Abstract
Microinvertebrates play a role as top consumers on glaciers. In this study we tested what kind of cryoconite material the animals inhabit (mud vs granules) on the edge of the Greenland ice sheet (GrIS) in the south-west. We also tested the links between the densities of micro-fauna in cryoconite material and selected biotic (algae, cyanobacteria, bacterial abundances) and abiotic (water depth, pH, ion content, radionuclides) factors. We collected 33 cryoconite samples. Tardigrada and Rotifera were found in 18 and 61% of samples, respectively. Invertebrates in this study were considerably less frequent and less abundant in comparison with High Arctic glaciers. The highest density of tardigrades and rotifers constituted 53 and 118 ind./ml, respectively. Generalized linear models showed no relationship between the densities of fauna and biotic and abiotic factors. The densities of animals were significantly higher in granules than in mud. The difference in the densities of animals between granules and mud reflects a simple mechanistic removal of invertebrates from the sediment during its erosion by flushing which leads to mud formation. These processes may influence a random distribution of micro-fauna without clear ecological interactions with biotic and abiotic variables at the edge of the GrIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaciers and ice sheets constitute an important element of the biosphere in terms of freshwater and carbon cycle (Hodson et al. 2008; Cook et al. 2015b). Despite extreme conditions such as low temperatures, a high melting rate of ice and high doses of UV radiation, the supraglacial zone is a habitable environment for various organisms from primary producers to higher trophic consumers like micro-fauna — the most frequent animals are tardigrades and rotifers (Mueller et al. 2001; Takeuchi et al. 2001a; Zawierucha et al. 2015; Grzesiak et al. 2015; Cook et al. 2015b; Gawor et al. 2016). Due to its unique species, a truncated food web and topographic as well as climatic features, glaciers and ice sheets are considered to be a distinct extreme biome with its highest biological activity observed within cryoconites (Anesio and Laybourn-Parry 2012).
Cryoconite consists of debris deposited on the ice surface by wind, water or rockfall. Microorganisms along with debris form special consortia called cryoconite granules. Cyanobacteria produce extracellular polymeric substances (EPS) which stick together mineral and organic matter to form granules (Takeuchi et al. 2001a, b; Hodson et al. 2010b; Langford et al. 2014; Cook et al. 2015b). These granules are a key component of glacial ecosystems (Hodson et al. 2008; Cook et al. 2015b), and together with dust and ice algae, they reduce surface albedo and increase the melting of glaciers (Wharton et al. 1985; Takeuchi et al. 2001a, b, 2010). The combination of cryoconite granules and solar radiation influences the formation of cryoconite holes — water-filled reservoirs on the surface of glaciers (Wharton et al. 1985; Hodson et al. 2008). Cryoconite holes are regarded as biodiversity hotspots on glaciers, constituting dynamic ecosystems with high primary production and organism assemblages adapted to such extreme conditions (e.g., Anesio and Laybourn-Parry 2012; Cook et al. 2015b; Zawierucha et al. 2015). The number of ecological studies on glacier ecosystems has increased in the last decade by surveys on the distribution of organisms in cryoconite holes (e.g., Uetake et al. 2010), biocryomorphological interactions (Cook et al. 2015a, 2016), the darkening of glaciers affected by algal blooming (e.g., Lutz et al. 2014), the interaction between organisms in cryoconite holes (e.g., Gokul et al. 2016), and even the biotechnological potential of cryoconite hole inhabitants (Singh et al. 2014). Surprisingly, data on the biggest organisms in glacial ecosystems, micro-animals, are scarce and mainly restricted to taxonomical snapshots (Dastych et al. 2003; Zawierucha et al. 2016a).
Microinvertebrates may play an important role as top consumers on glaciers (Zawierucha et al. 2016a). Animals (mainly tardigrades and rotifers) are ubiquitous occupants of cryoconite holes (Zawierucha et al. 2015, 2016a). Their densities may reach up to 168 ind. per cm3 and 624 ind. per g−1 of dry weight on High Arctic glaciers with a frequency of up to 90% of samples (Zawierucha et al. 2016b). Some of the Alpine and Himalayan invertebrates, e.g., tardigrades, have a black pigment, a protection against UV radiation (Zawierucha et al. 2015) which was proposed as a factor reducing albedo and influencing ice melting (Zawierucha et al. 2015). In spite of those facts, ecological studies of fauna on polar glaciers are limited to one survey in the Antarctic (Porazińska et al. 2004), and two in Svalbard (Vonnahme et al. 2015; Zawierucha et al. 2016b). Moreover, the relation between micro-animals and the flux of natural and anthropogenic pollution in glacial systems remains unexplored (Łokas et al. 2014, 2016), including radionuclides.
The main source of anthropogenic radionuclides is atmospheric testing of nuclear weapons which peaked in the early 1960s. The contamination from that source has been detected worldwide, including the High Arctic areas (AMAP 2015). Additionally, the European region of the Arctic has been subject to radioactive contamination from regional sources, such as the releases from nuclear industry and nuclear accidents (Dowdall et al. 2003; Johannessen et al. 2010; Paatero et al. 2012). After their deposition in the environment, artificial radionuclides can be remobilized and transported by the resuspension of snow, soil or marine aerosols, and with sea currents, ice pack or icebergs. Łokas et al. (2016) suggested a negative impact of radionuclides and heavy metals from cryoconite on downstream ecosystems.
