Abstract
The objective of this study was to explore the environmental factors having the greatest influence on the distribution and abundance of epipelic diatom species in different wetlands in southern Spain. We previously defined four groups of conductivity categories: fresh (< 0.8 mS cm−1), oligosaline (< 8 mS cm−1), mesosaline (8–30 mS cm−1) and eusaline (> 30 mS cm−1). A dbRDA analysis performed on a subset of 36 of the 53 wetlands, using a total of 25 environmental variables, showed that five environmental variables (conductivity, pH, wetland area, silicates, and total suspended solids) were the best explanatory variables for the diatom assemblage, with conductivity being the main explanatory variable. Nonmetric multidimensional scaling (nMDS) analysis performed on the set of 53 wetlands revealed significant differences in diatom composition among the four conductivity groups. The key species in the eusaline group were Tryblionella pararostrata, Halamphora sp.1 and Cocconeis euglypta, whereas in the mesosaline and oligosaline group, these were Navicula veneta, Tryblionella hungarica and Nitzschia inconspicua. Finally, in the fresh group dominated Achnanthidium minutissimum, Navicula veneta and Gomphonema exilissimum. This study on epipelic diatoms can therefore contribute to the knowledge of these organisms in a European region with a high diversity of wetland typologies.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Aquatic ecosystems are extensively widespread and can be found worldwide (Aponte et al., 2010). Among them, wetlands (Stenger-Kovács et al., 2014; Blanco et al., 2020) harbour a significant biodiversity (Nicolet et al., 2004; Biggs et al., 2005; Della Bella & Mancini, 2009) and provide valuable ecosystem services to humans (EPCN, 2008; Wallis et al., 2011), while also serving as indicators of global changes (Williamson et al., 2009; Gottschalk & Kahlert, 2012). However, anthropogenic pressures such as urbanization and agriculture are severely impacting these ecosystems, resulting in a decline in their quality and quantity (Biggs et al., 2005; EPCN, 2008; Arias & Gómez, 2015). Taxonomic studies have become an important focus due to the global biodiversity loss caused by the degradation of aquatic ecosystems (Pimm et al., 1995). In some cases, such as in saline wetlands, even small perturbations can have irreversible consequences (Hammer, 1990). To address this issue, several regulations, such as the European Water Framework Directive 2000/60/CE (WFD), the Ramsar list (Ramsar Convention Secretariat, 2016), the Habitat Directive 97/62/CE, or in Spain the WFD transposition (RD 817/2015, 2015), have been established. These documents aim to regulate the protection and restoration of aquatic systems.
In the Iberian Peninsula, especially in the south of Spain, studies of lakes, wetlands or reservoirs have been relatively numerous in the last 50 years (e.g. Margalef et al., 1976; Sánchez-Castillo, 1983, 1986, 1987, 1989; Aboal, 1988; Cambra et al., 1989; Carrillo et al., 1990; Pérez-Martínez and Sánchez-Castillo, 1990; de la Rosa, 1992; Comas-González et al., 2006; Delgado-Molina et al., 2009; Fanés-Treviño, 2009). However, studies on periphytic microalgae assemblages (e.g. epipelon, epilithon, episammon) have been relatively neglected compared to the phytoplankton, despite their important role in ecosystem functioning (Field et al., 1998; Sarthou et al., 2005; Armbrust, 2009) and their role as ecological indicators (Biggs, 1996; Stevenson, 2014). Among periphytic microalgae, diatoms are recognized as an important and diverse algal group (Levkov et al., 2005). In particular, periphytic diatoms, such as the epipelic ones, constitute one of the most important groups in terms of diversity, biomass, and ecosystem metabolism in aquatic habitats (Blanco et al., 2020).
In Spain, several studies have been conducted on periphytic diatoms, such as Aboal et al. (2003) and Cantoral-Uriza & Aboal (2008) in saline wetlands, as well as Blanco et al. (2014) in shallow wetlands. Additional studies on periphytic diatom communities have been conducted in Andalucía, South of Spain (e.g. Ubierna-León & Sánchez-Castillo, 1992; Linares-Cuesta, 2003), and some of them have led to the description of new species (Sánchez-Castillo, 1993; Blanco et al., 2013, 2019a, b, c; Fernández-Moreno et al., 2020, 2022). However, more datasets of periphytic diatoms from other wetlands in southern Spain are needed to improve our understanding of this group.
