spotted charr diverged from an ancestral lineage about 3.3–2.4 MYA in the Sea of Japan, and later colonized northward (Esin and Markevich 2018). A wide-ranging study of 588 single nucleotide polymorphisms (SNPs) revealed five geographic groups, several with slightly overlapping boundaries (Fig. 2; Yamamoto et al. 2023). However, these do not align perfectly with the four traditional subspecies, especially in central and southern Honshu where, based on SNPs, two groups assign to basins draining east to the Pacific Ocean and two others to basins draining west to the Sea of Japan. The northern lineage is largely congruent with S. l. leucomaenis in Hokkaido and northward, probably because migratory forms allowed gene flow among rivers. However, for the four southern lineages the evidence indicates that repeated colonization and isolation of landlocked forms, coupled with inter-drainage gene flow via stream capture caused by processes like uplift, erosion, and movement of active faults (Masuda et al. 2023) probably explains the complex zoogeography. Given this complex genetic structure and lack of contemporary gene flow, the most appropriate units for conservation are populations within river basins, rather than traditional subspecies (Yamamoto et al. 2023).
Southern Asian Dolly Varden (simply Dolly Varden hereafter, when referring to Japan) and white-spotted charr co-occur in Hokkaido (Fig. 1), although they overlap in relatively short stream segments. Dolly Varden are more cold-adapted and colonized river headwaters, whereas white-spotted charr are more warm-adapted and inhabit downstream segments and lowland habitats (Ishigaki 1984). The two species co-occur only in narrow zones of sympatry, or abut in parapatry, and the boundary is closely aligned with temperature isotherms (Fausch et al. 1994). For example, in streams of northeast Hokkaido on Shiretoko Peninsula, which extends into the cold North Pacific Ocean (mean annual air temperature [MAT] 5 °C), Dolly Varden dominate throughout and white-spotted charr are largely absent. Temperatures warm across the island toward the southwest, and the downstream limit of Dolly Varden and upstream limit of white-spotted charr gradually increase in elevation. On Oshima Peninsula of southwest Hokkaido (MAT 8–9 °C) white-spotted charr dominate and Dolly Varden are native only to one cold spring stream. Yamamoto et al. (2006a) reported evidence of introgression of white-spotted charr mtDNA into some Shiretoko Dolly Varden populations. They suggested hybridization was owing to smaller resident male Dolly Varden fertilizing eggs of a few large anadromous female white-spotted char that entered rivers there, either by paired mating or sneaking.
Individual behavior and ecology
During the 35 years since the Charr and Masu Salmon Symposium, Japanese charr have been studied intensively at the individual level, especially factors shaping their life history, the structure of dominance hierarchies and mechanisms driving foraging mode shifts, interactions with nonnative salmonids, and patterns of and mechanisms causing hybridization with nonnative species.
Life history. Dolly Varden and white-spotted charr have diverse and flexible life history patterns, based on costs of migration, food resources, and temperatures in different habitats. Like other salmonids, charr in Japan display more anadromy to the north and residency to the south (i.e., are landlocked), owing to more resources in marine environments to the north and in freshwaters to the south (Gross et al. 1988). Charrs are iteroparous and express life histories that include anadromous, fluvial, adfluvial, and resident fish, and precocious male parr. In white-spotted charr populations of Hokkaido that have both resident and migratory individuals (i.e., partial migration), males that exceed a threshold size attain sexual maturity, become resident, and fertilize eggs via sneaking behavior. This size threshold is a key life-history trait shaped by tradeoffs among migration costs and the benefits that determine fitness, and is highly variable and potentially locally adapted in salmonine fishes (Sloat et al. 2014). Migration costs increase with distance from the ocean, so male parr mature at a smaller size far from the ocean compared to near, about 145 mm versus 170 mm fork length in tributaries of the Kushiro River in eastern Hokkaido (Fig. 3; Sahashi and Morita 2013). A common garden experiment in the field revealed that male parr also matured at smaller sizes in narrower streams, probably because a greater number of visual refuges in small streams allows greater success for small sneaking males (Morita et al. 2009a).
A threshold model of partial migration in salmonids describing the size at which male parr remain resident vs. migrating to sea (upper right). The length at which half the parr are mature at first reproduction (L50) is reduced in populations farther from the sea (lower right) for both white-spotted charr (WSC; Salvelinus leucomaenis) and masu salmon (MS; Oncorhynchus masou) across 10 populations in the Kushiro River basin of eastern Hokkaido (left), demonstrating the flexibility of this life-history attribute with changes in migration cost (from Sahashi and Morita 2013, with permission)
Anadromy of white-spotted charr in Hokkaido is also complex and flexible. Data from archival tags and analysis of otolith Sr:Ca ratios showed that in eastern Hokkaido these charr made annual migrations, descending rivers to feed in the ocean in May–June and ascending to spawn and overwinter in rivers in August through November (Morita et al. 2013). However, in southwestern Hokkaido some adults can overwinter in the ocean (Kuroda and Miyashita 2022) because of warmer water temperatures brought by ocean currents and opportunities for growth (Takami et al. 1996).
In contrast, most Dolly Varden populations in Hokkaido are predominately resident, although anadromous forms occur in Shiretoko Peninsula (Maekawa 1973; Morita et al. 2009b; Morita 2019). Dolly Varden also show partial migration within a large river system in central Hokkaido. A majority of females adopted a fluvial life history and migrated from tributaries to the mainstem, whereas faster-growing males remained residents in tributaries (Koizumi et al. 2006b; Ayer et al. 2018), a pattern similar to other partially migratory salmonids that migrate to oceans or lakes (e.g., Jonsson and Jonsson 1993; Morita et al. 2014).
Spawning. Charr in Japan typically spawn from late September to late November, depending on water temperature (e.g., Taguchi et al. 2022). In Hokkaido, white-spotted charr spawning peaks in mid-October, about one month earlier than that of Dolly Varden in early November, but the 6-week spawning periods of the two species overlap (Ishigaki 1969; Komiyama 1987). Females excavate redds in water about 10–20 cm deep, with 10–15 cm ∙.s-1 velocity and in 10–30 mm diameter substrate (Kitano and Shimazaki 1995; Nakamura 1999a; Kishi and Tokuhara 2018). Male and female Dolly Varden in an isolated reach of a Shiretoko stream established dominance hierarchies during spawning, and larger females spawned earlier and excavated deeper redds than smaller fish (Kitano 1996). The largest male monopolized three-quarters of all spawning opportunities, whereas only half the subordinate males (the larger individuals) spawned, and several of those only by sneaking. Maekawa et al. (2001) reported a similar mating system among white-spotted charr in central Honshu, where relatively few large males monopolized most spawnings. Genetic analysis of offspring of Miyabe charr revealed that subordinate males that spawned by sneaking (about 1.5 satellite males per spawning event) fertilized about 25% of eggs (Maekawa and Onozato 1986).
Synchronous spawning within populations has been reported for both Dolly Varden (Koizumi and Shimatani 2016) and white-spotted charr (Sato and Harada 2008). An individual-based model of female Dolly Varden spawning dates among populations in 30 small tributaries supported the hypothesis that synchrony can be caused by social interactions rather than environmental factors or predator swamping, and may be a strategy to reduce subdominant males per female and hence egg cannibalism (Koizumi and Shimatani 2016).
Movement and mesohabitat selection. Stream salmonids including charr have been reported to move across stream segments to access habitats for spawning, feeding, and refuge (Gowan and Fausch 1996; Fausch et al. 2002; Rodríguez 2002). Movements of white-spotted charr detected by marking and recapturing fish multiple times per year over 4 years indicated about half remained in the same pools, whereas the other half moved to many different distances up to 1–3 km away (Nakamura et al. 2002). The investigators reported that this leptokurtic distribution of movement distances (cf. Radinger and Wolter 2014) suggested fish selected either sedentary or a range of mobile behaviors. Moreover, given that only 33% of tagged fish were recaptured, fish that moved longer distances outside the study area could have been missed, so the true proportion of long-distance movements could be even greater. In the same stream, more white-spotted charr were recaptured over year-long periods in pools that had refuges beneath boulders than pools without such refuges (Nakamura 2011). In an artificial stream, white-spotted charr selected overhead cover closer to the stream bed (10–20 cm) and cover with lateral visual isolation compared with cover farther from the bed or above the water surface (Terada et al. 2020).
Dominance and foraging behavior. White-spotted and Dolly Varden charr set up interspecific size-structured linear dominance hierarchies with each other, and with resident masu salmon (Oncorhynchus masou) in mesohabitats where they are syntopic in Japanese streams (Nakano and Furukawa-Tanaka 1994; Nakano 1995a; Fausch et al. 2021; see Fausch 2018 for a review of Nakano’s work). Within these local social groups, larger more dominant charr have the highest foraging success and growth, similar to dominance hierarchies of resident red-spotted masu salmon (“Amago”, Oncorhynchus masou ishikawae; Nakano 1995b). Dominance allows larger fish to select focal positions in velocity refuges near the upstream ends of pools, which provide access to the most drifting invertebrates for the least swimming cost and yield the highest net energy intake (Fausch 2014; Fausch et al. 2021).
Research by Shigeru Nakano and collaborators along a Hokkaido riverscape revealed mechanisms allowing coexistence of Dolly Varden and white-spotted charr in syntopy. Knockout experiments and statistical analysis of dominance hierarchies measured by snorkeling showed that for drift-foraging individuals neither species was dominant over the other, so dominance was based on size alone (Fausch et al. 2021). However, as streamflow and drifting invertebrates declined throughout summer, smaller Dolly Varden shifted from drift feeding to picking invertebrates from benthic substrates, a behavior facilitated by their subterminal mouth position (Fig. 4; Nakano and Furukawa-Tanaka 1994). The proportion of Dolly Varden that shifted to benthic foraging increased throughout summer, and the drift-rate threshold at which the shift occurred was nearly identical when measured by field experiment (Fausch et al. 1997) and an observational study over four summers in the same stream (Nakano et al. 1999a). In contrast, white-spotted charr, which have a terminal mouth position, switched to benthic feeding only at very low drift abundance.
Comparison (left) of head and jaw morphology of white-spotted charr (Salvelinus leucomaenis) and southern Asian Dolly Varden (S. curilus; illustration by S. Nakano; from Fausch 2015, with permission). Proportion of benthos foragers (right) of white-spotted charr and Dolly Varden (originally designated S. malma) measured by snorkeling during 10 periods over four summers in a 1.5-km segment of Poroshiri Stream, Hokkaido (from Nakano et al. 1999a, with permission)
The shift to benthic foraging by Dolly Varden is a mechanism of resource partitioning that stabilizes coexistence between the two species in zones of sympatry (Nakano et al. 1999a; Fausch et al. 2021). Moreover, this foraging-mode shift is facilitated by character displacement, whereby Dolly Varden in sympatry develop more subterminal mouths than those in allopatry, resulting in a shift in diet to forage more on benthic organisms (Nakano et al. 2020). This phenotypic plasticity is similar to other charrs across the Holarctic which produce two to seven distinct morphs that fill different trophic niches and habitats (e.g., Skúlason et al. 1999; Bertrand et al. 2008; Chavarie et al. 2015; Markevich et al. 2018). Current evidence indicates that the mechanism stems from environmental influences on gene regulation during development such that, for example, foraging by fry on benthic prey promotes development of blunt head shapes and subterminal jaws (Parsons et al. 2011; reviewed by Skúlason et al. 2019).
Behavioral interactions with other salmonids. White-spotted charr were subordinate to other native and nonnative salmonids in natural and laboratory streams. In a central Honshu stream, individual resident masu salmon (“Yamame”, Oncorhynchus masou masou) dominated charr up to 40% greater in mass, and the salmon grew faster during summer surpassing some charr in dominance rank (Nakano 1995a). In laboratory streams, nonnative brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss) clearly dominated white-spotted charr in individual pairwise contests (Hasegawa et al. 2004). When together, these nonnatives also forced the white-spotted charr and masu salmon to compete for similar microhabitats, and the competitively inferior charr were relegated to inferior foraging positions downstream (Hasegawa and Maekawa 2006). Visual isolation reduced interspecific aggression by brown trout on white-spotted charr, and allowed the charr to forage much more often on drifting prey (Hasegawa and Maekawa 2009). In two small headwater streams in central Honshu that had been invaded by nonnative brown trout and brook charr (Salvelinus fontinalis), about 25% of foraging attempts by white-spotted charr and brook charr were toward the benthos, probably owing to aggression by brown trout (Peterson et al. 2023). Moreover, the diet of white-spotted charr and brook charr overlapped almost completely. These interactions help explain why densities of white-spotted charr declined drastically in the region, and why they disappeared from four other streams studied.