The Greenland ice sheet (GrIS) is a dynamic and unique supraglacial ecosystem. The edge of the GrIS is characterized by supraglacial debris being washed away by meltwaters to subglacial and proglacial environments (e.g., Stibal et al. 2010; Cameron et al. 2016). Various studies have been performed on glaciers in south-west Greenland (Kangerlussuaq area) (see Cook et al. 2015a and literature cited herein), most of which have focused on the respiration, photosynthesis and cryoconite size (Cook et al. 2016; Irvine-Fynn et al. 2010; Stibal et al. 2010, 2012, 2015) with little emphasis on the combination of taxonomic and ecological data (Uetake et al. 2010). Furthermore, fauna has not been included in either of the previous ecological surveys. In this study we used an integrated approach of biotic and abiotic factors to determine the relation of tardigrades and rotifers and other biota and their environment in this area. These animals are the top consumers in cryoconite holes, and therefore they may be the key to understanding densities and interactions between cryoconite biota (potential food) in glacial ecosystems.
Materials and methods
Material and sampling
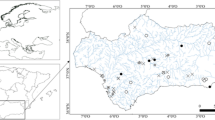
The GrIS is the second largest body of ice on Earth (Online Resources 1), and its ablation zone stretches up to 100 km inland, comprising all the ecological zones of glaciers: barren ice, slush and wet snow (Stibal et al. 2010; Hodson et al. 2010a). Cryoconite samples were collected from the 5th to the 6th of September 2015 from the edge of the GrIS (Online Resources 1, Fig. 1a–d) in the area of Kangerlussuaq (Online Resource 1). The samples were collected from the edge of the ice sheet towards ca. 1.5 km inland, from N67°08.177′ W50°02.331′ to N67°08.787′ W50°00.181′ (see Online Resources 1, 2). In total, 33 cryoconite samples were collected: four from cryoconite puddles, 28 from cryoconite holes and one (consisting of two subsamples) from a cryoconite lake (Online Resource 2, Fig. 1a–d). In this study, we considered (a) cryoconite holes to be reservoirs formed in the ice which are usually circular or oval-shaped and up to 30 cm in diameter (Fig. 1b), (b) cryoconite puddles to be shallow reservoirs or a deposition of cryoconite material in an irregular shape without clear borders (Fig. 1c), (c) cryoconite lakes to be large (more than a few square meters in size) water reservoirs with cryoconite material on the floor (Fig. 1d). The number of samples (N), the elevation above sea level (a.s.l.), pH, water content, the type of cryoconite material, and invertebrate densities were noted in Online Resource 2.
The cryoconite samples were collected with disposable plastic Pasteur pipettes and transferred into 15 cm3 plastic test tubes. For a faunistic and phycological analysis the collected samples were preserved using 96% ethylene alcohol. For a microbiological, biochemical, and radioactivity analysis the samples were collected separately. Microbiological samples were collected under sterile conditions, with sterile gloves and sterile plastic tubes. Then, the samples were frozen, placed in an icebox packed with ice and transported to the laboratory at Adam Mickiewicz University. The sediments were divided visually into two categories: mud and typical cryoconite granules (Fig. 2a–d). We considered the typical cryoconite granules as roundish or quasi-spherical particles with clearly visible organic parts (cyanobacteria) under a stereo microscope. Granules of that shape refer to those presented in Takeuchi et al. (2001b), and Cook et al. (2015b) (Fig. 2a, b). The height of water content in the center of holes (water depth) was measured using a ruler, while pH was measured at the bottom of the reservoirs close to the cryoconite material, using a Hanna HI 98129 probe.
Micro-animals, cyanobacteria, algae isolation and identification
From each sample, 1 cm3 of cryoconite material was scanned for tardigrades and rotifers with a stereomicroscope at 40× magnification. All tardigrades and their exuvia with eggs were mounted on microscopic slides in Hoyer’s medium and then examined with a phase contrast microscope (PCM), Olympus, model BX53. The taxon was identified using recent descriptions (Zawierucha et al. 2016a). Trophic groups were established based on the scheme presented in Guidetti et al. (2012).
Cyanobacterial and algal species observation was conducted with a Nikon Eclipse TE2000-S digital microscope equipped with a Nikon DS-Fi1 camera. Taxa were archived using NIS image analysis software with scalable object images. Due to a large amount of mineral deposits it was impossible to count the cyanobacterial and algal cells using the typical method of a sedimentation chamber. In order to conduct a quantitative analysis of species in the samples, the taxa were counted on slides. The so-called visible “calculation units” were counted and the following counted as calculation units: individual cells and 100-µm filaments (Klebsormidium filaments in the studied material often collapsed into individual cells, therefore the mean of seven cells was treated as 100-µm filaments — one calculation unit). The calculations were conducted in order, along the parallel specimen lines, through moving the field of vision by one unit. The calculations were conducted in ten repetitions, each time applying 0.1 ml volume on a slide and then counting mean values of units in 0.1 ml volume re-calculated to 1 ml volume (Napiórkowska-Krzebietke et al. 2012). The taxonomy of cyanobacteria and algae was based on Hoek et al. (1995). Cyanobacteria and algae were identified according to the following studies: Starmach (1972), Komárek and Anagnostidis (2005), Coesel and Meesters (2007).
Chemical analysis—ion concentrations
Cryoconite water and debris samples were transported to the laboratory of the Department of Analytical Chemistry of the Gdańsk University of Technology and stored at a temperature of 4 °C. In order to minimize the storage time, the analyses were performed immediately after the delivery of the samples to the laboratory, according to the analysis data presented in Online Resource S5. Before the analysis the samples of cryoconite water had been filtered through 0.45-µm filters.
Quantitative analyses of basic anions (F−, Cl−, NO2 −, Br−, NO3 −, PO4 3−, and SO4 2−) and cations (Li+, Na+, NH4 +, K+, Mg2+, and Ca2+) were determined by means of ion chromatography (ICS 3000 Dionex). The determination of various groups of analytes involved the application of demineralized Mili-Q water (Mili-Q® Ultrapure Water Purification Systems, Millipore® production). The analyses of ions were performed with the application of Standard Reference Material NIST, previously described in Lehmann et al. (2016).