Many studies indicate that conductivity is one of the main predictors associated with changes in the diatom assemblage composition (Potapova & Charles, 2003; Bere & Tundisi, 2011a, b; Mangadze et al., 2017; Stenger-Kovács et al., 2018; Rodríguez-Alcalá et al., 2020). This is because conductivity makes a significant contribution to the total variation of diatom data (Reed, 1998). For example, high conductivity values can be toxic for many species with less tolerance because of the reduction in the growth rates (Stenger-Kovács et al., 2023).
The main goal of the present study was to examine the composition of epipelic diatom assemblages from two datasets of wetlands in Andalucía (southern Spain) and their relationship with environmental variables. First, we analysed the epipelic diatom assemblages of a subset of 36 wetlands to explore their relationship with a set of 25 physicochemical and physiographical variables. Once the main predictor among these variables was determined, this was used to explain the distribution patterns in a second subset of 53 wetlands. Our specific objectives were (1) to establish the environmental parameters that have the greatest influence on the distribution and abundance of epipelic diatom species in the wetlands of Andalucía, (2) to describe the most characteristic species of epipelic diatoms based on the main explicative variable found in the previous objective.
Material and methods
Study area
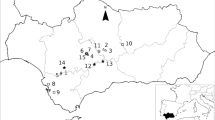
Our study area is the region of Andalucía in southern Spain, which is influenced by oceanic, continental, and Mediterranean climates (Fig. 1). Within the Andalusian region there are more than 200 wetlands, many of them considered important feeding and nesting refuges for birds and having different levels of government protection (Montes et al., 2002).
Geographical location of the study area. On the left, a map of Spain highlighting the Andalusian region; on the right, the Andalusian region displaying the 53 sampling sites. Wetlands were classified based on their conductivity level: eusaline (black circles), mesosaline (grey circles), oligosaline (grey crosses) and fresh (white circles)
Two subsets of wetlands were analysed in this study. First, a phycological survey was performed in 36 wetlands with the aim to relate diatom data to a previously published set of 25 environmental variables (Supplementary information Table S1). Second, a phycological survey was performed in 17 additional wetlands resulting in a total of 53 systems analysed during spring and summer between the years 2004 and 2007. Wetlands were in mid- to high mountain regions (1400–3000 m a.s.l.), endorheic areas (150–458 m a.s.l) or coastal regions (0 m a.s.l.) (Fig. 1).
The studied sites were subdivided into four conductivity groups (Supplementary information Table S2) based on the classification by Cowardin et al. (1979), which is one of the most used classifications for aquatic ecosystems worldwide (Ollis et al., 2015). Thus, we defined the groups as follow: fresh (< 0.8 mS cm−1), oligosaline (0.8–8 mS cm−1), mesosaline (8–30 mS cm−1), and eusaline (> 30 mS cm−1).
Water sampling and analysis
A dataset of 25 physical and chemical (Supplementary information S1) and basin variables from the first set of 36 wetlands was obtained from the Andalusian Environment Agency (Junta de Andalucía. Physical and chemical analyses 2002–2007). This dataset includes measurements (with an average of seven measurements) for each variable and each study site, taken at different times of the year between 2002 and 2007 (Table 1 and Supplementary information Table S1).
In the group of 53 sites, electric conductivity (EC) was measured in situ once in each of the wetlands during spring and early summer from 2004 to 2007 (Supplementary information Table S2) using a multiparameter set (pH/Cond 340i Portable Multi-Parameter, WTW model) (Table 1).