Hybridization with other species. White-spotted and Dolly Varden charr hybridize with several native and nonnative salmonids, producing hybrid swarms that can be sustained by movement. Reports of hybrids with nonnative salmonids are common, including white-spotted charr with brook charr or brown trout (Kitano et al. 2009, 2014; Fukui et al. 2016, 2018; Fukui and Koizumi 2020), Dolly Varden with brook charr (Fukui et al. 2021), and Miyabe charr with introduced masu salmon (Koizumi et al. 2005). The first hybrids are often asymmetrical, between male nonnative salmonids and female native charr (e.g., Kitano et al. 2009, 2014). Nonnative salmonids spawn later than native charr, so early-maturing male nonnative salmonids spawn with native female charr. As hybridization continues, later hybrids can be backcrosses with either species (Fukui et al. 2016).
Hybrids of white-spotted charr and brook charr in Hokkaido are less successful at reproduction but more successful at spreading nonnative genes. Based on parentage analysis, hybrids had much lower reproductive success than either pure species (Fukui et al. 2018). Male hybrids had much lower success than male brook charr owing to less pronounced secondary sexual characteristics (color, height of hump, kype) as well as outbreeding depression caused by other undetermined factors probably operating at multiple life stages. However, hybrid males also had more pronounced characteristics than white-spotted charr males, which increased asymmetrical backcrosses with female white-spotted charr. Despite their lower reproductive success, the survival, growth, and movement of hybrids was as high or higher than the parental species, which is believed to sustain introgression by continuing to spread nonnative genes (Fukui and Koizumi 2020).
Natural hybridization also occurs between white-spotted charr and masu salmon where their ranges overlap in Japan. For example, hybrids have been reported among Kirikuchi charr at the southern extent of the white-spotted charr range. Red-spotted masu salmon are expanding upstream into small, isolated populations of the charr, creating mating opportunities that produce intergeneric hybrids (Sato et al. 2008a, 2010). Similarly, in a river basin in central Honshu, 6% of 153 matings observed across 16 sites were between red-spotted masu salmon and white-spotted charr (Taguchi et al. 2022). Matings occurred during the period of overlap in the spawning seasons (salmon spawn earlier than charr), and females of both species engaged in intergeneric mating behavior. Sneaking behavior by smaller males of the two species is one mechanism causing hybridization.
Population ecology
Research on charr in Japan includes extensive studies over large spatial scales of factors influencing their distributions, as well as intensive studies over decade-long temporal scales and kilometer-long spatial scales of metapopulations and spatial ecology. Each revealed important information about how populations use habitat and persist across multiple spatial scales.
Effects of habitat at mesohabitat to riverscape scales. Swimming ability, thermal tolerance, and condition-specific competitive ability can explain the distribution of charrs and masu salmon in Hokkaido streams. In southern Hokkaido where Dolly Varden are scarce, white-spotted charr are more prevalent upstream and masu salmon downstream (Morita et al. 2016), a pattern of altitudinal niche partitioning correlated with water velocity and swimming stamina. Masu salmon are superior swimmers, and held positions in higher velocities that are prevalent downstream. In contrast, the charr held positions closer to the streambed and at lower velocities, microhabitats prevalent in upstream reaches.
The altitudinal niche partitioning of Dolly Varden and white-spotted charr described earlier (Fausch et al. 1994) can be accounted for by condition-specific competition, whereby different species dominate under different environmental conditions (Dunson and Travis 1991). A laboratory experiment revealed that white-spotted charr survived poorly at lower temperatures, with or without Dolly Varden, helping explain the upstream limit of their distribution and why it shifts with temperature across Hokkaido Island (Taniguchi and Nakano 2000). In contrast, at higher temperatures Dolly Varden were dominated by white-spotted charr and grew poorly in the laboratory and a field experiment (Watz et al. 2019), which can explain the downstream limit of their distribution. Dolly Varden also showed superior swimming performance over white-spotted charr at cold temperatures found upstream, whereas the two were equal at warmer temperatures found downstream, indicating that Dolly Varden can dominate in cold, steep headwaters where maximum velocities are high (Yamada et al. 2020). These equalizing mechanisms of temperature-dependent survival and condition-specific competition create the narrow zones of syntopy in which the stabilizing mechanisms of foraging mode shifts and character displacement by Dolly Varden can further balance fitness and promote coexistence when food resources are scarce (Nakano et al. 2020; Fausch et al. 2021).
Temperature and foraging opportunities also influence the life history and distribution of white-spotted charr in other locations. At the southern end of their distribution in Honshu, white-spotted charr landlocked at high elevations (>2,000 m) spawned earlier, grew slower, lived longer, and were older and larger at maturity compared to those in lower-elevation streams (Tsuboi et al. 2013a). In southwest Hokkaido, the rate of net energy intake calculated from a bioenergetic model as an index of habitat quality accounted for more than three-quarters of the variation (77%) in biomass of drift-feeding salmonids in 20 reaches of 4 streams (Urabe et al. 2010). White-spotted charr composed up to 70% of the biomass among reaches, and masu salmon the rest.
Spatial ecology. The spatial ecology of Japanese charr across mainstems and their tributaries is complex, driven by differences in relative survival, growth, and recruitment in habitats with contrasting disturbance regimes. A decade of research on small populations of Dolly Varden in 100 tributaries of a Hokkaido river using genetics and population surveys revealed a complex metapopulation structure (Koizumi and Maekawa 2004; Koizumi et al. 2006a, 2008; reviewed in Koizumi 2011). Subpopulations were largely independent, based on significant genetic divergence and little demographic synchrony among them, and showed many types of dynamics. These included a few large tributaries that acted as sources, dispersal among smaller ones that could rescue neighbors, and outlying small populations prone to extirpation, often from human effects like culverts and other barriers.
A decade-long study in central Honshu showed the importance of tributaries for sustaining small populations of white-spotted charr in the highly fragmented habitats found there (Tsuboi et al. 2020, 2022). More than 95,000 erosion-control dams that block upstream fish movements have been constructed throughout Japan, about 20,000 in Hokkaido alone (Morita and Yamamoto 2004; Tamate and Hayajiri 2008). In the 1-km stream network studied, these barriers occur about every 75 to 150 m in the mainstem, but not in two tributaries 145 and 280 m long (Fig. 5). Nursery habitat was more prevalent, and survival was higher in the more complex tributary habitats than the simpler mainstem habitats, but the higher density in tributaries resulted in lower growth than in the mainstem (Tsuboi et al. 2020). A matrix metapopulation model of these demographic data showed that the two small 2-m wide tributaries, which made up less than 20% of the total habitat area for charr, were source habitats critical to persistence of the entire metapopulation in this highly fragmented stream network. Without emigration of charr from tributaries to the mainstem, population growth rate would drop far below replacement (lambda = 0.88) and charr would be extirpated from sink habitats in the mainstem (Tsuboi et al. 2022). The mechanisms responsible for higher survival in tributaries are probably greater habitat complexity (more large wood and undercut banks) and providing a refuge from floods (Koizumi et al. 2013). This negative covariation in survival and growth between tributaries and mainstem habitats likely confers resilience on spatially structured charr populations (Terui et al. 2018).
Spatial demographics of white-spotted charr (Salvelinus leucomaenis japonicus; upstream, blue) and red-spotted masu salmon (Oncorhynchus masou ishikawae; downstream, red) in Sabusawa Stream, central Honshu (after Tsuboi et al. 2020, 2022; Y. Kanno images). Images show examples of high (solid lines) and low (dashed) erosion control dams along the mainstem (arrow shows flow direction). Higher survival and recruitment of fish in the tributaries (source habitats) prevent extirpation of populations in the mainstem fragments (sink habitats), despite the tributaries being less than half as wide as the mainstem (ca. 2 m vs. 5 m) and making up only 18% of the habitat area for charr and 12% for salmon
In contrast, in a northern-Hokkaido tributary with no barriers to movement, white-spotted charr displayed a leptokurtic distribution of movement distances over a 2-month summer period. Most fish were recaptured or detected within 60 m of their initial capture location, but the rest moved to many different distances up to 500 m away (Kanno et al. 2020). A sixth of the population (17%) moved downstream within 1–3 days and emigrated from the tributary permanently. Movements were influenced by body length and condition, but this varied across scales. Smaller charr were more likely to emigrate permanently. Within the tributary, larger fish in poorer condition were more likely to move to find better pools, whereas smaller fish in good condition were also more likely to move to find better opportunities for growth (Kanno et al. 2021). Similarly, in a fluvial population of Dolly Varden, migrants from two tributaries to the mainstem were in poorer condition than residents (Ayer et al. 2018).
Invasion biology
Rainbow trout, brook charr, and brown trout were first introduced in Japan starting nearly 150 years ago. They invaded streams and lakes that provide suitable environmental conditions and have displaced native salmonids. Rainbow trout were imported to a fish hatchery near Tokyo in 1877 from California (Japan Bureau of Fisheries 1927), one of the first two introductions of this species beyond North America (MacCrimmon 1971; Fausch et al. 2001). Brook charr were introduced in 1902 (Japan Bureau of Fisheries 1927). There is no official government record for the introduction of brown trout (Maruyama et al. 1987), but possible sources are a shipment of 20,000 eggs by the US Fish Commission in 1892–1893 (MacCrimmon et al. 1970) or inadvertent mixing of eyed eggs with those of rainbow trout or brook charr (Hasegawa 2020). Rainbow trout were introduced to Hokkaido in 1914 and began invading in the early 1970s, and brown trout began invading Hokkaido about 1980 (Takami and Aoyama 1999). Rainbow trout are now found in 36 of 47 prefectures in Japan, but reproduce naturally in only 9 of them (Hasegawa 2020), mainly in Hokkaido (Kitano 2004; Inoue et al. 2009). In contrast, brown trout reproduce in nearly three-quarters of the 18 prefectures where they occur. Brook charr are reported to reproduce in only five locations in Japan (Kitano 2018).
Flooding from monsoons and typhoons may be a key disturbance preventing natural reproduction of all three nonnative salmonid species, especially in Honshu. For example, white-spotted charr tend to reproduce and rear in small tributaries where flooding disturbance is less (Tsuboi et al. 2020, 2022). Rainbow trout invasion is limited or absent in streams with snowmelt and monsoon rain-driven flooding regimes that coincide with fry emergence in early summer (Fausch et al. 2001), and tends to be restricted to streams dominated by groundwater discharge, especially in Hokkaido (Inoue et al. 2009). In contrast, the timing of emergence of brown trout fry, which occurs in April through May, is closer to the February-to-April fry emergence of native white-spotted charr and masu salmon. Therefore, although floods in Japanese rivers begin in April (Fausch et al. 2001), brown trout may be better suited than rainbow trout to invade these habitats (Hasegawa 2020). This may be similar to invasions by nonnative brook charr and brown trout in Colorado, USA, where spring snowmelt floods reduce recruitment somewhat, but populations nevertheless reach high densities (Fausch 2008).
Several studies have revealed mechanisms at various scales by which rainbow trout and brown trout exclude white-spotted charr from habitats. An early analysis at pool and riverscape scales (Morita et al. 2004), followed by sophisticated statistical analysis of a 16-year time series (Morita 2018) showed that introduction and invasion first by brown trout followed by expansion of rainbow trout displaced white-spotted charr from a southwest Hokkaido stream (Fig. 6). Torrential rains during summer when fry of the nonnative trout are small and vulnerable to displacement apparently limited the pace of invasions, based on a stock-recruitment analysis of the full 20 years of data (Morita 2022). Hence, the charr are limited by invasion of the nonnative trout, whereas the nonnatives themselves are limited by environmental factors. In another Hokkaido stream brown trout invaded when a dam was breached, and displaced white-spotted charr over a 9-year period (Hasegawa 2017), but the trout were limited upstream by cold temperatures (Hasegawa and Maekawa 2008a). The charr were relegated to pools with more structural complexity when brown trout were present (Hasegawa and Maekawa 2008b).
Densities of native white-spotted charr (Salvelinus leucomaenis) and nonnative rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta) measured by angling (upper panel; Tsuboi and Morita 2004; J. Tsuboi, National Research Institute of Fisheries Science, Nikko, Japan, written communication of unpublished data, 17 June 2023) and snorkeling (middle panel; modified from Morita 2022, with permission) over two decades in the Hekirichi River, southern Hokkaido (lower panel; K. Morita image). Both sampling methods revealed similar declines in the proportion of charr and increases in nonnative trout
In an interesting twist, white-spotted charr have become “native invaders” (Carey et al. 2012) in several locations in the southern islands of Shikoku and Kyushu, beyond their native range (Fig. 1; Kondou et al. 1999; Inoue et al. 2023). In three tributaries of the Shikoku stream, white-spotted charr invaded upstream to an erosion-control dam and a natural waterfall over a 15-year period, using headwater habitats similar to those in their native range and displacing the native red-spotted masu salmon there. Spawning habitat is limited in headwater habitats, so one possible mechanism is superimposition of salmon redds by the later-spawning charr (Togaki et al. 2023).
Community and ecosystem ecology
Pioneering studies by Shigeru Nakano and his colleagues using large-scale manipulations in the field to measure effects of charr on reciprocal stream-forest food web interactions made major advances in stream and fish ecology (Fausch 2018). This research inspired other researchers to use a similar approach to study the direct and indirect effects of nonnative trout and nematomorph parasites on terrestrial and aquatic invertebrates that drive these food web effects.