Radiometric analysis
Samples were dried at 105 °C and subsequently measured for gamma (137Cs, 210Pb), alpha (238Pu, 239+240Pu) and beta (90Sr) spectrometry. For gamma analyses, a planar HPGe (high-purity germanium) detector (home-made by the Institute of Nuclear Physics PAS Krakow and electronics by Silena S.p.A.) was used. The activities of 137Cs were determined via the 137mBa emission peak at 662 keV but for 210Pb the 46.6-keV peak was used as the analytical signal. The activities of 238Pu, 239+240Pu and 90Sr were determined for 0.34–0.78 g of the dried samples. The samples were digested using concentrated HF, HNO3, HCl, and a small addition of H3BO3. Finally, the sample solution was converted to 1 M HNO3. The radiochemical procedure consisted of a Pu separation step by anion exchange in 8 M HNO3 after oxidation-state adjustment to Pu(IV). The separation of strontium from the effluent from the Dowex 1X8 column (8 M HNO3) took place on another column which was filled with Sr-resin (Triskem). The chemical procedure that followed was based on that previously described (Łokas et al. 2016). The measurements of plutonium isotope activities were determined using alpha particle spectrometers with semiconductor, passivated planar silicon detectors (Canberra) on a Silena Alphaquattro spectrometer (Silena S.p.A). 90Sr was measured using a Wallac 1414 Guardian LSC spectrometer. A reference material (IAEA 447) was analyzed to ensure the quality of measurements (Online Resource S6). The obtained results corresponded well with the certified values of activities calculated in November 2009.
Quantitative real-time PCR (qPCR)
Metagenomic DNA was extracted from 1 g (wet weight) of each sample by heat lysis and a Genomic Mini kit (A&A Biotechnology). DNA quality and quantity were assessed spectrophotometrically and electrophoretically. The purified DNA samples were then stored at −20 °C for further analyses.
The qPCR was performed to quantify the bacterial 16S rRNA gene in total DNA. The 16S rRNA gene copy numbers were determined using the forward primer 5′-CGCAACGAGCGCAACCC-3′ and reverse primer 5′CGTAAGGGCCATGAKGA-3′ set described previously (Xi et al. 2009). The total number of bacteria was calculated as the copy number of 16S rRNA gene/4, with four being the average number of copies of the gene encoding 16S rRNA per bacterial cell, according to the ribosomal RNA database (Klappenbach et al. 2001; Stalder et al. 2012). Serial dilutions of standard templates ranging from 109 to 102 gene copies were used to generate the qPCR standard curve. The specificity of amplification was determined by a melt curve analysis and gel electrophoresis. The qPCR amplifications were performed in a 96-well plate in a final volume of 20 ml with Luminaris HiGreen qPCR Master Mix (Thermo Scientific) using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad).
Data analysis
We obtained a full set of descriptors: the count of each phylum (densities), water depth of the cryoconite sample areas, water pH and the height above mean sea level for 33 samples. We computed all the analyses in R software version 3.3.1 (R Core Development Team 2016).
We tested for a relationship between Tardigrada, Rotifera, cyanobacteria, and algae count by building generalized linear models (GLMs) with the abundance of Tardigrada (models set 1) and Rotifera (models set 2) as response variables, and the abundance of the other three groups as explanatory variables. We used a log-link, Poisson family error distribution and corrected for residual overdispersion. We also removed an outlier with exceptionally high cyanobacteria abundance from the analysis (101 individuals, mean no. of individuals and SD for the dataset: 12 ± 25) as model validation showed that it had a Cook’s distance value above 1. For each group, we computed models consisting of all the possible combinations of explanatory variables, and evaluated them with quasi-AIC (QAIC) using the ‘MuMIn’ package (Bartoń 2016).
We tested for a relationship between Tardigrada, Rotifera, cyanobacteria, and algae count and water depth of the cryoconite sample areas, as well as water pH and the height above mean sea level by Poisson family (corrected for overdispersion), log-link GLMs with the count of each group as a response variable. For each group, we computed models consisting of all the possible combinations of explanatory variables, and evaluated them with quasi-AIC (QAIC). We followed an information-theoretic approach to identify the most parsimonious models (Burnham and Anderson 2002). The model with the lowest QAIC within the set was considered the best, given the data (Burnham and Anderson 2002), but we discarded models with uninformative parameters that were more complex versions of a competitive model with fewer parameters whose QAIC < 2 (Arnold 2010). The best candidate model had a ΔQAIC of zero. As a rule of thumb, models with a substantial empirical support had ΔQAIC < 2; models with ΔQAIC between 4 and 7 had considerably less support, and models with ΔQAIC > 10 were essentially not supported (Burnham and Anderson 2002). In both analyses based on the QAIC we discarded models with uninformative parameters that were more complex versions of a competitive model with fewer parameters whose ΔAICc was < 2 (a detailed explanation of why these parameters are uninformative can be found in Arnold 2010).
For a subsample of cryoconite holes we obtained data on water biochemistry (N = 12). Here, we first performed the principal component analysis (PCA) on the concentration of cations and anions to reduce the 11 ion concentration variables to a few essential components. Next, we included the principal components that explained most variance (see the results) as explanatory variables into Poisson (corrected for overdispersion), log-link GLMs with an abundance of each phylum as a response variable. Here, we arrived at the final model structure by sequentially removing non-significant variables (Zuur et al. 2009).
Results
Invertebrate, algological, radiometrical, chemical and microbiological descriptive statistics are presented in Online Resources 2, 4, 6, 7, 8, 9, respectively.