Diatom sampling, analysis, and identification
Epipelon samples were collected from the littoral zone (< 10 cm depth) of each of the 53 wetlands. Three to five transects of 3 m in length were used along the shoreline. Samples were obtained by suction through a one-metre-long glass tube following standard methods (Round, 1953; Polge et al., 2010). All transect samples from each sampling site were combined to create a single sample per wetland. In the laboratory, each one of the epipelon sample per wetland was extended in a 120 mm Petri dish. After an hour, the supernatant was removed with a pipette, and the remaining material was covered with 7–8 coverslips per sample. Petri dishes were then placed under a zenithal light for at least 12 h to allow diatoms to adhere to the coverslips through phototactic movement. Once diatoms were attached to the coverslips, they were then transferred to plastic bottles and preserved with formaldehyde (4%). Afterwards, samples were washed and oxidized in glass tubes with 30% w/w hydrogen peroxide to remove organic matter. Calcium carbonate inclusions were removed by adding a few drops of 1N hydrochloric acid. Samples were then washed with distilled water four times by centrifugation (3000 rpm, 5 min), and the supernatant was removed, leaving a clean pellet. Permanent slides were mounted with a synthetic resin (Naphrax®) with a high refractive index (1.74). Diatom counting was performed using a light microscope (LM—Leica DMI3000 B light) equipped with phase contrast and a 1000× oil immersion objective with a numerical aperture of 1.32. At least 300 valves per sample were counted following Battarbee (1986) and Soeprobowati (2016). The diatom floras used for identification was extensive, reflecting the high diversity of the studied wetlands. It included freshwater inland floras such as Krammer and Lange-Bertalot (1986, 1988, 1991a; b), Lange-Bertalot (2000–2002; 2001, 2017) and Levkov (2009). Additional regional floras, including those from East Africa (Gasse, 1986), saline wetlands in Canada (Cumming et al., 1995), marine habitats such as documented by Witkowski et al. (2000), mountain wetlands in Sierra Nevada as studied by Linares-Cuesta (2003), estuarine waters (Ribeiro, 2010) and ephemeral freshwater pools in Sardinia (Lange-Bertalot et al., 2003), were also utilized.
Data analyses
PRIMER7 v.7 software (Clarke & Gorley, 2015) with the PERMANOVA + add-on package (Anderson et al., 2008) and R 4.2.0 (R Foundation for Statistical Computing) were used for statistical ordination and multivariate analyses. Vegan MuMIn (Bartoń, 2014; Oksanen et al., 2015) packages of R 4.2.0 were used for ordination and model selection analyses, respectively.
The ordination and model selection analysis included all diatom taxa found in at least two wetlands with a relative abundance of ≥ 1% or taxa found in any wetland with a relative abundance of 10%.
Statistical analysis for the subset of 36 wetlands and 25 environmental variables
For multivariate analysis, the percentage species values were log (x + 1) transformed to stabilize their variances. The dataset for the 25 environmental variables used in this work for the subset of 36 wetlands, had 900 values with 13 missing values distributed among seven variables. To estimate the missing values, the regression imputation method was employed. This method involves estimating the missing values based on their close relationship with other variables by regression analysis. Before conducting the analyses, all environmental variables were tested for skewness. If needed, variables were log (x + 1) transformed (Legendre & Birks, 2012). To ensure comparability, potential explanatory variables for diatom assemblages were standardized using a relative-scale transformation (yʹ = yi/ymax). This transformation was necessary because variables were expressed in different units (Legendre & Birks, 2012).
Prior to multivariate ordination analysis, the diatom taxa underwent detrended correspondence analysis (DCA) to determine the gradient length along the DCA axis. The results (gradient length axis 1 > 4 standard deviation units) indicated that Euclidean distance was not appropriate for ordination methods.
To explore relationships between diatom assemblages and the environmental variables, automatic stepwise models for dbRDA (with forward- and backward selection) were built, selecting those that best described the species distribution. Akaike’s information criterion (AIC) (Akaike, 1973, 1974) was used to extract the environmental variables that significantly increased the amount of explained variation of diatom assemblages, thereby optimizing the global AIC. This analysis was performed on 16 variables after using the “vifstep” function of the usdm R package to remove variables with high multicollinearity (Variance Inflation Factors, VIFs > 5). Monte Carlo permutation tests (999 unrestricted permutations) were then used to establish the significance of each variable and the percentage of the variance explained, with a significance level set at P < 0.05. Significant environmental variables were incorporated into the final model and subjected to dbRDA once again.