Charr piscivory. White-spotted charr prey on hatchery-reared fry of anadromous chum salmon (Oncorhynchus keta) and masu salmon that are stocked in Hokkaido rivers, selecting the smaller individuals (Hasegawa et al. 2021). These fry provide a pulsed food resource that temporarily increases the food intake and growth rate of the charr (Hasegawa and Fukui 2022). Experiments revealed that predation on masu salmon fry could be reduced substantially if fry were raised to exceed 40% of the length of white-spotted charr predators (Miyamoto and Araki 2017).
Role in food webs. Dolly Varden and white-spotted charr have strong effects on linked stream-riparian food webs through predation on aquatic insects that emerge into riparian zones, and terrestrial arthropods that fall into streams. Terrestrial arthropods are an important resource subsidy for charr, making up 50% of the total annual energy intake for white-spotted charr and 23% for Dolly Varden in a southern Hokkaido stream (Nakano and Murakami 2001). When Dolly Varden alone were enclosed in 25-m long reaches during a field experiment in the same stream, more than half their summer diet was terrestrial arthropods (Nakano et al. 1999b).
Shigeru Nakano and his colleagues pioneered using mesh greenhouses to experimentally exclude this terrestrial arthropod subsidy (Fig. 7), which caused Dolly Varden charr to initiate cascading food web interactions sufficient to influence riparian predators through indirect effects (Nakano et al. 1999b; Baxter et al. 2004). When Dolly Varden were enclosed in fenced reaches covered with greenhouses, they switched to foraging on benthic invertebrates, reducing their biomass by 46%. This caused benthic algae to bloom (a 49% increase in biomass), and emergence of adult aquatic insects to decrease by 79% (Fausch et al. 2010). Loss of insect emergence, such as virtually eliminating it using mesh greenhouses, caused most riparian spiders that rely entirely on this subsidy to leave or die (an 83% decline; see also Kato et al. 2003), and bat foraging to decline drastically (Fukui et al. 2006). In another experiment where fish were free to move, half the biomass of salmonids (including both charr species) left reaches covered by mesh greenhouses compared to control reaches (Kawaguchi et al. 2003), showing the importance of the terrestrial arthropod subsidy.
Effects of charr in food webs subjected to habitat alteration and nonnative trout invasions. Shigeru Nakano pioneered the use of mesh greenhouses (upper left) to mimic habitat alteration of riparian zones that supply terrestrial arthropods to streams. Nonnative rainbow trout (Oncorhynchus mykiss; lower left) have a similar effect, usurping this prey subsidy from Dolly Varden (Salvelinus curilus). This treatment resulted in cascading effects throughout the linked stream-riparian food web (shown in red), ultimately causing reduction of riparian spiders by two-thirds, and an increase in benthic algae by two-thirds. Figure and images are from Baxter et al. (2004, with permission)
Nonnative rainbow trout had a similar effect as the greenhouses, usurping most terrestrial invertebrates and dominating Dolly Varden, which also caused the charr to shift to benthic feeding (Nakano et al. 1999c; Baxter et al. 2004, 2007). Much like the effect of greenhouses, adding rainbow trout resulted in a 36% reduction in benthic herbivores, a 67% increase in streambed algae, a 35% reduction in emerging aquatic insects, and a 65% decrease in riparian spiders (Fig. 7; Baxter et al. 2004; Fausch et al. 2010).
Integrating all the information from studies of this stream-riparian ecosystem in a systems model revealed that the seasonal subsidies of terrestrial invertebrates favored rainbow trout and white-spotted charr, which resulted in exclusion of Dolly Varden. Terrestrial prey inputs in summer and fall supported 95% of the trophic basis of production of rainbow trout and white-spotted charr, increasing their abundance and biomass (Marcarelli et al. 2020). In turn, during winter and spring these fish depleted nearly all the production of benthic insects, which supported about 75% of the production of these two fish species during these seasons. Overall, this forced Dolly Varden to compete with sculpin for scarce benthic prey in summer, so production of these charr fell to nearly zero. The lack of food resources caused by invasion of rainbow trout and their dominance over Dolly Varden likely explains why the nonnative trout have excluded these charr from various spring-fed streams in Hokkaido (Baxter et al. 2007).
Several parasites also can change the foraging of charr, either indirectly or directly, affecting stream food webs. In southern Honshu, nematomorph (horsehair worm) parasites enhance the terrestrial arthropod subsidy to Kirikuchi charr by manipulating behavior of orthopterans (camel crickets and grasshoppers). The final adult stage of the horsehair worm causes these large terrestrial insects to jump into streams to reach the reproductive habitat of the nematomorph during mid-August to mid-November, supplying 60% of the annual energy intake of charr in one stream (Fig. 8; Sato et al. 2008b, 2011a) and accounting for the variation in terrestrial subsidies and charr and resident masu salmon diets across nine streams studied (Sato et al. 2011b). In turn, a manipulative field experiment showed that this subsidy reduced red-spotted masu salmon foraging on benthic invertebrates. As a result, invertebrate grazers reduced periphyton by approximately 50% in a trophic cascade, and shredders increased leaf breakdown rate by 30% compared to the treatment where crickets were excluded (Sato et al. 2012). Another parasite, a mouth-attaching copepod (genus Salmincola) reduced condition of Dolly Varden in Shiretoko Peninsula streams in Hokkaido (Hasegawa et al. 2022), and could potentially alter food-web interactions by changing charr foraging behavior.
Influence of horsehair worm (nematomorph) parasites in manipulating behavior of terrestrial orthopterans that jump into streams and are eaten by Kirikuchi charr (Salvelinus leucomaenis japonicus) in southern Honshu (modified from Sato et al. 2011a, with permission). Percentages of the annual energy intake of charr made up by infected orthopterans, other terrestrial invertebrates, and benthic invertebrates are shown. A field experiment showed that this subsidy reduces charr predation on benthic insect shredders and grazers, thereby increasing leaf breakdown rate and reducing periphyton
Anthropogenic effects
A series of detailed studies of white-spotted charr populations isolated in short stream fragments are among the best examples worldwide showing effects of habitat fragmentation on stream fish populations. Coupled with studies on the effects of forestry, hydropower production, roads, stocking hatchery fish, recreational angling, and climate change, there is now a rich literature on effects of human activities on Japanese charr.
Habitat fragmentation. Many streams throughout Japan are highly fragmented by dams of various heights, most constructed for erosion control and nearly all insurmountable by charr. These can cause extirpation of charr populations isolated above them from genetic deterioration, demographic factors, and stochastic environmental events, as well as overfishing. Effective population size (Ne) is less than 50 individuals in many fragmented charr populations (e.g., Sato and Harada 2008; Yamamoto et al. 2016, 2019), which can lead to further genetic diversity loss, inbreeding, and consequently increased risk of extirpation. Three case studies highlight the range of effects.
Extensive studies of small, isolated populations of white-spotted charr in Oshima Peninsula showed increased rates of extirpation above dams, owing to reduced egg production, lower growth rates, and stochastic demographic and environmental factors (Morita et al. 2000, 2009c, 2019; Morita and Yamamoto 2002). Predictions from an early incidence function based on watershed area of extirpations in smaller fragments (Morita and Yamamoto 2002) were confirmed by sampling 15 years later (Morita et al. 2019), and an updated incidence function was nearly identical to the original model. In contrast, a fish ladder installed in one stream allowed recolonization of a fishless fragment by charr and masu salmon.
Comparison of the genetic structure of white-spotted charr populations above and below dams at 23 of these locations over a 15-year interval showed that charr populations above dams had lower genetic diversity (heterozygosity and allelic richness) than those below dams, and in half the cases genetic diversity and effective population size declined through time (Yamamoto et al. 2019). Populations above dams also had higher developmental instability of morphological characters compared to connected populations (Yamamoto et al. 2013a). The reduced genetic diversity in restricted habitats above dams was primarily owing to founder effects just after fragmentation and random genetic drift. Effective population sizes were less than 50, leading to risks of inbreeding to add to those from stochastic environmental effects such as severe floods and debris flows resulting from typhoons (Sato 2006a) and predicted demographic effects (Morita and Yokota 2002).
In a second case study of Fuji River headwater tributaries in central Honshu, more than 30,000 dams have been constructed, most since 1980. Tsuboi et al. (2010) reported that 356 dams had been constructed in 27 streams they surveyed, a density of 3.8 per km. Native red-spotted masu salmon (Amago) and white-spotted charr (Yamato-iwana) are sympatric in this watershed, but genetically pure charr are found only in small headwater streams above the dams (Tsuboi et al. 2010, 2013b) owing to widespread stocking of nonnative Nikko-iwana. Habitat fragmentation apparently disrupts coexistence of native salmon and charr, because in four of five streams with both species in syntopy one species became extirpated from the 1970s to 2004, and in the intensively studied Sabusawa Stream Amago was extirpated or declined over 4 years in the upper sections (Tsuboi et al. 2010). The two native species were found in only about 20 streams each in the entire watershed, and each occupied only 1–2% of thermally suitable habitat. A Population Viability Analysis (PVA) based on empirical data for survival, maturity, and fecundity yielded estimates of probability of extirpation in 100 years of 48% for the charr (Tsuboi et al. 2013b). However, a modest increase in adult survival rates reduced this predicted extirpation probability to only 5.5%, which could be achieved by saving only two mature female charr per year from angling mortality in the longest fragments of Sabusawa Stream that measured 250–400 m between dams.
A third case study in the Totsu River headwaters on the Kii Peninsula in south-central Honshu showed that endangered Kirikuchi charr (Sato 2007) were lost from most of their range owing to habitat destruction, overharvest, and hybridization with nonnative Nikko-iwana and Ezo-iwana. Kirikuchi charr are genetically distinct from other subspecies and forms. In addition, two genetically pure populations only 40 km apart in reaches isolated above natural waterfalls and erosion-control dams are clearly different genetically, potentially owing to genetic drift during long isolation, founder effects, or invasion by different anadromous populations (Sato et al. 2010; Yamamoto et al. 2023). Effective population sizes were low, ranging only 38–68 and 55–158 for the two populations based on a stochastic individual-based PVA model. An estimated 20–50% of their genetic variation will be lost over 200 years, reducing it far below the 90–95% heterozygosity needed for sustained population viability (Sato and Harada 2008). The incidence of morphological deformities was high (3–17% over 3 years), and annual survival rates were about half those of intact fish (Sato 2006b; see also Morita and Yamamoto 2000, Morita et al. 2009c, Yamamoto et al. 2013a). Sato et al. (2010) proposed that because fish in each stream originated from one population, pure fish could be translocated among isolated reaches within each stream to increase genetic diversity.
Land and water uses. Forestry, hydropower production, and road construction also reduce charr populations by increasing road density and erosion control dams, and reducing flows, all of which degrade habitat. Forestry plantations are common in Japan, and forestry activities can increase the number of erosion control dams and road density in the riparian zone, which reduces canopy cover. In streams of Shiretoko Peninsula, one of the most undisturbed regions in Japan, plantations led to higher stream temperatures and lower biomass of periphyton and Dolly Varden (Kishi et al. 2004). Streams on the peninsula with Dolly Varden have been fragmented by 238 dams without fish ladders. Streams with more dams had higher temperatures, more shallow habitats with fine sediment, and fewer Dolly Varden than reference streams with few or no dams (Kishi and Maekawa 2009). In contrast, in southwestern Japan where native vegetation is deciduous broadleaved forest, plantations of coniferous cedar and cypress exclude most light from smaller streams year-round. This leads to little periphyton production during the leaf-free season (fall through spring) compared to streams draining deciduous forests (M. Inoue, pers. observation). Furthermore, terrestrial arthropod input from conifer plantations is much lower than from natural deciduous forests, resulting in lower fish abundance in streams draining conifer plantation (Inoue et al. 2013).
Depletion of flow for hydropower production, and roads constructed to access the dams also degrade charr habitats. In a central Japan stream, depletion of 83% of the flow from a 1-km segment during about 9 months per year for hydropower production resulted in a 33% decrease in density of age-1 and older white-spotted charr, and a 42% reduction in biomass (Nakamura 2013). A 4-yr mark-recapture study revealed that more than half the charr recaptured had remained in the same pools in a 3-km free-flowing section upstream compared to only about 30–40% of those in the section where flow was depleted (Nakamura et al. 2002). Throughout this segment, white-spotted charr declined to low levels after rocks and sand were dumped into the channel during road construction, which filled pools and homogenized habitat, although abundance rebounded rapidly after angling was prohibited (Nakamura et al. 1994, 2001).
Stocking hatchery charr. Stocked charr are larger than wild fish and can dominate them, and likely reduce their survival, but the stocked charr themselves survive poorly. Both in enclosures and two natural headwater streams of central Japan, white-spotted charr that were wild or semi-wild (stocked in the stream as eyed eggs) dominated genetically similar hatchery charr when fish were of equal length. However, the hatchery charr stocked in streams are 14% larger than the semi-wild fish, and dominated wild individuals in enclosures when the hatchery charr were more than 11% larger (Nakamura and Doi 2014). Moreover, the apparent survival rate of hybrids between wild native males and domesticated females was 2.5 times that of domesticated charr in five isolated sections of a central Honshu mountain watershed (Yamashita et al. 2020). This represents a worst-case scenario, where larger hatchery-reared charr dominate less domesticated semi-wild fish and yet survive poorly themselves (Weber and Fausch 2003).