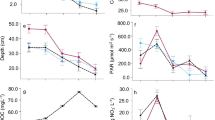
Two groups of invertebrates were found in this study: Tardigrada and Rotifera (Online Resource 3a-b), and no other microscopic animals (nematodes or arthropods) were found. Only one taxon of Tardigrada was found — Pilatobius sp. Rotifers were not identified. Tardigrada and Rotifera were found in 6 samples (18% of all samples) and 20 samples (61%), respectively. The highest density of tardigrades and rotifers constituted 53 and 118 ind./ml, respectively (on average, 3.5 tardigrades and 10.4 rotifers per milliliter of cryoconite). The density of both groups was significantly higher in water reservoirs with granules than in those with mud (Tardigrada: χ 2 = 14.97, df = 1, p < 0.001, Rotifera: χ 2 = 11.79, df = 1, p < 0.001, see Fig. 3).
Cryoconite cyanobacteria and algae composition
Thirteen cyanobacterial and algal taxa were recorded belonging to 3 phyla (Online Resource 4). The most numerous were cyanobacteria (five species, representing more than 38% of all the identified species), including the orders Oscillatoriales (31%, four species), Nostocales (7%, one species), and Heterokontophyta (five species, more than 38%) represented by the class Bacillariophyceae. Chlorophyta were the least species-diverse (three taxa representing 24% of all the identified species) belonging to classes Klebsormidiophyceae, Zygnematophyceae and Chlorophyceae (Online Resource 1). Klebsormidium sp. (Chlorophyta) was recorded in almost all the samples. The next dominants were Leptolyngbya sp. and Pseudanabaena frigida.
Radionuclides
Activity concentration of anthropogenic (137Cs, 239+240Pu and 90Sr) and natural (210Pb) radionuclides in cryoconite samples was detected (Online Resource 6). In most cases, the activity concentration of cesium was below the minimal detectable concentration (MDC) but in two samples (9 and 30) activity concentrations varied between 43 and 123 Bq/kg dw. The MDC values of 137Cs varied between 13 and 280 Bq/kg dw depending on a very small sample mass. The activity concentrations of 238Pu in each sample were below the minimal detectable concentration. In most samples, the activity concentrations of 239+240Pu were also below MDC (0.03–0.11 Bq/kg dw), but in three samples (4, 24 and 30) this varied from 0.03 to 0.82 Bq/kg. The activity concentrations of 90Sr were below MDC (7.4–11.3 Bq/kg dw) except for sample 23 (23.2 ± 7.3 Bq/kg dw). 210Pb is a natural, airborne radionuclide which binds to and is transported with aerosol particles. Atmospheric fallout of 210Pb varies spatially depending mainly on rainfall and geographical location. The 210Pb activity concentrations ranged from 63 to 1561 Bq/kg.
Relations of biota to abiotic factors (water depth, pH, a.s.l.)
Tardigrada and Rotifera counts were neither related to one another, nor to counts of the other groups (cyanobacteria and algae) in the samples. In both cases, the null model received the most QAIC support (Online Resource 10). We found no relationship between Tardigrada, Rotifera and cyanobacteria count and the measured abiotic factors as the null models received most QAIC support in all three cases (Online Resource 12). The best models for the Rotifera and cyanobacteria count contained the positive effect of water depth but in both cases the null model received similar support (ΔQAIC < 2). The best model for the algae count included the negative effects of water pH (Online Resource 11), with regression coefficient 95% confidence intervals equal to: −2.25 to −0.21.
Relation of biota to water biochemical parameters
The PCA resulted in 11 principal components, three of which had an eigenvalue above 1 (Online Resource 13). These first three components explained 43.7, 24.3, and 13.6% of variance, respectively. The positive values of PC1 represented an environment rich in PO4 3−, NO3 −, Na+, Cl−, Mg2+, while PC1 negative values represented an environment rich in NH4 +, F−, NO2 −, and SO4 2− (Table 2S). The positive values of PC2 represented an environment with a relatively high Mg2+ and Ca2+ concentration, while the negative PC2 values correlated with a high concentration of Na+, Cl−, NH4 +, K+, NO3 −, PO4 3−, and SO4 2−. The positive values of PC3 represented a relatively high concentration of K+ and PO4 3−, and the negative PC3 values an environment relatively rich in all the other measured ions (Online Resource 12). However, the counts of the studied groups were not related to any PC (p > 0.20). In the case of Tardigrada, PC2 had a significant negative effect but the model validation showed that this was driven by two observations that had a Cook’s distance value above 1. Refitting the model without these observations resulted in no significant relationships.
Discussion
Tardigrade and rotifer distribution and densities
The density of invertebrates was higher in cryoconite granules. Greater melt rates and marginally steeper surface slopes result in a frequent or periodic disruption and redistribution of cryoconite holes and their contents (Hodson et al. 2007, 2008). The mud often comes from cryoconite reworked by streaming water after being flushed from cryoconite holes (a typical process during rain events). The difference in the abundances of animals does not reflect a specific habitat preference but may reflect a simple mechanistic removal of animals from the sediment during its erosion. Therefore, the distribution of tardigrades and rotifers on the edge of the GrIS seems to be random. Cryoconite granules are a consortium of archaea, cyanobacteria, heterotrophic bacteria, algae and fungi (Takeuchi et al. 2001a, b, 2010; Langford et al. 2014). In laboratory cultures tardigrades belonging to Hypsibioidea (e.g., genus Pilatobius) feed mainly on algae (e.g., Altiero and Rebecchi 2001; Kosztyła et al. 2016). Thus, those granules may be an abundant and stable source of food for invertebrates. Moreover, cryoconite granules ease the locomotion of animals to a greater extent than mud. Strong relations between a grain size and micro-animal densities were detected for meiofauna in other freshwater ecosystems (Beier and Traunspurger 2003).