To summarize changes in diatom community composition across different study sites, detrended correspondence analysis (DCA) was used. The length of the first DCA axis served as a measure of beta diversity, reflecting the heterogeneity of species composition among wetlands. DCA axis 1 site scores were correlated and regressed with the environmental variables to explore the factors influencing species turnover across wetlands. Therefore, a generalized linear model was performed with the 16 environmental variables, using forward and backward stepwise selection model based on Akaike information criterion (AIC).
Statistical analysis for the set of 53 wetlands and conductivity variable
Similarity among diatom assemblages was analysed using a nonmetric multidimensional scaling ordination (nMDS) analysis. A Bray–Curtis similarity matrix was constructed with a log (x + 1) transformed data (Legendre & Birks, 2012) and considering the four conductivity groups as defined above. To evaluate whether there were significant differences in assemblage composition among conductivity groups, analysis of similarity (ANOSIM, Clarke, 1993) was used. Finally, a SIMPER analysis (SIMilarity PERcentage; cut-off 90%) was performed to assess the degree of similarity between the diatom assemblages among conductivity groups.
Results
Physical and chemical basin features of the set of 36 wetlands
Average electrical conductivity values range from fresh to eusaline conditions. (0.1–76.4 mS cm−1) (Table 1). Most of the wetlands in this study exhibited chloride-dominated anions, while sulphate and carbonate were less abundant. Additionally, there was a tendency towards [Na+] dominance rather than [Ca2+]. The wide range in some variables (e.g. Cl−, SO42−, Na+, TSS-total suspended solids, elevation) reflects the diversity of wetlands, which have different ecological status, altitude, or geology. The remaining variables analysed (alkalinity, total phosphorous, total nitrogen, pH, PO43−, DIN—dissolved inorganic nitrogen, O2, HCO3−, CO3−, silicates, chlorophyll-a or salinity) showed a lower data dispersion.
Physical and chemical basin features of the set of 53 wetlands
The main variable measured in situ was electric conductivity, ranging from fresh to eusaline conditions (0.009–84 mS cm−1) (Table 1). The average conductivity value was 12.7 mS cm−1, which was consistent with the average values observed in the set of 36 wetlands based on data from the Andalusian Environment Agency. Geographical variables, such as wetland area and elevation, exhibited high variability in both sets of wetland data in Andalucía.
Diatom composition
The diatom dataset from the 53 wetlands included 366 diatom taxa, 76 of them with more than 10% of abundance in at least one system, and 80 different genera (Supplementary information Table S3).
The diatom community found was largely dominated by several genera, with Navicula (17.0% of all counted valves), Nitzschia (14.6%), Tryblionella (9.9%), Halamphora (6.3%), Gomphonema (4.5%), Achnanthidium (4.5%), Amphora (3.7%), Cocconeis (3.6%), Staurosirella (2.2%), Planothidium (2.2%), Anomoeoneis (2%) and Staurosira (1.1%) collectively accounting for over 70% of the entire diatom community (Table 2). In terms of species richness, the genera Nitzschia (51 taxa), Navicula (40 taxa), and Pinnularia (28 taxa) exhibited the highest values. When considering the different conductivity ranges in this study, the fresh group (with 14 wetlands) was dominated by the genera Navicula (14 taxa), Nitzschia (26 taxa), Pinnularia (23 taxa) and Stauroneis (9 taxa). The oligosaline group (with 20 wetlands) was dominated by Navicula (15 taxa), Nitzschia (28 taxa), Fragilaria and Tryblionella (both 8 taxa). Meanwhile, in the mesosaline and eusaline group (with 8 and 10 wetlands, respectively), the main genera were Navicula (13 and 16 taxa, respectively), Nitzschia (both 16 taxa) and Halamphora (7 and 6 taxa, respectively).
Influence of environmental variables on diatom community variation in the subset of 36 wetlands
Nine of the original 25 environmental variables were excluded from the analyses by applying the “vifstep” function (VIF > 5). These excluded variables exhibited high correlation coefficients with other variables. Therefore, ordination analyses were performed with 16 environmental variables: wetland area, altitude, longitude, latitude, pH, alkalinity, conductivity, dissolved oxygen, sulphates, phosphates, total phosphorous (TP), dissolved inorganic nitrogen (DIN), total nitrogen (TN), silicates, suspended solids, and chlorophyll-a.