Response to recreational angling. Both charr species are susceptible to traditional Japanese fishing in mountain streams, so regulations are required to sustain populations. Dolly Varden and white-spotted charr, along with resident masu salmon, provide popular sport fisheries in Japan, especially for anglers that practice traditional “Keiryu” (mountain stream) fishing using live benthic invertebrates as bait. White-spotted charr are more vulnerable to angling than sympatric Dolly Varden, probably because white-spotted charr orient more toward the water column and water surface whereas Dolly Varden feed more frequently from the benthos when syntopic (Tsuboi et al. 2021).
Larger charr are more vulnerable to angling than smaller ones, and fish caught once are more likely to be caught again (Tsuboi and Morita 2004; Tsuboi et al. 2021). A moderate effort of bait fishing (0.5 min ∙ m-2 of pool area) captured a third or more of charr in Hokkaido mountain streams, on average, and catchability increased as density decreased (Tsuboi et al. 2021; Hasegawa and Koizumi 2023). Hence, populations of charr in small streams are prone to collapse with unregulated bait fishing, so regulations on harvest will be required to sustain sport fisheries.
Miyabe charr in Lake Shikaribetsu are a popular sport fish, but by 2000 had declined owing to overharvest. Regulations that restrict fishing to about half the lake, restrict gear, and allow catch-and-release angling during only 50 days per year have increased abundance more than seven times and nearly tripled catch rates (Yoshiyama et al. 2017, 2023). Anglers attracted from throughout Japan provide a strong economic boost to the local economy (Yoshiyama et al. 2018).
Climate change. Charr are stenothermal fishes, highly susceptible to changes in water temperature. In an early analysis based on simple models of climate warming effects on groundwater temperature, Nakano et al. (1996) predicted that an increase of 2 °C in air temperature would lead to loss of more than 65% of habitat for Dolly Varden and 20% for white-spotted charr in the Japanese archipelago. Moreover, their analysis showed that as charr retreat to higher elevations they become restricted to shorter habitat fragments in headwater streams, further increasing risk of extirpation. Empirical data show that stream drying can also eliminate charr, such as a 2014 drought in central Japan that killed 5% of white-spotted charr and 9% of red-spotted masu salmon in one headwater tributary (Kishi et al. 2020).
Conservation and restoration of charr
As in most countries, management of charr in Japan occurs on a template of habitat degradation from past activities, ongoing stocking of hatchery fish, invasions of nonnative species, and growing influences of climate change. It also must operate within constraints imposed by local traditions and a complex mixture of jurisdictions over land, water, and fish populations. Overlain on a long history of intensive land and water use, especially in Honshu, various management actions have been used to conserve native charr, and new methods are being proposed.
Status of charr in Japan. A few rare forms of charr in Japan are considered vulnerable to extinction at the international or national level, and others are listed as such in certain prefectures. The seven remaining populations of Kirikuchi charr are the southernmost in the genus Salvelinus, and are listed as Endangered by the International Union for Conservation of Nature (Sato 2007) and on the Japan Red List (Ministry of the Environment of Japan 2020). The lineage of white-spotted charr called Gogi (Salvelinus leucomaenis imbrius), all southern Asian Dolly Varden, and Miyabe charr in Lake Shikaribetsu are listed as Vulnerable on the Japan Red List, indicating an increasing risk of extinction. Most prefectures with native white-spotted charr list their subspecies on the regional Red List as at some level of extinction risk (S. l. pluvius are listed in 15 prefectures, and S. l. japonicus and S. l. imbrius are listed in 5 prefectures each; Japan Red Data Retrieval System 2023). However, charr populations are often not protected in national parks in Japan, where only scenery is protected (Nakamura et al. 1994), and there is no systematic accounting of protected charr populations in Japan.
Management to conserve and restore native charr. Four key threats affecting many native charr populations in Japan are habitat fragmentation by erosion-control dams which causes loss of genetic diversity and increased risk of extirpation, introgression from stocking hatchery charr, invasions by nonnative salmonids, and overfishing (Morita 2019). Adding fishways to dams can be effective in restoring upstream migration (Morita et al. 2019) and gene flow and genetic diversity (Yamamoto et al. 2006b). Fishways have been constructed on some dams in various watersheds, including portable fishways and some handmade by citizens (Machida et al. 2019; Sato et al. 2021). Narrow slits have been cut in other dams to allow fish passage (Morita 2019). Nevertheless, the more than 95,000 dams constructed throughout Japan resulted in stream fragments with medians of only 94 and 375 m in two Honshu basins (Fig. 9; Nakamura 2001; Endou et al. 2006), and only 140 m even in relatively undisturbed watersheds of Shiretoko Peninsula (Kishi and Maekawa 2009; but see Nakamura and Komiyama 2010). Nevertheless, breaching barriers must be considered carefully, because in many watersheds specific dams prevent invasions from downstream by hybridized charr or nonnative salmonids, a classic invasion-isolation tradeoff (Fausch et al. 2009).
Cumulative frequency distributions of stream habitat fragment lengths (m) occupied by four native charr in Japan and North America. Points for 34 frequency bins were calculated from the empirical data and used to fit logistic curves. An empirical median is shown for each curve. Data for each species and region are from the following sources: white-spotted charr [WSC; Salvelinus leucomaenis ] in central Honshu (Endou et al. 2006; range: 16–3,245 m, N = 154), Dolly Varden [DV; S. curilus] on Shiretoko Peninsula (Kishi and Maekawa 2009; range: 10–8,130 m, N = 144), WSC in northern Honshu (Nakamura 2001; range: 125–3,750 m, N = 77), southern Appalachian brook charr (S. fontinalis; M. Kulp, National Park Service, Gatlinburg, Tennessee, USA, written communication of GIS layers, 25 April 2023; range: 65–11,758 m, N = 152), and bull charr (S. confluentus) in Oregon watersheds (Chelgren et al. 2023; range: 1,400–64,322 m, N = 104). Fragment lengths from Nakamura (2001) were interpolated from a histogram and so are approximate
Artificial spawning channels, and adding gravel to natural channels, are another form of habitat restoration, and have been successful in attracting white-spotted charr to construct redds, and spawn and incubate eggs in several central Honshu mountain streams (Nakamura 1999b; Kishi and Tokuhara 2017). Many anglers participated in creating these spawning sites, which helps engage local stakeholders in conserving wild fish.
In Honshu, hatchery white-spotted charr (primarily Nikko-iwana) have been widely stocked by anglers and fisheries cooperatives, causing introgression with other native forms (e.g., Sato et al. 2010). In Yamanashi Prefecture, anglers are prohibited from stocking salmonids, and in Sabusawa Stream Nikko-iwana were eradicated to help conserve native Yamato-iwana. In contrast, for pure populations isolated in short stream fragments genetic restoration (Whiteley et al. 2015) is possible, as demonstrated by translocating white-spotted charr from below dams into isolated populations above barriers in two Hokkaido streams (Yamamoto et al. 2006b). Genetic diversity was restored to the same levels as pure fish below barriers by rapid introgression of genes into the isolated populations.
Nonnative rainbow and brown trout are widespread in Japan and cannot be eradicated from most habitats, but regulations to prevent stocking them are rare. Rainbow trout are officially stocked in 31 of 47 prefectures throughout Japan, and regulations prohibit their stocking only in Saga prefecture in Kyushu (Kitano 2004). Regulations prevent stocking brown trout and brook charr only in Hokkaido and Shiga prefecture around Lake Biwa, but no regulations prevent moving those stocked previously. Artificial ponds for angling are common sources of salmonid invasions because nonnative species and hybrids are widely stocked and can easily escape. Smoltified rainbow trout and anadromous brown trout have been reported in Japan (Hasegawa 2020), which could create another vector causing widespread invasions into new rivers. Direct eradication is difficult but might be feasible in small streams (but see Meyer et al. 2006), or by targeting spawning or winter aggregations (Kitano 2004; Koizumi et al. 2017; Furusawa et al. 2022). In cases where an invasive species is already widespread, another option could be managing physical and biological factors that modify impacts of invaders, such as modifying temperature and flow regimes to hamper nonnative salmonids (Dunham et al. 2020).
Fishing has long been prohibited in some small headwater tributaries in Japan (termed “Tanezawa”), primarily for rare forms of white-spotted charr such as Kirikuchi, Gogi, and “Nagaremon”, a unique morphotype (Nakamura et al. 1994; Kikko et al. 2022). Spatially arranged fisheries regulations can be a highly successful management option for addressing overfishing and conserving native salmonids. For example, in the Zako River in central Honshu, high catch rates of native salmonids have been sustained in the mainstem by preventing harvest in a group of small tributaries that apparently supply recruits (Yamamoto et al. 2013b), further supporting the importance of tributary habitats (Tsuboi et al. 2022). Nakamura (2008; Nakamura et al. 2012) proposed a general plan for conserving native charr and salmon in watersheds by creating three zones (Fig. 10): 1) selected headwaters closed to fishing or designated catch-and-release, and not stocked with hatchery charr, 2) middle segments under normal angling regulations, and 3) downstream segments where domesticated fish are stocked as eggs, fry, or adults for angling. Some fisheries cooperatives are now implementing such plans.
A general scheme for managing and conserving native charr in Japan streams (modified from Nakamura 2008, with permission; Nakamura et al. 2012). Headwaters are designated as conservation zones for pure native charr and salmon, with some tributaries protected by fishing bans or catch-and-release regulations. Downstream segments are under normal angling regulations, and some zones are designated for put-and-take angling of stocked adult fish
Considering a future for charr in Japan
Diligent research by Japanese and some foreign fish biologists has generated a large body of information on the charr of Japan since the 1988 symposium, yet progress to conserve the diversity of forms and their habitats has been modest. Some of this is owing to the declining human population and interest in charr in rural areas of Japan, coupled with reduced numbers of fisheries staff to conduct research or management. In addition, as in many regions it takes time for research to influence management, and some current actions conflict with conservation of native charr. Here we compare the management of charr in Japan to two charr species in North America, and consider what steps and new information might advance the conservation of Japanese charr.
Bull charr (Salvelinus confluentus) of the Pacific Northwest and brook charr of the southern Appalachian Mountains represent ends of a spectrum for charr conservation in North America. Bull charr are listed as a Threatened species under the U.S. Endangered Species Act, and the approximately 600 populations in the conterminous USA typically occupy drainages from less than 10 km to over 200 km in spatial extent (i.e., length of the longest continuous river segment, Figs. 9, 11; U.S. Fish and Wildlife Service 2015). The native range of bull charr includes the Pacific coast from southern Oregon to southeast Alaska, and inland to the Yukon, Northwest Territories, Alberta, Montana, Idaho, and Nevada (Dunham et al. 2008). In the conterminous USA populations are arranged in 6 management units and 109 core areas (subwatersheds) for conservation and recovery, based on substantial genetic diversity among populations and relatively low diversity and low effective population sizes within them (e.g., 76% of U.S. populations sampled had Ne < 50; Ardren et al. 2011). No hatchery bull charr are stocked for angling but some populations are invaded by brook charr causing risk of introgression, especially by sneaking males (Kitano et al. 1994; Kanda et al. 2002). Many populations have been inventoried and evaluated for purity (U.S. Fish and Wildlife Service 2015). Their threatened status has fostered active management for more than 25 years, including removing barriers to increase connectivity (Brenkman et al. 2019), removing nonnative brook charr (Buktenica et al. 2013), translocating pure fish into watersheds where they were lost (Hayes and Banish 2017; Benjamin et al. 2019), and modeling climate change risks (Falke et al. 2015; Isaak and Young 2023). Recent research using decision support tools to analyze tradeoffs showed that the greatest benefits will be from targeted translocations coupled with enhancing diverse life histories and spawning and rearing habitat to allow adapting to rather than removing nonnative brook charr (Brignon et al. 2018; Dunham et al. 2022). Nevertheless, removing brook charr can be effective in some cases (Buktenica et al. 2013).