We did not find a clear relationship between the 16S rRNA gene copy number and tardigrade and rotifer abundance (Online Resource 9). However, this pattern was based on too small a sample size and could not statistically assess the data. Further studies on the relation between micro-fauna and bacteria on glaciers could be a fruitful avenue of future research.
In the previously published studies, the presence and density of rotifers and tardigrades were correlated with each other (De Smet and Van Rompu 1994; Grøngaard et al. 1999; Porazińska et al. 2004). In this study, we did not find a correlation between those two groups, and we noted a significantly lower density of animals than in the previous works. The density of tardigrades in High Arctic glaciers reached up to 168 ind./ml and 624 ind. g−1 of dry weight, with their frequency of up to 90% of samples (Zawierucha et al. 2016b). Those differences between High Arctic glaciers and the edge of the GrIS in the south-west may be related to a strong flushing of sediments at the margin. The proportion of eroded granules to mud and stable granules confirms this hypothesis (seven to 26 samples).
Cyanobacteria and algae in cryoconite holes
Cyanobacteria and algae are characterized by a broad range of ecological tolerance which allows them to colonize all environments, even the most extreme ones. Among the many studies on cryoconite holes in Greenland (Cook et al. 2016; Hodson et al. 2010a; Stibal et al. 2010) few describe cyanobacteria and algae assemblages (Uetake et al. 2010, 2016; Kaczmarek et al. 2016). The Klebsormidium genus has been often recorded in extreme Arctic habitats and has a high tolerance to gradients of light, temperature and UVR (Kitzing and Karsten 2015). This probably results from the fact that when forming akinetes filled with reserve material and covered with a thick membrane, this species efficiently adapts to the harsh Arctic conditions. Due to an easy vegetative multiplication by filament division into individual cells, it often occurs in large quantities (Starmach 1972).
In this study, Klebsormidium sp., a filamentous chlorophyte, was the dominant genus. Uetake et al. (2010, 2016) also noticed a clear domination of a filamentous chlorophyta species. It was accompanied by the desmidia of Cylindrocystis brebissonii, also identified in one of the studied locations, which may suggest that it is a species characteristic of cryoconite holes. Klebsormidium, which occupies an environment where both seasonal and diurnal variations of water availability prevail, is well-adapted to freezing and desiccation injuries (Elster et al. 2008). Cylindrocystis brebissonii belongs to the group of snow algae, i.e., species which can be designated as cold-resistant (psychrotolerant), with its highest metabolic activities between 10 and 20 °C (Komárek and Nedbalová 2007).
Invertebrates and abiotic factors
Ecological studies on invertebrates in cryoconite holes were conducted on high mountain, valley or tidewater glaciers which are relatively flat and are gradually reducing their elevation above sea level (Dastych et al. 2003; Porazińska et al. 2004; Zawierucha et al. 2016a). The ice sheet in this study is characterized by numerous hills and small valleys, thus it is not surprising that we did not find the effect of altitude on invertebrate density. Differences in the distribution of sampling points (small hills vs valleys) along with a high flushing rate on the edge of the GrIS (e.g., Stibal et al. 2010) may strongly influence the micro-fauna distribution pattern.
Water chemistry has been found to be an important abiotic factor influencing the occurrence of animals. Porazińska et al. (2004) showed that tardigrade variation was best explained by NH4 +, NO3 − and Mg2+ (Porazińska et al. 2004). Zawierucha et al. (2016a) showed that the occurrence of tardigrades seems to be related to the concentration of Mg2+ and other cations, including K+ and Ca2+. Those cations may play an important role as nutrients, especially in tardigrades and bdelloid rotifers whose CaCO3 incrustations have also been reported in rotifer trophi and a tardigrade buccal apparatus (Melone et al. 1998; Guidetti et al. 2012). In this study, Tardigrada and Rotifera were not related to water chemistry. This may be the result of locality (the edge of the GrIS), ice melting, flushing and the dilution of nutrients influencing a random distribution of micro-fauna. Stibal et al. (2010) suggested that a high flushing rate from the margin of the ice sheet may negatively influence nutrient concentration.
Therefore, activity concentrations of radionuclides obtained in this study have been compared with data from cryoconites from SW Spitsbergen (Łokas et al. 2016), and these activity concentrations are distinctly lower. The relation between micro-animals and the flux of natural and anthropogenic pollution in glacial systems and freshwater trophic webs remains unexplored (Łokas et al. 2014, 2016), including radionuclides. Łokas et al. (2016) showed that heavy metals and radionuclides do not have a negative impact on tardigrades and rotifers on the Hans Glacier (Svalbard) and animals inhabited these cryoconite holes in high densities (Łokas et al. 2016; Zawierucha et al. 2016b). Micro-fauna (as a top consumer) and cryoconite granules (cyanobacteria which produce EPS) may cumulate pollutants from the atmosphere. In our study, cryoconite granules were mostly eroded to cryoconite mud and animals were less frequent in comparison with other locations, thus leading to a low radionuclide content.