Automatic stepwise analyses for dbRDA identified five environmental variables that significantly explained the variation in diatom data: conductivity, pH, wetland area, silicates, and TSS-total suspended solids (Table 2). Conductivity emerged as the best explanatory variable for the diatom assemblages across the study sites in the dbRDA model (Table 2). The dbRDA axes separated study sites with low electric conductivity levels towards their negative side, while eusaline wetlands were located towards the positive side (Fig. 2). Meso- and oligosaline wetlands were mainly located in the positive zone of axis 1 (characterized by high silicate levels and pH values) and the negative zone of axis 2 (associated with low TSS values). Furthermore, the dbRDA axes revealed that high conductivity wetlands tended to be larger (Fig. 2).
DCA axis 1 sample scores track the main diatom assemblage changes. We used the difference in standard deviation units (SD units) between two wetlands as a measure of beta diversity. Generalized model selection analyses were conducted to explore explanatory variables for species turnover among wetlands. The results revealed that conductivity was the main predictor variable for diatom species turnover for the set of 36 wetlands. Additionally, pH and total suspended solids were also found to be significant predictor variables (Table 3).
Influence of conductivity on diatom community variation in the set of 53 wetlands
The nMDS indicated significant differences in diatom composition among the four conductivity groups (ANOSIM, R = 0.334; P = 0.0001). Eusaline samples were plotted to the left of the axis 1, while oligosaline and fresh samples were plotted to the right (Fig. 3), showing a decreasing conductivity gradient along the first axis. Significant differences were found among all groups except between mesosaline and oligosaline (Table 4).
Regarding the four conductivity classes, the fresh group had the most heterogeneous species composition. Thus, cluster comprises 15 wetlands, but taxonomic similarity was only 9.98%. Twenty-eight species contributed to 90% of this similarity, particularly Achnanthidium minutissimum, Navicula veneta and Gomphonema exilissimum (Table 5). The oligosaline group included 20 wetlands with a species similarity of 18.57%. In this group, 19 species contributed to 90% of the diatom assemblage similarity, including Navicula veneta, Tryblionella hungarica, Nitzschia inconspicua and T. piculatea. Mesosaline group included eight wetlands and shows the highest taxonomic similarity with 24.47%. In this group, ten species contributed to 90% of this similarity, namely Navicula veneta, Tryblionella hungarica and Nitzschia inconspicua. Finally, in the eusaline group, the species similarity among the ten wetlands was 15.68%. Twelve species contributed to 90% of the similarity in diatom assemblage, with taxa such as Tryblionella pararostrata, Halamphora sp., Cocconeis euglypta and Navicula salinicola (Table 5).
Discussion
This study assesses the contribution of environmental factors to the compositional variation of epipelic diatom communities in different wetlands of southern Spain. The results of the dbRDA in the subset of 36 wetlands indicated, from a set of 25 parameters, that conductivity is the main explanatory variable of the diatom assemblages in Andalusian wetlands. Moreover, species turnover in these wetlands is mainly explained by the variations in conductivity, this being revealed as an important factor driving variation in diatoms communities as reported by different studies in both lotic and lentic systems (Reed, 1998; Potapova & Charles, 2003; Bere & Tundisi, 2011a, b; Mangadze et al., 2017), including some studies in epipelic communities such as in Stenger-Kovács et al. (2014, 2018).
Electric conductivity is associated with ionic concentration, but differences in ionic composition can also determine species associations in ecosystems (Cumming et al., 1995; Saros & Fritz, 2000; Bere & Tundisi, 2009). Potapova & Charles (2003) reported that the prevalence of the chloride anion (Cl−) is more common in high than in low salinity ecosystems. This also happens in our study sites, particularly in groups with high conductivity levels (mesosaline and eusaline). There are also wetlands where the dominant anion is SO4− (such as Laguna Honda and Archidona). According to Potapova & Charles (2003) and Urrea & Sabater (2009), the dominance of this anion influences diatom assemblages, with certain species (Ctenophora pulchella or Navicymbula pusilla) preferentially inhabiting fresh and mesosaline wetlands, respectively.