Comparison of typical domains of spatial connectivity and biotic interactions for bull charr (Salvelinus confluentus) and southern Appalachian brook charr (BK; S. fontinalis) in North America and white-spotted charr (WSC; S. leucomaenis) and Dolly Varden (S. curilus) in Japan. Genetically pure populations number about 600 for bull charr and at least 500 for southern Appalachian brook charr, but most charr populations in Japan have not been inventoried. Current and planned management actions are described for each species. A conservation goal represented by the arrow is to move populations towards the lower right, by restoring habitats with greater connectivity and fewer biotic interactions with nonnative species and forms
Brook charr are widespread throughout eastern North America, occurring from Labrador, Canada inland to Minnesota, USA and south along the Adirondack and Appalachian mountains to northern Georgia. Similar to white-spotted charr, the phylogeography of brook charr is complex, and populations in the southern Appalachian Mountains are highly divergent from those to the north (Kazyak et al. 2022). They are also highly differentiated from each other, apparently owing to diversity lost through genetic drift in the small headwater stream fragments they inhabit. Introductions of nonnative rainbow trout and brown trout began about 140 years ago, along with stocking hatchery brook charr of northern origin, thereby eliminating pure native charr from most segments downstream from barriers (Hudy et al. 2008). As a result, non-introgressed southern Appalachian brook charr occur in short stream segments about 0.2–10 km in extent above natural waterfalls or barriers that protect them from nonnative trout invasions (Figs. 9, 11). Like charr in Japan, effective population sizes are small, 60% of them less than 30. Many populations have been inventoried and undergone genetic analysis for purity (Kazyak et al. 2018; Pregler et al. 2018), of which at least 500 represent native lineages (M. Kulp and J. Rash, pers. communication). Active management to remove nonnative trout and translocate pure southern brook charr into headwater enclaves has been ongoing for 45 years (Larson and Moore 1985).
By contrast to these charr in North America, most populations of Dolly Varden and white-spotted charr in Japan are isolated in short stream fragments with medians of about 100–375 m and maxima of 3 to 8 km in total extent (Fig. 9), although white-spotted charr in Hokkaido occupy some connected rivers that are much longer (Fig. 11). Most white-spotted charr populations in Honshu have been subjected to introgressive hybridization from nonnative lineages stocked for angling, or competition with masu salmon stocked beyond their native ranges, and some are threatened by invasions of nonnative rainbow trout and brown trout (Morita 2019; Hasegawa 2020). Populations have been inventoried in only a few regions, and except for Kirikuchi charr (Sato et al. 2010) few populations have been tested for genetic purity. However, in recent years the genetic characteristics of wild charr populations are becoming better understood, and new nation-wide databases of genetic characteristics are being created (e.g., Yamamoto et al. 2004, 2006a, 2014, 2023). Stocking by local fisheries cooperatives and by some anglers is ongoing, but some cooperatives are now focusing on sustaining endemic charr lineages and their local adaptations. Recent efforts are beginning to increase connectivity for pure populations by removing barriers or installing fishways while retaining downstream barriers that prevent invasions by nonnative forms, and a modest number of translocations for reintroduction or genetic restoration above barriers have been accomplished or are planned. Angling regulations are set by local fisheries cooperatives, which typically close fishing from October through February and set minimum size limits, but bag limits are either unrestricted or limited only to large numbers (e.g., 20 stocked fish per day; Rahel and Taniguchi 2019). Catch-and-release regulations are relatively uncommon in Japan, but prohibiting fishing in selected tributaries has been found effective at increasing adult fish densities (Yamashita et al. 2023).
Given this, what options are available for sustaining lineages of native charr in Japan into the future? For regions where pure native charr are less common, such as throughout most of Honshu, a first step is to inventory charr populations and their genetic purity, as well as the barriers that isolate them in each drainage basin so that management can be prioritized (Fig. 12). At present, in only two prefectures have comprehensive surveys been completed (Tochigi and Yamanashi) and an eDNA survey is planned in one of the basins as a follow up after two decades. However, local anglers and others may have extensive knowledge, so there may be opportunities to engage these stakeholders in innovative ways to collect or provide data on species distribution and abundance (e.g., Zhou et al. 2022; Chelgren et al. 2023).
A second step is to identify appropriate groups of populations for management, often grouped into conservation units that are substantially reproductively isolated and represent important evolutionary legacies of the species (Waples 1995). The phylogeography of white-spotted charr indicated five such evolutionarily significant units (Fig. 2), and revealed that within each one, drainage basins are the appropriate units of conservation owing to substantial genetic diversity among them (Yamamoto et al. 2023). A similar conclusion was reached for bull charr (U.S. Fish and Wildlife Service 2015) and southern Appalachian brook charr (Kazyak et al. 2022). Though less studied, Dolly Varden also vary across Hokkaido (Yamamoto et al. 2014, 2021), including the unique Miyabe charr, and in most regions have been long isolated in headwater segments, so drainage basins are also the appropriate unit for their conservation. Once conservation units are designated, a key step will be to educate members of fisheries cooperatives, local anglers, and the public about the importance of this genetic diversity and the adaptive life-history variation it supports, and to involve all stakeholders in setting guidelines or regulations to prevent introgression of the remaining pure lineages from stocking hatchery charr.
A third step is to consider options for managing populations within conservation units, which must necessarily consider key factors of the spatial scale of connectivity and biotic interactions (Figs. 11, 12). For example, when stocking has been widespread and invasions are prevalent, as for white-spotted charr in Honshu and southern Appalachian brook charr, downstream barriers that isolate these pure charr populations must remain in place to protect them from nonnative salmonids. Under this scenario, management options include removing nonnatives and introgressed forms, optimizing the number, size, and distribution of these habitat fragments and their tributaries, and restoring habitat quality (Fausch et al. 2009). In contrast, downstream from barriers and in larger watersheds already invaded by nonnative brown trout or rainbow trout where controlling them is not feasible, their impacts might be modified by enhancing the expression of charr life history diversity (e.g., fluvial, adfluvial, and partial-migration life histories) and managing the physical environment to favor the native charr (e.g., restoring coldwater refuges, managing flow regimes), as has been proposed for bull charr (Dunham et al. 2020, 2022). This life-history diversity will be increasingly important for reducing risks of extirpation in a changing environment, as well as providing many other benefits to ecosystems.
The fourth step, and most important, is the early and extensive engagement of stakeholders in the management process, and education of children and youth about the importance of evolutionary legacies of their local biota like native charr. Illegal stocking of nonnative charr or trout by anglers or others can reverse expensive and time-consuming management actions like those described above, so it is critical that this step be taken early and continued throughout (Bamzai-Dodson et al. 2021; Brignon et al. 2023). For example, engaging interested and knowledgeable anglers in inventorying pure native charr populations in their local basin will engender pride in, and knowledge about these irreplaceable ancient evolutionary legacies. Likewise, fisheries cooperatives might become involved in culturing rare lineages for translocation to suitable habitats, with the goal of eventually developing a catch-and-release fishery to allow anglers to see and hold the charr that are native to their home waters. This could engender an ethic for conserving native charr, allowing it to evolve in the minds of those in the community who are interested (Leopold 1949) and be culturally transmitted to future generations.
References
Ardren WR, DeHaan PW, Smith CT, Taylor EB, Leary R, Kozfkay CC, Godfrey L, Diggs M, Fredenberg W, Chan J, Kilpatrick CW, Small MP, Hawkins DK (2011) Genetic structure, evolutionary history, and conservation units of bull trout in the coterminous United States. Trans Am Fish Soc 140:506–525
Ayer CG, Katahira H, Fukui S, Koizumi I (2018) Seasonal patterns of downstream movement in partially migratory stream-dwelling Dolly Varden. Ecol Freshw Fish 27:247–254
Bamzai-Dodson A, Cravens AE, Wade AA, McPherson RA (2021) Engaging with stakeholders to produce actionable science: a framework and guidance. Weather Clim Soc 13:1027–1041
Baxter CV, Fausch KD, Murakami M, Chapman PL (2004) Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 85:2656–2663
Baxter CV, Fausch KD, Murakami M, Chapman PL (2007) Invading rainbow trout usurp a terrestrial prey subsidy from native charr and reduce their growth and abundance. Oecologia 153:461–470
Benjamin JR, Brignon WR, Dunham JB (2019) Decision analysis for the reintroduction of bull trout into the lower Pend Oreille River, Washington. N Am J Fish Manag 39:1026–1045
Bertrand M, Marcogliese DJ, Magnan P (2008) Trophic polymorphism in brook charr revealed by diet, parasites and morphometrics. J Fish Biol 72:555–572
Brenkman SJ, Peters RJ, Tabor RA, Geffre JJ, Sutton KT (2019) Rapid recolonization and life history responses of bull trout following dam removal in Washington's Elwha River. N Am J Fish Manag 39:560–573
Brignon WR, Davis MB, Gunckel S, Dunham J, Meeuwig MH, Allen C, Clements S (2023) Engaging stakeholders to develop a decision support model of conservation risk and management capacity to prioritize investments in bull trout recovery. N Am J Fish Manag 43:821–833
Brignon WR, Peterson JT, Dunham JB, Schaller HA, Schreck CB (2018) Evaluating trade-offs in bull trout reintroduction strategies using structured decision making. Can J Fish Aquat Sci 75:293–307
Buktenica MW, Hering DK, Girdner SF, Mahoney BD, Rosenlund BD (2013) Eradication of nonnative brook trout with electrofishing and antimycin-A and the response of a remnant bull trout population. N Am J Fish Manag 33:117–129
Carey MP, Sanderson BL, Barnas KA, Olden JD (2012) Native invaders create novel challenges for science, management, society and policy. Front Ecol Environ 10:373–381
Chavarie L, Howland K, Harris L, Tonn W (2015) Polymorphism in lake trout in Great Bear Lake: intra-lake morphological diversification at two spatial scales. Biol J Linn Soc Lond 114:109–125
Chelgren ND, Dunham JB, Gunckel SL, Hockman-Wert DP, Allen CS (2023) Combining expert knowledge of a threatened trout distribution with sparse occupancy data for climate-related projection. N Am J Fish Manag 43:839–858
Dunham JB, Arismendi I, Murphy C, Koeberle A, Olivos JA, Pearson J, Pickens F, Roon D, Stevenson J (2020) What to do when invaders are out of control? WIREs Water 7:e1476
Dunham J, Baxter C, Fausch K, Fredenberg W, Kitano S, Koizumi I, Morita K, Nakamura T, Rieman B, Savvaitova K, Stanford J, Taylor E, Yamamoto S (2008) Evolution, ecology, and conservation of Dolly Varden, white-spotted char, and bull trout. Fisheries 33:537–550
Dunham JB, Benjamin JR, Lawrence DJ, Clifford K (2022) Resist, accept, and direct responses to biological invasions: a social-ecological perspective. Fish Manag Ecol 29:475–485
Dunson WA, Travis J (1991) The role of abiotic factors in community organization. Am Nat 138:1067–1091