Summary and conclusion
The knowledge of animal diversity and ecology in glacial habitats remains scarce (Zawierucha et al. 2015, 2016a). We found higher densities of invertebrates in cryoconite granules than in mud in the material collected in 2015. The difference in densities of animals between granules and mud may reflect a simple mechanistic removal of fauna from the sediment during its erosion by flushing which leads to mud formation at the edge of the GrIS. The proportion of eroded granules to mud and stable granules does not falsify this hypothesis. Moreover, cryoconite granules are the consortia of various microorganisms (Takeuchi et al. 2001a, b; Hodson et al. 2010b) which may prevail in more stable cryoconite reservoirs (undisturbed) and constitute an abundant and stable source of food for invertebrates — algae, cyanobacteria, heterotrophic bacteria and fungi. Thirteen cyanobacterial and algal taxa were recorded and Klebsormidium sp., a filamentous chlorophyte, was the dominant genus. Activity concentrations of anthropogenic (137Cs, 239+240Pu and 90Sr) and natural (210Pb) radionuclides in cryoconite samples were found, but were distinctly lower than on the other glaciers. Micro-animals (top consumers in cryoconites) and cyanobacteria, which produce EPS and form granules, may cumulate pollutants from the atmosphere. In our study, cryoconite granules were mostly eroded to cryoconite mud and animals were removed by flushing, thus most probably leading to a low radionuclide content. As opposed to the previous studies conducted on High Arctic glaciers, the frequency and density of invertebrates were low, and surprisingly, only one taxon of tardigrades (Pilatobius sp.) has been found. The lack of clear pattern between biotic and abiotic components of cryoconite holes and significantly lower abundance and frequency of invertebrates in comparison to High Arctic glaciers may result from sampling location and a fast melting of the GrIS surface (Doyle et al. 2015); Stibal et al. (2010, 2012) and Cameron et al. (2016) suggested that nutrients increased with the distance from the margin of the GrIS, and it is a place of rapid flushing, the dilution of nutrients and a main way for microbes to travel to downstream ecosystems. A periodic disruption, redistribution and erosion of cryoconite material led to a random distribution of tardigrades and rotifers without clear ecological interactions with biotic and abiotic variables at the edge of the GrIS. Additionally, we suggest that the relation between animals and the 16S rRNA gene copy number on glaciers may be a fruitful avenue of future ecological research in undisturbed cryoconite holes (bacteria as food). Studies on glacial ecosystems should focus on investigations into cryoconite hole animal ecology to a greater extent. Those animals (top consumers on glaciers) may be the key to understanding densities and interactions between cryoconite biota in glacial ecosystems.
References
Altiero T, Rebecchi L (2001) Rearing tardigrades: results and problems. Zool Anzg 240:217–221
Anesio AM, Laybourn-Parry J (2012) Glaciers and ice sheets as a biome. TrEE 4:219–225
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildl 74:1175–1178
AMAP assessment (2015) Radioactivity in the Arctic. Arctic monitoring and assessment programme (AMAP), Oslo, Norway
Bartoń K (2016) MuMIn: multi-model inference. R package version 1.15.6. https://CRAN.R-project.org/package=MuMIn
Beier S, Traunspurger W (2003) Temporal dynamics of meiofauna communities in two small submountain carbonate streams with different grain size. Hydrobiol 498:107–131
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. 2nd edn. Springer, New York
Cameron AK, Stibal M, Hawkings JR, Mikkelsen AB, Telling J, Kohler TJ, Gözdereliler E, Zarsky JD, Wadham JL, Jacobsen CS (2016) Meltwater export of prokaryotic cells from the Greenland ice sheet. Environ Microbial. doi:10.1111/1462-2920.13483
Coesel PFM, Meesters J (2007) Desmids of the lowlands. Mesotaeniaceae and Desmidiaceae of the European Lowlands. KNNV Publishing, The Netherlands., p 351
Cook JM, Edwards A, Hubbard A (2015a) Biocryomorphology: integrating microbial processes with ice surface hydrology, topography, and roughness. Front Earth Sci 3:78
Cook JM, Edwards A, Takeuchi N, Irvine-Fynn T (2015b) Cryoconite. The dark biological secret of the cryosphere. Prog Phys Geogr 40:1–46
Cook JM, Edwards A, Bulling M, Mur LAJ, Cook S, Gokul JK, Cameron KA, Sweet M, Irvine-Fynn TDL (2016) Metabolome-mediated biocryomorphic evolution promotes carbon fixation in Greenlandic cryoconite holes. Environ Microbiol. 18(12):4674–4686
Dastych H, Kraus HJ, Thaler K (2003) Redescription and notes on the biology of the glacier tardigrade Hypsibius klebelsbergi Mihelcic, 1959 (Tardigrada), based on material from Ötztal Alps, Austria. Mitt Hamb Zool Mus Inst 100:73–100
De Smet WHE, Van Rompu A (1994) Rotifera and Tardigrada from some cryoconite holes on a Spitsbergen (Svalbard) glacier. Belg J Zool 124:27–37
Dowdall M, Gerland S, Lind B (2003) Gamma-emitting natural and anthropogenic radionuclides in the terrestrial environment of Kongsfjord, Svalbard. Sci Total Environ 305:229–240