High ionic concentration, dominated by either Cl− or SO42−, causes osmotic pressure that limits diatom growth (Cleave et al., 1981). This factor determines the structure and composition of diatom communities in wetlands with high conductivity values. ANOSIM results showed that the resulting conductivity groups differed also in species composition, especially when comparing fresh and oligosaline with eusaline. However, these differences do not occur between mesosaline and oligosaline.
Wetlands belonging to the fresh group include siliceous and calcareous mountain wetlands, located at high altitude (Supplementary information Table S2) and mostly small. As previously mentioned, there were no significant taxonomic differences between the oligosaline and mesosaline wetlands located < 800 m a.s.l. Some of these ecosystems are located near the marine littoral and, according to CMA (2005), many of them receive wastewater inputs, being surrounded by farmland and thus disturbed by human activities including horse and cattle breeding. As a result, nutrient inputs from fertilizers can reach these wetlands, leading in a decrease in salt concentration. Stenger-Kovács et al. (2023) suggest that the degradation of these ecosystems will lead to a reduction in the number of species adapted to high salinity, resulting in an altered representation of the original status of saline wetlands. The eusaline wetlands are also surrounded by cultivated areas, thus intensifying the influence of conductivity over organisms respect to natural areas (Stewart et al., 2000; Carrino-Kyker & Swanson, 2007).
Although our results demonstrate shifts in epipelic taxa and the presence of rare and presumably new species along different gradient of conductivity, the knowledge about the taxa needs improvement. There is a lack of knowledge on the identity of several species, especially those characteristics of the eusaline group such as Halamphora sp1, cf. Entomoneis sp. or cf. Caloneis sp.
Achnanthidium minutissimum and Gomphonema exilissimum, were two of the most common species within the fresh group. Other species are regarded as rare or uncommon, such as Stauroneis cf. arctorussica, as well as endemic species (e.g. Hantzschia gadorensis S. Blanco, Pinnularia baetica Fernández-Moreno & Sánchez-Castillo). It is noteworthy that, according to SIMPER analysis, taxonomic similarity within the fresh group does not reach 10% but shows the highest number of species contributing to this similarity. This is because the fresh group exhibits a high degree of heterogeneity among wetlands, many of which are located in isolated mountainous areas and thus harbouring specialist species with narrow niche breadths (Blanco et al., 2020).
The oligo and mesosaline groups exhibit similar species composition, with Navicula veneta, Tryblionella hungarica and Nitzschia inconspicua being the main contributors to the group similarity in both groups, according to SIMPER analysis. However, the mesosaline category contains more halophilous species, such as Tryblionella pararostrata, Halamphora cf. pertusa, and Seminavis pusilla, albeit with a low relative abundance. The dominance of Navicula veneta, a species known for its tolerance to nutrient-rich environments (Hausmann et al., 2016; Lange-Bertalot, 2001) and salinity (Pienitz et al., 1995; Frost et al., 2023), suggests that disturbances are affecting oligo and mesosaline wetlands. Navicula veneta is present in a broad range of conductivity levels in our wetlands (0.87–17.3 mS cm−1), with maximum abundance at conductivities of 9.28 mS cm−1 (Laguna de las Marismillas). Actually, this species occurs in a total of 35 locations (out of a total of 53 wetlands) in all conductivity groups (Supplementary Information Table S3), with relative abundances > 10% in 17 wetlands belonging to the fresh, oligosaline, and mesosaline groups. Variations in diatom communities along gradients of ionic concentration and composition may also be influenced by nutrient availability (Mangadze et al., 2017). Additionally, nutrient inputs may expand the upper end of a taxon’s salinity tolerance range (Saros & Fritz, 2000). For example, Cirić et al. (2021) found that freshwater diatoms were dominant in saline wetlands highly degraded by nutrient pollution. This fact may explain the high presence of N. veneta in polluted wetlands across a wide range of conductivities.