Endou S, Tsuboi J, Iwata T (2006) Effects of damming on the persistence of white-spotted charr and red-spotted masu salmon populations. Japan J Conserv Ecol 11:4–12 [in Japanese]
Esin EV, Markevich GN (2018) Evolution of the charrs, genus Salvelinus (Salmonidae). 1. Origins and expansion of the species. J Ichthyol 58:187–203
Falke JA, Flitcroft RL, Dunham JB, McNyset KM, Hessburg PF, Reeves GH (2015) Climate change and vulnerability of bull trout (Salvelinus confluentus) in a fire-prone landscape. Can J Fish Aquat Sci 72:304–318
Fausch KD (2008) A paradox of trout invasions in North America. Biol Invasions 10:685–701
Fausch KD (2014) A historical perspective on drift foraging models for stream salmonids. Environ Biol Fishes 97:453–464
Fausch KD (2015) For the love of rivers: a scientist’s journey. Oregon State Univ Press, Corvallis, Oregon
Fausch KD (2018) Crossing boundaries: Shigeru Nakano’s enduring legacy for ecology. Ecol Res 33:119–133
Fausch KD, Baxter CV, Murakami M (2010) Multiple stressors in north temperate streams: lessons from linked forest-stream ecosystems in northern Japan. Freshw Biol 55(Suppl 1):120–134
Fausch KD, Nakano S, Ishigaki K (1994) Distribution of two congeneric charrs in streams of Hokkaido Island, Japan: considering multiple factors across scales. Oecologia 100:1–12
Fausch KD, Nakano S, Kitano S (1997) Experimentally induced foraging mode shift by sympatric charrs in a Japanese mountain stream. Behav Ecol 8:414–420
Fausch KD, Nakano S, Kitano S, Kanno Y, Kim S (2021) Interspecific social dominance networks reveal mechanisms promoting coexistence in sympatric charr in Hokkaido, Japan. J Anim Ecol 90:515–527
Fausch KD, Rieman BE, Dunham JB, Young MK, Peterson DP (2009) Invasion versus isolation: trade-offs in managing native salmonids with barriers to upstream movement. Conserv Biol 23:859–870
Fausch KD, Taniguchi Y, Nakano S, Grossman GD, Townsend CR (2001) Flood disturbance regimes influence rainbow trout invasion success among five Holarctic regions. Ecol Appl 11:1438–1455
Fausch KD, Torgersen CE, Baxter CV, Li HW (2002) Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes. BioScience 52:483–498
Fukui D, Murakami M, Nakano S, Aoi T (2006) Effect of emergent aquatic insects on bat foraging in a riparian forest. J Anim Ecol 75:1252–1258
Fukui S, Kasugai K, Sawada A, Koizumi I (2021) Evidence for introgressive hybridization between native Dolly Varden (Salvelinus curilus (syn. Salvelinus malma)) and introduced brook trout (Salvelinus fontinalis) in the Nishibetsu River of Hokkaido, Japan. Zoolog Sci 38:247–251
Fukui S, Koizumi I (2020) Hybrids as potential mediators spreading non‐native genes: comparison of survival, growth, and movement among native, introduced and their hybrid salmonids. Ecol Freshw Fish 29:280–288
Fukui S, May-McNally SL, Katahira H, Kitano S, Koizumi I (2016) Temporal change in the distribution and composition of native, introduced, and hybrid charrs in northern Japan. Hydrobiologia 783:309–316
Fukui S, May‐McNally SL, Taylor EB, Koizumi I (2018) Maladaptive secondary sexual characteristics reduce the reproductive success of hybrids between native and non‐native salmonids. Ecol Evol 8:12173–12182
Furusawa C, Suehiro-Kanazawa Y, Tanaka Y, Fukui S, Yamazaki C, Hosoki TK, Koizumi I (2022) Local factors affecting winter habitat use of non-native rainbow trout in a boreal stream in northern Japan. Ichthyol Res 69:125–131
Gowan C, Fausch KD (1996) Mobile brook trout in two high-elevation Colorado streams: re-evaluating the concept of restricted movement. Can J Fish Aquat Sci 53:1370–1381
Gross MR, Coleman RM, McDowall RM (1988) Aquatic productivity and the evolution of diadromous fish migration. Science 239:1291–1293
Hasegawa K (2017) Displacement of native white-spotted charr Salvelinus leucomaenis by non-native brown trout Salmo trutta after resolution of habitat fragmentation by a migration barrier. J Fish Biol 90:2475–2479
Hasegawa K (2020) Invasions of rainbow trout and brown trout in Japan: a comparison of invasiveness and impact on native species. Ecol Freshw Fish 29:419–428
Hasegawa K, Fukui S (2022) Pulsed supplies of small fish facilitate time-limited intraguild predation in salmon-stocked streams. R Soc Open Sci 9:220127
Hasegawa K, Honda K, Yoshiyama T, Suzuki K, Fukui S (2021) Small biased body size of salmon fry preyed upon by piscivorous fish in riverine and marine habitats. Can J Fish Aquat Sci 78:631–638
Hasegawa K, Maekawa K (2006) The effects of introduced salmonids on two native stream-dwelling salmonids through interspecific competition. J Fish Biol 68:1123–1132
Hasegawa K, Maekawa K (2008a) Different longitudinal distribution patterns of native white-spotted charr and non-native brown trout in Monbetsu stream, Hokkaido, northern Japan. Ecol Freshw Fish 17:189–192
Hasegawa K, Maekawa K (2008b) Potential of habitat complexity for mitigating interference competition between native and non-native salmonid species. Can J Zool 86:386–393
Hasegawa K, Maekawa K (2009) Role of visual barriers on mitigation of interspecific interference competition between native and non-native salmonid species. Can J Zool 87:781–786
Hasegawa K, Yamamoto T, Murakami M, Maekawa K (2004) Comparison of competitive ability between native and introduced salmonids: evidence from pairwise contests. Ichthyol Res 51:191–194
Hasegawa R, Ayer CG, Umatani Y, Miura K, Ukumura M, Katahira H, Koizumi I (2022) Potential negative effects and heterogeneous distribution of a parasitic copepod Salmincola edwardsii (Copepoda: Lernaeopodidae) on Southern Asian Dolly Varden Salvelinus curilus in Hokkaido, Japan. Parasitol Int 87:102529
Hasegawa R, Koizumi I (2023) Parasites either reduce or increase host vulnerability to fishing: a case study of a parasitic copepod and its salmonid host. Sci Nat 110:10
Hayes MF, Banish NP (2017) Translocation and reintroduction of native fishes: a review of bull trout Salvelinus confluentus with applications for future reintroductions. Endanger Species Res 34:191–209
Hudy M, Theiling T, Gillespie N, Smith EP (2008) Distribution, status, and land use characteristics of subwatersheds within the native range of brook trout in the eastern United States. N Am J Fish Manag 28:1069–1085
Inoue M, Ichimori D, Abe H, Mizuno N (2023) Complementary distribution of non‑native white‑spotted charr and native red‑spotted masu salmon in Shikoku Island, southwestern Japan: a consequence of interspecific interactions? Ichthyol Res 70:82–90
Inoue M, Miyata H, Tange Y, Taniguchi Y (2009) Rainbow trout (Oncorhynchus mykiss) invasion in Hokkaido streams, northern Japan, in relation to flow variability and biotic interactions. Can J Fish Aquat Sci 66:1423–1434
Inoue M, Sakamoto S, Kikuchi S (2013) Terrestrial prey inputs to streams bordered by deciduous broadleaved forests, conifer plantations and clear-cut sites in southwestern Japan: effects on the abundance of red-spotted masu salmon. Ecol Freshw Fish 22:335–347
Isaak DJ, Young M (2023) Cold-water habitats, climate refugia, and their utility for conserving salmonid fishes. Can J Fish Aquat Sci 80:1187–1206
Ishigaki K (1969) Ecological and morphological studies on charrs in Hokkaido. Ph.D. Dissertation, Hokkaido University [in Japanese with English summary]
Ishigaki K (1984) Exploring the mystery of charrs. Iwanami-shoten, Tokyo [in Japanese]
Japan Bureau of Fisheries (1927) Report of a survey on fishery stock enhancement. Ministry Agric Forestry Japan, Tokyo [in Japanese]
Japan Red Data Retrieval System (2023) Searchable database of Red List data for organisms in Japan. http://jpnrdb.com/. Accessed 12 Sep 2023
Jonsson B, Jonsson N (1993) Partial migration: niche shift versus sexual maturation in fishes. Rev Fish Biol Fish 3:348–365
Kanda N, Leary RF, Allendorf FW (2002) Evidence of introgressive hybridization between bull trout and brook trout. Trans Am Fish Soc 121:772–782
Kanno Y, Harris AC, Kishida O, Utsumi S, Uno H (2021) Complex effects of body length and condition on within-tributary movement and emigration in stream salmonids. Ecol Freshw Fish 31:317–329
Kanno Y, Yui N, Mamiya W, Sakai R, Yabuhara Y, Miyazaki T, Utsumi S, Kishida O, Uno H (2020) A multistate mark–recapture approach to characterize stream fish movement at multiple spatial scales. Can J Fish Aquat Sci 77:1090–1100
Kato C, Iwata T, Nakano S, Kishi D (2003) Dynamics of aquatic insect flux affects distribution of riparian web-building spiders. Oikos 103:113–120
Kawaguchi Y, Nakano S, Taniguchi Y (2003) Terrestrial invertebrate inputs determine the local abundance of stream fishes in a forested stream. Ecology 84:701–708
Kawanabe H (1989) Japanese char(r(r))s and masu-salmon problems: a review. Physiol Ecol Japan Spec 1:13–24
Kawanabe H, Yamazaki F, Noakes DLG (1989) Biology of charrs and masu salmon. Physiology and Ecology Japan, Special volume 1. Kyoto University, Kyoto
Kazyak DC, Lubinski BA, Kulp MA, Pregler KC, Whiteley AR, Hallerman E, Coombs JA, Kanno Y, Rash JM, Morgan RP II, Habera J, Henegar J, Weathers TC, Sell MT, Rabern A, Rankin D, King TL (2022) Population genetics of brook trout in the Southern Appalachian Mountains. Trans Am Fish Soc 151:127–149
Kazyak DC, Rash J, Lubinski BA, King TL (2018) Assessing the impact of stocking northern-origin hatchery brook trout on the genetics of wild populations in North Carolina. Conserv Genet 19:207–219
Kikko T, Sugahara K, Kataoka Y, Ishizaki D, Yoshioka T, Tsuboi J, Morita K, Kuwahara M, Iguchi K, Kai Y, Nakayama K (2022) Current genetic status of nagaremon-charr, a threatened morphotype of Salvelinus leucomaenis in the Ane River, Lake Biwa system, central Japan, with comments on its conservation. Zoolog Sci 39:242–252
Kishi D, Maekawa K (2009) Stream-dwelling Dolly Varden (Salvelinus malma) density and habitat characteristics in stream sections installed with low-head dams in the Shiretoko Peninsula, Hokkaido, Japan. Ecol Res 24:873–880
Kishi D, Murakami M, Nakano S, Taniguchi Y (2004) Effects of forestry on the thermal habitat of Dolly Varden (Salvelinus malma). Ecol Res 19:283–290
Kishi D, Ohara K, Tsuji H, Tokuhara T (2020) Effects of desiccation on stream-dwelling salmonids in a drought year in a small stream in Gifu, central Japan. Limnol Tokai Region Japan 87:39–44 [in Japanese]
Kishi D, Tokuhara T (2017) Habitat characteristics and egg survival of charr (Salvelinus leucomaenis) in an artificial spawning channel constructed in Maze, Gero City, Gifu Prefecture, central Japan. Ecol Civil Eng 19:221–231 [in Japanese]
Kishi D, Tokuhara T (2018) Water depth, current velocity and substrate of redds of Japanese charr Salvelinus leucomaenis, masu salmon Oncorhynchus masou masou and red spotted masu salmon O. m. ishikawae in streams of the Hida region, Gifu, central Japan. Rep Gifu Prefect Res Inst Fish Aquat Environ 63:1–6 [in Japanese]
Kitano S (1996) Size‐related factors causing individual variation in seasonal reproductive success of fluvial male Dolly Varden (Salvelinus malma). Ecol Freshw Fish 5:59–67
Kitano S (2004) Ecological impacts of rainbow, brown and brook trout in Japanese inland waters. Global Environ Res 8:41–50
Kitano S (2018) Salmonid fish profiles - 16 Brook trout. Salmon Inf 12:37–40 [in Japanese]
Kitano S, Hasegawa K, Maekawa K (2009) Evidence for interspecific hybridization between native white-spotted charr Salvelinus leucomaenis and non-native brown trout Salmo trutta on Hokkaido Island, Japan. J Fish Biol 74:467–473
Kitano S, Maekawa K, Nakano S, Fausch KD (1994) Spawning behavior of bull trout in the upper Flathead drainage, Montana, with special reference to hybridization with brook trout. Trans Am Fish Soc 123:988–992
Kitano S, Ohdachi S, Koizumi I, Hasegawa K (2014) Hybridization between native white-spotted charr and nonnative brook trout in the upper Sorachi River, Hokkaido, Japan. Ichthyol Res 61:1–8
Kitano S, Shimazaki K (1995) Spawning habitat and nest depth of female Dolly Varden Salvelinus malma of different body size. Fish Sci 61:776–779
Koizumi I (2011) Integration of ecology, demography and genetics to reveal population structure and persistence: a mini review and case study of stream-dwelling Dolly Varden. Ecol Freshw Fish 20:352–363
Koizumi I, Kanazawa Y, Tanaka Y (2013) The fishermen were right: experimental evidence for tributary refuge hypothesis during floods. Zoolog Sci 30:375–379
Koizumi I, Kanazawa Y, Yamazaki C, Tanaka Y, Takaya K (2017) Extreme winter aggregation of invasive rainbow trout in small tributaries: implications for effective control. Ichthyol Res 64:197–203