Doyle SH, Hubbard A, van de Wal RSW, Box JE, van As D, Scharrer K, Meierbachtol TW, Smeets PCJP, Harper JT, Johansson E, Mottram RH, Mikkelsen AB, Wilhelms F, Patton H, Christoersen P, Hubbard B (2015) Amplified melt and flow of the Greenland ice sheet driven by late-summer cyclonic rainfall. Nat Geosci 8:647–653
Elster J, Degma P, Kováčik Ľ, Valentová L, Šramková K, Batista Pereira A (2008) Freezing and desiccation injury resistance in the filamentous green alga Klebsormidium from the Antarctic, Arctic and Slovakia. Biologia 63(6):843–851
Gawor J, Grzesiak J, Sasin-Kurowska J, Borsuk P, Gromadka R, Górniak D, Świątecki A, Aleksandrzak-Piekarczyk T, Zdanowski MK (2016) Evidence of adaptation, niche separation and microevolution within the genus Polaromonas on Arctic and Antarctic glacial surfaces. Extremophiles 20:403–413
Gokul JK, Hodson AJ, Saetnan ER, Irvine-Fynn TD, Westall PJ, Detheridge AP, Takeuchi N, Bussell J, Mur LA, Edwards A (2016) Taxon interactions control the distributions of cryoconite bacteria colonizing a High Arctic ice cap. Mol Ecol 25:3752–3767
Grøngaard A, Pugh PJA, McInnes SJ (1999) Tardigrades, and other cryoconite biota, on the Greenland ice sheet. Zool Anz 238:211–214
Grzesiak J, Górniak D, Świątecki A, Aleksandrzak-Piekarczyk T, Szatraj K, Zdanowski MK (2015) Microbial community development on the surface of Hans and Werenskiold Glaciers (Svalbard, Arctic): a comparison. Extremophiles 19:885–897
Guidetti R, Altiero T, Marchioro T, Sarzi Amade L, Avdonina AM, Bertolani R, Rebecchi L (2012) Form and function of the feeding apparatus in Eutardigrada (Tardigrada). Zoomorphology 131:127–148
Hodson A, Anesio AM, Ng F, Watson R, Quirk J, Irvine-Fynn T, Dye A, Clark Ch, McCloy P, Kohler J, Sattler B (2007) A glacier respires: quantifying the distribution and respiration CO2 flux of cryoconite across an entire Arctic supraglacial ecosystem. J Geophys Res 112(G4):G04S36. doi:10.1029/2007JG000452
Hodson A, Anesio AM, Tranter M, Fountain A, Osborn M, Priscu J, Laybourn-Parry J, Sattler B (2008) Glacial ecosystems. Ecol Monogr 78:41–67
Hodson A, Bøggild C, Hanna E, Huybrechts P, Langford H, Cameron K, Houldsworth A (2010a) The cryoconite ecosystem on the Greenland ice sheet. Ann Glaciol 51(56):123–129
Hodson A, Cameron K, Bøggild C, Irvine-Fynn T, Langford H, Pearce D, Ban-Wart S (2010b) The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. J Glaciol 56:349–362
Hoek C, Mann DG, Johns HM (1995) Alga: an introduction to phycology. Great Britain at University Press, Cambridge, p 623
Irvine-Fynn TDL, Bridge JW, Hodson AJ (2010) Rapid quantification of cryoconite: granule geometry and in situ supraglacial extents, using examples from Svalbard and Greenland. J Glaciol 56:297–306
Johannessen OM, Volkov VA, Pettersson LH, Maderich VS, Zheleznyak MJ, Gao Y, Bobylev LP, Stepanov AV, Neelov IA, Tishkov VP, Nielsen SP (2010) Radioactivity and pollution in the Nordic Seas and Arctic Region. Observations, modeling and simulation. Springer, New York
Kaczmarek Ł, Jakubowska N, Celewicz-Gołdyn S, Zawierucha K (2016) Cryoconite holes microorganisms (algae, Archaea, bacteria, cyanobacteria, fungi, and Protista)—a review. Polar Rec 52:176–203
Kitzing Ch, Karsten U (2015) Effects of UV radiation on optimum quantum yield and sunscreen contents in members of the genera Interfilum, Klebsormidium, Hormidiella and Entransia (Klebsormidiophyceae, Streptophyta). Eur J Phycol 50:279–287. doi:10.1080/09670262.2015.1031190
Klappenbach JA, Saxman PR, Cole JR, Schmidt TM (2001) rrnDB: the Ribosomal RNA Operon Copy Number Database. Nucl Acids Res 29:181–184
Komárek J, Anagnostidis K (2005) Cyanoprocaryota; Oscillatoriales II. In Büdel AB, Krienitz L, Gärtner G, Schagerl M (eds) Süβwasserflora von Mitteleuropa, 19.2, Spektrum Akademischer Verlag 759
Komárek J, Nedbalová L (2007) Green cryosestic algae. Algae Cyanobacteria Extrem Environ 11:321–342
Kosztyła P, Stec D, Morek W, Gąsiorek P, Zawierucha K, Michno K, Ufir K, Małek D, Hlebowicz K, Laska A, Dudziak M, Frohme M, Prokop ZM, Kaczmarek Ł, Michalczyk Ł (2016) Experimental taxonomy confirms the environmental stability of morphometric traits in a taxonomically challenging group of microinvertebrates. Zool J Linn Soc 178(4):765–775
Langford HJ, Irvine-Fynn TDL, Edwards A, Banwart SA, Hodson AJ (2014) A spatial investigation of the environmental controls over cryoconite aggregation on Longyearbreen glacier, Svalbard. Biogeosciences 11:5365–5380
Lehmann S, Gajek G, Chmiel S, Polkowska Ż (2016) Do morphometric parameters and geological conditions determine chemistry of glacier surface ice? Spatial distribution of contaminants present in the surface ice of Spitsbergen glaciers (European Arctic). Environ Sci Pollut Res 23(23):23385–23405
Łokas E, Bartmiński P, Wachniew P, Mietelski JW, Kawiak T, Srodoń J (2014) Sources and pathways of artificial radionuclides to soils at a High Arctic site. Environ Sci Pollut Res 21:12479–12493
Łokas E, Zaborska A, Kolicka M, Różycki M, Zawierucha K (2016) Accumulation of atmospheric radionuclides and heavy metals in cryoconite holes on an Arctic glacier. Chemosphere 160:162–172
Lutz S, Anesio AM, Villar SEJ, Benning LG (2014) Variations of algal communities cause darkening of a Greenland glacier. FEMS Microbiol Ecol. doi:10.1111/1574-6941.12351
Melone G, Ricci C, Segers H (1998) The trophi of Bdelloidea (Rotifera): a comparative study across the class. Can J Zool 76:1755–1765