The major contributors to the eusaline group were Halamphora sp. 1 and T. pararostrata, in addition to Halamphora cf. pertusa and Navicula salinicola, which are known to inhabit hypersaline environments (Reed, 1998; Clavero, 2009; Stepaneck & Kocioleck, 2015). In this group, uncommon species were found, including those mentioned above, as well as recently described species such as Navicula maiorpargemina. This may be attributed to the limited number of species that can survive under such salinity stress conditions (Stenger-Kovács et al., 2023), rather than the geographic isolation characterizing these high mountain locations. Noteworthy, N. veneta is almost absent in this group, unlike in the oligo and mesosaline systems. It is possible that the high conductivity of these wetlands is toxic to this species, preventing it from reaching high abundances. However, further studies are needed to determine its maximum tolerance range.
This study demonstrates that conductivity has a significant impact on the composition of diatom epipelic communities in Andalusian wetlands. The results highlight the high species diversity present in these wetlands and confirm the presence of key species that are dependent on the conductivity gradient. Even within the same conductivity group, we found a high level of dissimilarity in taxonomic composition. This can be attributed to various factors, including the type of ionic composition, wetland elevation, the influence of livestock, wastewater discharge, and the strong agriculture development in the surrounding areas. Therefore, to ensure proper wetland management and conservation in Andalucía, it is essential to have a comprehensive understanding of the diatom species present in these ecosystems and to assess the level of wetland degradation. This will enable us to take appropriate action towards better management and conservation of these ecosystems.
Data availability
Enquiries about data availability should be directed to the authors.
References
Aboal, M., 1988. Aportación al conocimiento de las algas epicontinentales del S.E de España VII. Clorofíceas (Chlorophyceae Wille in Warming, 1884). Candoella 43: 521–548.
Aboal, M., M. A. Cobelas & J. Cambra, 2003. Floristc List of the Non-marine Diatoms (Bacillariophyceae) of Iberian Peninsula, Balearic Islands and Canary Islands, Gantner Verlag, Ruggell, Liechtenstein:
Akaike, H., 1973. "Information theory and an extension of the maximum likelihood principle", in Petrov, B. N.; Csáki, F. (eds.), 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, USSR, September 2–8, 1971, Budapest: Akadémiai Kiadó, pp. 267–281. Republished in Kotz, S.; Johnson, N. L., eds. (1992), Breakthroughs in Statistics, vol. I, Springer-Verlag, pp. 610–624.
Akaike, H., 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control 19(6): 716–723. https://doi.org/10.1109/TAC.1974.1100705.
Armbrust, E. V., 2009. The life of diatoms in the world’s oceans. Nature 459: 185–192. https://doi.org/10.1038/nature08057.
Anderson, M.J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth, UK.
Aponte, C., G. Kazakis, D. Ghosn & V. P. Papanastasis, 2010. Characteristics of the soil seed bank in Mediterranean temporary ponds and its role in ecosystem dynamics. Wetlands Ecology and Management 18(3): 243–253.
Arias-García, J. & J. Gómez-Zotano, 2015. La planificación y gestión de los humedales de Andalucía en el marco del Convenio Ramsar. Investigaciones Geográficas 63: 117–129.
Barton, K., 2014. MuMIn: Multi-model inference. R package version 1.10.0. Retrieved May 14, 2014, from http://cran.r-project.org/package=MuMIn
Battarbee, R. W., 1986. Diatom analysis. In Berglund (ed), Handbook of Holocene Paleoecology and Paleohydrology Wiley, London: 527–570.
Bere, T. & J. G. Tundisi, 2009. Weighted average regression and calibration of conductivity and pH of benthic diatoms in streams influenced by urban pollution – Sao Carlos/SP Brazil. Acta Limnol. Brasil. 21: 317–325.
Bere, T. & J. G. Tundisi, 2011a. Influence of ionic strength and conductivity on benthic diatom communities in a tropical river (Monjolinho), São Carlos-SP, Brazil. Hydrobiologia 661: 261–276.
Bere, T. & J. G. Tundisi, 2011b. Influence of land-use patterns on benthic diatom communities and water quality in the tropical Monjolinho hydrological basin, São Carlos-SP, Brazil. Water SA 37: 93–102.
Biggs, B.J.F., 1996. Patterns in benthic algae of streams. In Algal Ecology: Freshwater Benthic Ecosystems; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Eds.; Academic Press: San Diego, CA, USA: 31–56.