Koizumi I, Kobayashi H, Maekawa K, Azuma N, Nagase T (2005) Occurrence of a hybrid between endemic Miyabe charr Salvelinus malma miyabei and introduced masu salmon Oncorhynchus masou masou in the Lake Shikaribetsu system, Hokkaido, Japan. Ichthyol Res 52:83–85
Koizumi I, Maekawa K (2004) Metapopulation structure of stream-dwelling Dolly Varden charr inferred from patterns of occurrence in the Sorachi River basin, Hokkaido, Japan. Freshw Biol 49:973–981
Koizumi I, Shimatani IK (2016) Socially induced reproductive synchrony in a salmonid: an approximate Bayesian computation approach. Behav Ecol 27:1386–1396
Koizumi I, Yamamoto S, Maekawa K (2006a) Decomposed pairwise regression analysis of genetic and geographic distances reveals a metapopulation structure of stream-dwelling Dolly Varden charr. Mol Ecol 15:3175–3189
Koizumi I, Yamamoto S, Maekawa K (2006b) Female-biased migration of stream-dwelling Dolly Varden in the Shiisorapuchi River, Hokkaido, Japan. J Fish Biol 68:1513–1529
Koizumi I, Yamamoto S, Nomoto K, Maekawa K (2008) Synchrony in local population dynamics of stream-dwelling Dolly Varden: do genetically similar groups show similar demography? Popul Ecol 50:367–377
Komiyama E (1987) Spawning behaviors of Salvelinus spp. In: Miyadi D, Kaiko T, Yamamoto S (eds) One hundred forms of charrs. Chikumashobo Ltd, Tokyo, pp 16–29 [in Japanese]
Kondou T, Sakata K, Takeshita N, Nakazono A, Kimura S (1999) Range extension and reproduction of introduced iwana-charr Salvelinus leucomaenis in the upper reaches of the Kuma River in Kyushu Island, Japan. Jpn J Ichthyol 46:121–125 [in Japanese with English abstract]
Kuroda M, Miyashita K (2022) Winter migratory pattern for anadromous white‑spotted char (Salvelinus leucomaenis) in southwestern Hokkaido, Japan. Environ Biol Fishes 105:1–11
Larson GL, Moore SE (1985) Encroachment of exotic rainbow trout into stream populations of native brook trout in the southern Appalachian Mountains. Trans Am Fish Soc 114:195–203
Leopold A (1949) A Sand County almanac. Oxford University Press, Oxford
MacCrimmon HR (1971) World distribution of rainbow trout (Salmo gairdneri). J Fish Res Board Can 28:663–704
MacCrimmon HR, Marshall TL, Gots BL (1970) World distribution of brown trout, Salmo trutta: further observations. J Fish Res Board Can 27:811–818
Machida Y, Yamamoto A, Akiyama Y, Nomoto K, Kanaiwa M, Jinbo T, Iwase H, Hashimoto M (2019) Did multiple handmade fishways contribute to salmonid fish habitat recovery? Ecol Civil Eng 21:181–189 [in Japanese with English abstract]
Maekawa K (1973) On a silvery smolt of the Dolly Varden, Salvelinus malma, collected from the Shiretoko Peninsula, Hokkaido. Jpn J Ichthyol 20:245–247 [in Japanese]
Maekawa K (1984) Life history pattern of the Miyabe charr in Shikaribetsu Lake, Japan. In: Johnson L, Burns B (eds) Biology of the Arctic charr. University of Manitoba Press, Winnipeg, pp 223–250
Maekawa K (1985) Homing of lacustrine charr in a small lake with a few inlet creeks. Jpn J Ichthyol 32:355–358
Maekawa K, Koseki Y, Iguchi K, Kitano S (2001) Skewed reproductive success among male white-spotted charr land-locked by an erosion control dam: implications for effective population size. Ecol Res 16:727–735
Maekawa K, Onozato H (1986) Reproductive tactics and fertilization success of mature male Miyabe charr, Salvelinus malma miyabei. Environ Biol Fishes 15:119–129
Marcarelli AM, Baxter CV, Benjamin JR, Miyake Y, Murakami M, Fausch KD, Nakano S (2020) Magnitude and direction of stream-forest community interactions change with time scale. Ecology 101:e03064
Markevich G, Esin E (2019) Trout and char of Russia. In: Kershner JL, Williams JE, Gresswell RE, Lobón-Cervia J (eds) Trout and char of the world. American Fisheries Society, Bethesda, pp 517–571
Markevich G, Esin E, Anisimova L (2018) Basic description and some notes on the evolution of seven sympatric morphs of Dolly Varden Salvelinus malma from the Lake Kronotskoe Basin. Ecol Evol 8:2554–2567
Maruyama T, Fujii K, Kijima T, Maeda H (1987) Introduction of foreign fish species into Japan. Fisheries Agency Japan, Tokyo [in Japanese]
Masuda T, Shimono Y, Kishi D, Koizumi I (2023) Systematic headwater sampling of white-spotted charr reveals stream capture events across dynamic topography. J Biogeogr 50:453–466
Meyer KA, Lamansky JA Jr, Schill DJ (2006) Evaluation of an unsuccessful brook trout electrofishing removal project in a small Rocky Mountain stream. N Am J Fish Manag 26:849–860
Ministry of the Environment of Japan (2020) Japan Red list, 4th edition. https://www.env.go.jp/press/107905.html. Accessed 12 Sep 2023
Miyamoto K, Araki H (2017) Differentiated predation risk on hatchery-reared juvenile masu salmon by white-spotted charr with different body size. Fish Sci 83:245–250
Mori T (1936) Studies on the geographical distribution of freshwater fishes in Chosen. Bull Biogeogr Soc Jpn 6(7):35–61
Morita K (2018) Assessing the long-term causal effect of trout invasion on a native charr. Ecol Indic 87:189–192
Morita K (2019) Trout and char of Japan. In: Kershner JL, Williams JE, Gresswell RE, Lobón-Cervia J (eds) Trout and char of the world. American Fisheries Society, Bethesda, pp 487–515
Morita K (2022) Ups and downs of non-native and native stream-dwelling salmonids: lessons from two contrasting rivers. Ecol Res 37:188–196
Morita K, Morita SH, Fukuwaka M, Nagasawa T (2009b) Offshore Dolly Varden charr (Salvelinus malma) in the North Pacific. Environ Biol Fishes 86:451–456
Morita K, Morita SH, Nagasawa T, Kuroki M (2013) Migratory patterns of anadromous white-spotted charr Salvelinus leucomaenis in eastern Hokkaido, Japan: the solution to a mystery? J Ichthyol 53:809–819
Morita K, Morita SH, Yamamoto S (2009c) Effects of habitat fragmentation by damming on salmonid fishes: lessons from white-spotted charr in Japan. Ecol Res 24:711–722
Morita K, Sahashi G, Miya M, Kamada S, Kanbe T, Araki H (2019) Ongoing localized extinctions of stream-dwelling whitespotted charr populations in small dammed-off habitats of Hokkaido Island, Japan. Hydrobiologia 840:207–213
Morita K, Sahashi G, Tsuboi J (2016) Altitudinal niche partitioning between white-spotted charr (Salvelinus leucomaenis) and masu salmon (Oncorhynchus masou) in a Japanese river. Hydrobiologia 783:93–103
Morita K, Tamate T, Kuroki M, Nagasawa T (2014) Temperature-dependent variation in alternative migratory tactics and its implications for fitness and population dynamics in a salmonid fish. J Anim Ecol 83:1268–1278
Morita K, Tsuboi J, Matsuda H (2004) The impact of exotic trout on native charr in a Japanese stream. J Appl Ecol 41:962–972
Morita K, Tsuboi J, Nagasawa T (2009a) Plasticity in probabilistic reaction norms for maturation in a salmonid fish. Biol Lett 5:628–631
Morita K, Yamamoto S (2000) Occurrence of a deformed white-spotted charr, Salvelinus leucomaenis (Pallas), population on the edge of its distribution. Fish Manag Ecol 7:551–553
Morita K, Yamamoto S (2002) Effects of habitat fragmentation by damming on the persistence of stream-dwelling charr populations. Conserv Biol 1:1318–1323
Morita K, Yamamoto S (2004) Consequences of riverine fragmentation by damming—rivers are corridors between forest and the sea. In: Maekawa K (ed) Ecology and evolution of salmonids (in Japanese). Bunichi Sougou Shuppan, Tokyo, pp 281–312
Morita K, Yamamoto S, Hoshino N (2000) Extreme life history change of white-spotted charr (Salvelinus leucomaenis) after damming. Can J Fish Aquat Sci 57:1300–1306
Morita K, Yokota A (2002) Population viability of stream-resident salmonids after habitat fragmentation: a case study with white-spotted charr (Salvelinus leucomaenis) by an individual based model. Ecol Modell 155:85–94
Nakamura F, Komiyama E (2010) A challenge to dam improvement for the protection of both salmon and human livelihood in Shiretoko, Japan’s third Natural Heritage Site. Landsc Ecol Eng 6:143–152
Nakamura T (1999a) Comparison of physical characteristics of spawning redds between the fluvial Japanese charr Salvelinus leucomaenis and the masu salmon Oncorhynchus masou masou in the headwaters of the Kinu River, central Japan. Nippon Suisan Gakkaishi 65:427–433 [in Japanese with English abstract]
Nakamura T (1999b) Spawning activities of the fluvial Japanese charr Salvelinus leucomaenis and incubation of their eggs at the artificial spawning sites. Nippon Suisan Gakkaishi 65:434–440 [in Japanese with English abstract]
Nakamura T (2001) Estimation of the distribution of genetically pure populations of Japanese charr by inquiring survey. J Japan Soc Erosion Control Eng 53:3–9 [in Japanese with English abstract]
Nakamura T (2008) Toward sustainable use of charr: new ways to protect, propagate, and utilize an elusive fish. Fly Magazine, Tokyo [in Japanese]
Nakamura T (2011) Relationships between physical characteristics of pools and residency of stream-dwelling white-spotted charr. Aquacult Sci 59:427–433
Nakamura T (2013) Effects of flow reduction on a whitespotted char population in a Japanese mountain stream. N Am J Fish Manag 33:1142–1148
Nakamura T, Doi T (2014) Do stocked hatchery-reared juveniles ecologically suppress wild juveniles in Salvelinus leucomaenis? J Fish Biol 84:1289–1299
Nakamura T, Kishi D, Tokuhara T, Kubota H, Kikko T, Tsuboi J (2012) Present status and future conservation of fluvial charr (Salvelinus spp.) and salmon (Oncorhynchus spp.) populations in Japanese mountain streams. Jpn J Ichthyol 59:163–167 [in Japanese]
Nakamura T, Maruyama T, Nozaki E (1994) Seasonal abundance and the re-establishment of iwana charr Salvelinus leucomaenis f. pluvius after excessive sediment loading by road construction in the Hakusan National Park, central Japan. Environ Biol Fishes 39:97–107
Nakamura T, Maruyama T, Watanabe S (2001) Population increase of fluvial Japanese charr Salvelinus leucomaenis after fishing prohibition. Nippon Suisan Gakkaishi 67:105–107 [in Japanese]
Nakamura T, Maruyama T, Watanabe S (2002) Residency and movement of stream-dwelling Japanese charr, Salvelinus leucomaenis, in a central Japanese mountain stream. Ecol Freshw Fish 11:150–157
Nakano S (1995a) Competitive interactions for foraging microhabitats in a size-structured interspecific dominance hierarchy of two sympatric stream salmonids in a natural habitat. Can J Zool 73:1845–1854
Nakano S (1995b) Individual differences in resource use, growth and emigration under the influence of a dominance hierarchy in fluvial red-spotted masu salmon in a natural habitat. J Anim Ecol 64:75–84
Nakano S, Fausch KD, Kitano S (1999a) Flexible niche partitioning via a foraging mode shift: a proposed mechanism for coexistence in stream-dwelling charrs. J Anim Ecol 68:1079–1092
Nakano S, Fausch KD, Koizumi I, Kanno Y, Taniguchi Y, Kitano S, Miyake Y (2020) Evaluating a pattern of ecological character displacement: charr jaw morphology and diet diverge in sympatry versus allopatry across catchments in Hokkaido, Japan. Biol J Linn Soc Lond 129:356–378
Nakano S, Furukawa-Tanaka T (1994) Intra- and interspecific dominance hierarchies and variation in foraging tactics of two species of stream-dwelling chars. Ecol Res 9:9–20
Nakano S, Kawaguchi Y, Taniguchi Y, Miyasaka H, Shibata Y, Urabe H, Kuhara N (1999c) Selective foraging on terrestrial invertebrates by rainbow trout in a forested headwater stream in northern Japan. Ecol Res 14:351–360
Nakano S, Kitano F, Maekawa K (1996) Potential fragmentation and loss of thermal habitats for charrs in the Japanese archipelago due to climatic warming. Freshw Biol 36:711–722
Nakano S, Miyasaka H, Kuhara N (1999b) Terrestrial-aquatic linkages: riparian arthropod inputs alter trophic cascades in a stream food web. Ecology 80:2435–2441
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci U S A 98:166–170
Noakes DLG (1989) Symposium to be remembered. Environ Biol Fishes 24:313–317
Parsons KJ, Sheets HD, Skúlason S, Ferguson MM (2011) Phenotypic plasticity, heterochrony and ontogenetic repatterning during juvenile development of divergent Arctic charr (Salvelinus alpinus). J Evol Biol 24:1640–1652
Peterson MI, Kitano S, Yamamoto S, Kando T, Tsuda Y (2023) Species-specific foraging behavior and diets of stream salmonids: An implication for negative impacts on native charr by nonnative trout in Japanese mountain streams. Ecol Res. https://doi.org/https://doi.org/10.1111/1440-1703.12419