Mueller DR, Vincent WF, Pollard WH, Fritsen CH (2001) Glacial cryoconite ecosystems: a bipolar comparison of algal communities and habitats. Nova Hedwig Beih 123:173–197
Napiórkowska-Krzebietke A, Pasztaleniec A, Hutorowicz A (2012) Phytoplankton metrics response to the increasing phosphorus and nitrogen gradient in shallow lakes. J Elem 17:289–303. doi:10.5601/jelem.2012.17.2.11
Paatero J, Vira J, Siitari-Kauppi M, Hatakka J, Holmén K, Viisanen Y (2012) Airborne fission products in the High Arctic after the Fukushima nuclear accident. J Environ Radioac 114:41–47
Porazińska DL, Fountain AG, Nylen TH, Tranter M, Virginia RA, Wall DH (2004) The biodiversity and biogeochemistry of cryoconite holes from McMurdo Dry Valley Glaciers, Antarctica. Arct Antarct Alp Res 36:84–91
Singh P, Hanada Y, Singh SM, Tsuda S (2014) Antifreeze protein activity in Arctic cryoconite bacteria. FEMS Microbiol Lett 35:14–22
Stalder T, Barraud O, Casellas M, Dagot C, Ploy MC (2012) Integron involvement in environmental spread of antibiotic resistance. Front Microbiol 3:119
Starmach K, (1972) Flora słodkowodna Polski: Chlorophyta III. Zielenice nitkowate 10. PWN, Warszawa, Kraków 751
Stibal M, Lawson EC, Lis GP, Mak KM, Wadham JL, Anesio AM (2010) Organic matter content and quality in supraglacial debris across the ablation zone of the Greenland ice sheet. Ann Glaciol 51:1–8
Stibal M, Telling J, Cook J, Mak KM, Hodson A, Anesio AM (2012) Environmental controls on microbial abundance and activity on the Greenland ice sheet: a multivariate analysis approach. Microbial Ecol 63:74–84
Stibal M, Schostag M, Cameron KA, Hansen LH, Chandler DM, Wadham JL, Jacobsen CS (2015) Different bulk and active bacterial communities in cryoconite from the margin and interior of the Greenland Ice Sheet. Environ Microbiol Rep 7:293–300
Takeuchi N, Kohshima S, Shiraiwa T, Kubota K (2001a) Characteristics of cryoconite (surface dust on glaciers) and surface albedo of a Patagonian glacier, Tyndall Glacier, Southern Patagonia Icefield. Bull Glaciol Res 18:65–69
Takeuchi N, Kohshima S, Seko K (2001b) Structure, formation, and darkening process of albedo-reducing material (cryoconite) on a Himalayan glacier: a granular algal mat growing on the glacier. Arct Antarct Alp Res 33:115–122
Takeuchi N, Nishiyama H, Li Z (2010) Structure and formation process of cryoconite granules on Ürümqiglacier No. 1, Tien Shan, China. Ann Glaciol 51(56):9–14
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Uetake J, Naganuma T, Hebsgaard MB, Kanda H, Kohshima S (2010) Communities of algae and cyanobacteria on glaciers in west Greenland. Polar Sci 4:71–80
Uetake J, Tanaka S, Segawa T, Takeuchi N, Nagatsuka N, Motoyama H, Aoki T (2016) Microbial community variation in cryoconite granules on Qaanaaq Glacier, NW Greenland. FEMS Microbiol Ecol 92:1–10
Vonnahme TR, Devetter M, Zárský JD, Sabacká M, Elster J (2015) Controls on microalgal community structures in cryoconite holes upon High Arctic glaciers, Svalbard. Biogeosciences 12:11751–11795
Wharton RA, McKay CP, Simmons GM, Parker BC (1985) Cryoconite holes on glaciers. Bioscience 35:449–503
Xi C, Zhang Y, Marrs YL, Ye W, Simon C, Foxman B, Nriagu J (2009) Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl Environ Microbiol 75:5714–5718
Zawierucha K, Kolicka M, Takeuchi N, Kaczmarek Ł (2015) What animals can live in cryoconite holes? A faunal review. J Zool 295:159–169
Zawierucha K, Ostrowska M, Vonnahme TR, Devetter M, Nawrot AP, Lehmann S, Kolicka M (2016a) Diversity and distribution of Tardigrada in Arctic cryoconite holes. J Limnol 75:545–559
Zawierucha K, Vonnahme TR, Devetter M, Kolicka M, Ostrowska M, Chmielewski S, Kosicki JZ (2016b) Area, depth and elevation of cryoconite holes in the Arctic do not influence Tardigrada densities. Pol Polar Res 37:325–334
Zuur A, Leno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
K.Z. would like to thank his guide, William Guzman, for all his patience during sampling, and to Piotr Klimaszyk for lending a probe for the pH measurements. The authors would like to thank Marta Kołowrotkiewicz, Katherine Short (British Antarctic Survey) and the Cambridge Proofreading company for proofreading the manuscript. We are also grateful to two anonymous reviewers for their comments on the manuscript. K.Z. would also like to thank Joseph Cook (University of Sheffield) and the journal editor professor Jamie Kneitel for their comments on the manuscript. The studies were supported by the National Science Center grant no. NCN 2013/11/N/NZ8/00597 to K.Z. M.B. was supported by the National Science Center grant “Etiuda” no. 2015/16/T/NZ8/00018 and by the Foundation for Polish Science scholarship “Start”. Radionuclide analyses were supported by the National Science Center grant “Opus 11” no. 2016/21/B/ST10/02327 to E.Ł. Ion analyses were supported by the National Science Center grant “Preludium 9” no. 2015/17/N/ST10/03177.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Jamie Kneitel.
Electronic supplementary material
Below are the links to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zawierucha, K., Buda, J., Pietryka, M. et al. Snapshot of micro-animals and associated biotic and abiotic environmental variables on the edge of the south-west Greenland ice sheet. Limnology 19, 141–150 (2018). https://doi.org/10.1007/s10201-017-0528-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-017-0528-9