Pregler KC, Kanno Y, Rankin D, Coombs JA, Whiteley AR (2018) Characterizing genetic integrity of rear-edge trout populations in the southern Appalachians. Conserv Genet 19:1487–1503
Radinger J, Wolter C (2014) Patterns and predictors of fish dispersal in rivers. Fish Fish 15:456–473
Rahel FJ, Taniguchi Y (2019) A comparison of freshwater fisheries management in the USA and Japan. Fish Sci 85:271–283
Rodríguez MA (2002) Restricted movement in stream fish: the paradigm is incomplete, not lost. Ecology 83:1–13
Sahashi G, Morita K (2013) Migration costs drive convergence of threshold traits for migratory tactics. Proc R Soc B 280:20132539
Sato K (1980) Genus Salvelinus spp. in the Korean Peninsula. Special Issue on Freshwater Fish: Salvelinus. Freshwater Fish Protection Association, Osaka, pp 86–87 [in Japanese]
Sato M, Minatoya K, Tsuboi J (2021) Development of portable fishways for extending the upstream migration area of masu salmon Oncorhynchus masou. Nippon Suisan Gakkaishi 87:160–162 [in Japanese]
Sato T (2006a) Dramatic decline in population abundance of Salvelinus leucomaenis after a severe flood and debris flow in a high gradient stream. J Fish Biol 69:1849–1854
Sato T (2006b) Occurrence of deformed fish and their fitness-related traits in Kirikuchi Charr, Salvelinus leucomaenis japonicus, the southernmost population of the genus Salvelinus. Zoolog Sci 23:593–599
Sato T (2007) Threatened fishes of the world: Kirikuchi charr, Salvelinus leucomaenis japonicus (Oshima 1961) (Salmonidae). Environ Biol Fishes 78:217–218
Sato T, Arizono M, Sone R, Harada Y (2008b) Parasite-mediated allochthonous input: do hairworms enhance subsidized predation of stream salmonids on crickets? Can J Zool 86:231–235
Sato T, Demise T, Kubota H, Nagoshi M, Watanabe K (2010) Hybridization, isolation, and low genetic diversity of Kirikuchi Char, the southernmost populations of the genus Salvelinus. Trans Am Fish Soc 139:1758–1774
Sato T, Egusa T, Fukushima K, Oda T, Ohte N, Tokuchi N, Watanabe K, Kanaiwa M, Murakami I, Lafferty KD (2012) Nematomorph parasites indirectly alter the food web and ecosystem function of streams through behavioural manipulation of their cricket hosts. Ecol Lett 15:786–793
Sato T, Harada Y (2008) Loss of genetic variation and effective population size of Kirikuchi charr: implications for the management of small, isolated salmonid populations. Anim Conserv 11:153–159
Sato T, Watanabe K, Arizono M, Mori S, Nagoshi M, Harada Y (2008a) Intergeneric hybridization between sympatric Kirikuchi Char and Red-Spotted Masu Salmon in a small Japanese mountain stream. N Am J Fish Manag 28:547–566
Sato T, Watanabe K, Kanaiwa M, Niizuma Y, Harada Y, Lafferty KD (2011a) Nematomorph parasites drive energy flow through a riparian ecosystem. Ecology 92:201–207
Sato T, Watanabe K, Tokuchi N, Kamauchi H, Harada Y, Lafferty KD (2011b) A nematomorph parasite explains variation in terrestrial subsidies to trout streams in Japan. Oikos 120:1595–1599
Skúlason S, Parsons KJ, Svanbäck R, Räsänen K, Ferguson MM, Adams CE, Amundsen P, Bartels P, Bean C, Boughman J, Englund G, Guðbrandsson J, Hooker O, Hudson A, Kahilainen KK, Knudsen R, Kristjánsson BK, Leblanc C, Jónsson Z, Öhlund G, Smith C, Snorrason SS (2019) A way forward with eco evo devo: an extended theory of resource polymorphism with postglacial fishes as model systems. Biol Rev Camb Philos Soc 94:1786–1808
Skúlason S, Snorrason SS, Jonsson B (1999) Sympatric morphs, populations and speciation in freshwater fish with emphasis on Arctic charr. In: Magurran AE, May RM (eds) Evolution of biological diversity. Oxford University Press, Oxford, pp 70–92
Sloat MR, Fraser DJ, Dunham JB, Falke JA, Jordan CE, McMillan JR, Ohms HA (2014) Ecological and evolutionary patterns of freshwater maturation in Pacific and Atlantic salmonines. Rev Fish Biol Fish 24:689–707
Taguchi E, Kishi D, Ito K (2022) Intergeneric mating behavior between red-spotted masu salmon Oncorhynchus masou ishikawae and char Salvelinus leucomaenis in small mountain streams, Gifu Prefecture, central Japan. Ichthy (Nat Hist Fishes Japan) 27:87–94 [in Japanese with English abstract]
Takami T, Aoyama T (1999) Distributions of rainbow and brown trouts in Hokkaido, northern Japan. Wildl Conserv Japan 4:41–48 [in Japanese]
Takami T, Murakami Y, Mori M (1996) Growth and feeding habits of anadromous white-spotted charr (Salvelinus leucomaenis) in southwestern Hokkaido, Japan. Sci Rep Hokkaido Fish Hatch 50:37–44
Tamate T, Hayajiri M (2008) The relationship between the number of main dams and the coastal catch of masu salmon (Oncorhynchus masou) in Hokkaido: implications for river ecosystem conservation. Water Sci 52:72–84 [in Japanese]
Taniguchi Y, Nakano S (2000) Condition-specific competition: implications for the altitudinal distribution of stream fishes. Ecology 81:2027–2039
Terada K, Nakamura T, Yokota M (2020) Test of cover selection by white-spotted charr Salvelinus leucomaenis in a pool of an artificial stream. Nippon Suisan Gakkaishi 86:460–465 [in Japanese with English abstract]
Terui A, Ishiyama N, Urabe H, Ono S, Finlay JC, Nakamura F (2018) Metapopulation stability in branching river networks. Proc Natl Acad Sci U S A 115:E5963–E5969
Togaki D, Sunohara A, Inoue M (2023) Overlap in spawning habitat characteristics between two salmonids in relation to stream size: redd superimposition hypothesis on longitudinal species replacement. Can J Fish Aquat Sci 80:840–850
Tsuboi J, Endou S, Morita K (2010) Habitat fragmentation by damming threatens coexistence of stream-dwelling charr and salmon in the Fuji River, Japan. Hydrobiologia 650:223–232
Tsuboi J, Iwata T, Morita K, Endou S, Oohama H, Kaji K (2013b) Strategies for the conservation and management of isolated salmonid populations: lessons from Japanese streams. Freshw Biol 58:908–917
Tsuboi J, Morita K (2004) Selectivity effects on wild white-spotted charr (Salvelinus leucomaenis) during a catch and release fishery. Fish Res 69:229–238
Tsuboi J, Morita K, Koseki Y, Endo S, Sahashi G, Kishi D, Kikko T, Ishizaki D, Nunokawa M, Kanno Y (2020) Spatial covariation of fish population vital rates in a stream network. Oikos 129:924–937
Tsuboi J, Morita K, Koseki Y, Endo S, Sahashi G, Kishi D, Kikko T, Ishizaki D, Nunokawa M, Kanno Y (2022) Small giants: tributaries rescue spatially structured populations from extirpation in a highly fragmented stream. J Appl Ecol 59:1997–2009
Tsuboi J, Morita K, Sahashi G, Kuroki M, Baba S, Arlinghaus R (2021) Species-specific vulnerability to angling and its size-selectivity in sympatric stream salmonids. Can J Fish Aquat Sci 78:1470–1478
Tsuboi J, Yamamoto S, Morita K, Mitsui K, Ashizawa A, Hirose K (2013a) Life history traits of white-spotted charr in an alpine environment: implications for local adaptation along an altitude gradient. J Ichthyol 53:884–888
Urabe H, Nakajima M, Torao M, Aoyama T (2010) Evaluation of habitat quality for stream salmonids based on a bioenergetics model. Trans Am Fish Soc 139:1665–1676
U.S. Fish and Wildlife Service (2015) Recovery plan for the coterminous United States population of bull trout (Salvelinus confluentus). Portland, Oregon
Waples R (1995) Evolutionarily significant units and the conservation of biological diversity under the Endangered Species Act. Am Fish Soc Symp 17:8–27
Watz JR, Otsuki Y, Nagatsuka K, Hasegawa K, Koizumi I (2019) Temperature dependent competition between juvenile salmonids in small streams. Freshw Biol 64:1534–1541
Weber ED, Fausch KD (2003) Interactions between hatchery and wild salmonids in streams: differences in biology and evidence for competition. Can J Fish Aquat Sci 60:1018–1036
Whiteley A, Fitzpatrick SW, Funk WC, Tallmon DA (2015) Genetic rescue to the rescue. Trends Ecol Evol 30:42–49
Yamada T, Koizumi I, Urabe H, Nakamura F (2020) Temperature-dependent swimming performance differs by species: implications for condition-specific competition between stream salmonids. Zoolog Sci 37:1–5
Yamamoto S, Denda I, Shigekura M, Kohno N, Ogawa S, Ueshima G, Kitano S (2013b) Stock assessment of stream-dwelling white-spotted charr Salvelinus leucomaenis in the Zako River. Bull Nagano Pref Fish Exp Sta 14:1–6 [in Japanese]
Yamamoto S, Kitano S, Maekawa K, Koizumi I, Morita K (2006a) Introgressive hybridization between Dolly Varden Salvelinus malma and white-spotted charr Salvelinus leucomaenis on Hokkaido Island, Japan. J Fish Biol 68(Suppl A):68–85
Yamamoto S, Kubota H, Hasegawa K, Nakamura T (2016) Census and effective population sizes of white-spotted charr (Salvelinus leucomaenis) in a fragmented landscape. Ecol Freshw Fish 25:612–621
Yamamoto S, Maekawa K, Morita K, Crane PA, Oleinik AG (2014) Phylogeography of the salmonid fish, Dolly Varden Salvelinus malma: multiple glacial refugia in the North Pacific rim. Zoolog Sci 31:660–670
Yamamoto S, Maekawa K, Tamate T, Koizumi I, Hasegawa K, Kubota H (2006b) Genetic evaluation of translocation in artificially isolated populations of white-spotted charr (Salvelinus leucomaenis). Fish Res 78:352–358
Yamamoto S, Morita K, Kitano S, Tabata R, Watanabe K, Maekawa K (2023) Phylogeography of a salmonid fish, white-spotted charr (Salvelinus leucomaenis), in a historically non-glaciated region in the northwestern North Pacific. Biol J Linn Soc Lond 139:115–130
Yamamoto S, Morita K, Kitano S, Watanabe K, Koizumi I, Maekawa K, Takamura K (2004) Phylogeography of white-spotted charr (Salvelinus leucomaenis) inferred from mitochondrial DNA sequences. Zoolog Sci 21:229–240
Yamamoto S, Morita K, Sahashi G (2019) Spatial and temporal changes in genetic structure and diversity of isolated white-spotted charr (Salvelinus leucomaenis) populations. Hydrobiologia 840:35–48
Yamamoto S, Morita K, Sahashi G, Maekawa K, Oleinik A, Bondar E, Brykov V (2021) Introgressive hybridization between southern Asian Dolly Varden, Salvelinus curilus, and northern Dolly Varden, S. malma malma, on Sakhalin Island. Russ J Genet 57:361–370
Yamamoto S, Morita K, Yokoyama R, Miyamoto K, Sato M, Maekawa K (2013a) Incidence of a skeletal deformity (truncated upper jaw) in an isolated population of whitespotted charr Salvelinus leucomaenis. J Ichthyol 53:889–893
Yamashita Y, Iwasaki Y, Matsubara T, Suzuki K, Kanzawa Y, Okuda T, Nishina K, Strüssmann CA (2020) Comparison of survival rates between domesticated and semi-native char using Bayesian multi-variate state-space model. Fish Res 221:105380
Yamashita Y, Matsubara T, Kanzawa Y, Suzuki K, Strüssmann CA (2023) Quantification of the size effect of recreational fishing prohibition on stock enhancement of resident stream salmonids. Nippon Suisan Gakkaishi 89:345–352 [in Japanese with English abstract]
Yoshimura C, Omura T, Furumai H, Tockner K (2005) Present state of rivers and streams in Japan. River Res Appl 21:93–112
Yoshiyama T, Tsuboi J, Matsuishi T (2017) Recreational fishery as a conservation tool for endemic Dolly Varden Salvelinus malma miyabei in Lake Shikaribetsu, Japan. Fish Sci 83:171–180
Yoshiyama T, Tsuboi J, Matsuishi T (2018) Consumption activities and expenditure of anglers targeting endangered fishes in Hokkaido lakes, Japan. Nippon Suisan Gakkaishi 84:858–871 [in Japanese with English abstract]
Yoshiyama T, Tsuboi J, Matsuishi T (2023) Interactions between fish and angler behaviour affect species-specific catchability of sympatric native charr and two introduced trout. Fish Res 259:106547
Zhou Z, Hitt NP, Letcher BH, Shi W, Li S (2022) Pigmentation-based visual learning for Salvelinus fontinalis individual re-identification. 2022 IEEE International Conference on Big Data (Big Data), Osaka, Japan. https://doi.org/10.1109/BigData55660.2022.10020966
Acknowledgments
We are grateful to the organizers of the 2023 Charr Symposium in Nikko, Japan, for inviting the senior author to present this work as the keynote speaker and providing funding for travel. We dedicate this paper to Dr. Hiroya Kawanabe, who was instrumental in organizing the 1988 Symposium on Charrs and Masu Salmon, and to the late Dr. Shigeru Nakano for catalyzing much research on charrs in Japan. We thank N. Chelgren, M. Kulp, T. Nakamura, and J. Rash for supplying data and information on habitat fragments for charr, and N. Hitt and two anonymous reviewers for helpful comments on the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Fausch, K.D., Morita, K., Tsuboi, Ji. et al. The past, present, and a future for native charr in Japan. Ichthyol Res (2024). https://doi.org/10.1007/s10228-024-00955-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10228-024-00955-3