Biggs, J., P. Williams, M. Whitfield, P. Nicolet & A. Weatherby, 2005. 15 years of pond assessment in Britain: results and lessons learned from the work of Pond Conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 693714. https://doi.org/10.1002/aqc.745.
Blanco, S., I. Álvarez-Blanco, C. Cejudo-Figueiras, J. M. R. Espejo, C. B. Barrera, E. Bécares, F. D. Del Olmo & C. Artigas, 2013. The diatom flora in temporary ponds of Doñana National Park (southwest Spain): five new taxa. Nordic Journal of Botany 31(4): 489–499.
Blanco, S., C. Cejudo-Figueiras, I. Álvarez-Blanco, E. Van Donk, E. M. Gross, L.-A. Hansson, K. Irvine, E. Jeppesen, T. Kairesalo, B. Moss, T. Nõges & E. Bécares, 2014. Epiphytic diatoms along environmental gradients in Western European shallow lakes. Clean Soil, Air, Water 42(3): 229–235. https://doi.org/10.1002/clen.201200630.
Blanco, S., A. Olenici, I. De Vicente & F. Guerrero, 2019a. Contribution to the inventory of Iberian diatoms: Encyonema nevadense S. Blanco and al. sp. nov. (Cymbellales, Gomphonemataceae). Anales Del Jardín Botánico De Madrid 76(088): 2. https://doi.org/10.3989/ajbm.2519.
Blanco, S., A. Olenici, F. Ortega, F. Jiménez-Gómez & F. Guerrero, 2019b. Taxonomía y morfología de Craticula gadorensis sp. nov. (Bacillariophyta, Stauroneida-ceae). Bol. Soc. Argent. Bot. 54(1): 1–5.
Blanco, S., A. Olenici, F. Jiménez-Gómez, F. Ortega & F. Guerrero, 2019c. Una nueva especie del género Hantzschia (Bacillariaceae) en Almería, España. Caldasia 41(2): 343–348. https://doi.org/10.15446/caldasia.v41n2.74979.
Blanco, S., A. Olenici, F. Ortega, F. Jiménez-Gómez & F. Guerrero, 2020. Identifying environmental drivers of benthic diatom diversity: the case of Mediterranean mountain ponds. Peer Journal 8: e8825. https://doi.org/10.7717/peerj.8825.
Cambra, J., J. Nolla & S. Sabater, 1989. Composición fitoplanctónica en embalses de pequeño volumen del este de la Península Ibérica. Boletim Da Sociedade Broteriana 62: 5–18.
Cantoral-Uriza, E. A. & M. Aboal, 2008. Diatomeas (Bacillariophyceae) del marjal Oliva-Pego, (Comunidad Valenciana, España). Anales Jardín Botánico De Madrid 65(1): 111–128.
Carrillo, P., L. Cruz Pizarro & P. M. Sánchez Castillo, 1990. Analysis of phytoplankton-zooplankton relationships in an oligotrophic lake under natural and manipulated conditions. Hydrobiologia 200(201): 49–58.
Carrino-Kyker, S. R. & A. K. Swanson, 2007. Seasonal physicochemical characteristics of thirty northern Ohio temporary pools along gradients of GIS-delineated human land-use. Wetlands 27: 749–760.
Ćirić, M., B. Gavrilović, J. Krizmanić, B. P. Dojčinović & D. Vidaković, 2021. Can a benthic diatom community complement chemical analyses and discriminate between disturbed and undisturbed saline wetland habitats? A study of seven soda pans in Serbia. Wetlands Ecology and Management 29: 451–466.
Clarke, K. R., 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x.
Clarke, K. R., Gorley, R. N, 2015. PRIMER v7: User Manual/Tutorial. PRIMER-E. Plymouth, United Kingdom.
Clavero i Oms, E., 2009. Diatomees d’ambients hipersalins costaners: taxonomia. distribució i empremtes en el registre sedimentari. Institut d’Estudis Catalans. Barcelona, p 432.
Cleave, M. L., D. B. Porcella & V. D. Adams, 1981. The application of batch bioassay techniques to the study of salinity toxicity to freshwater phytoplankton. Water Research 15: 573–584.
Comas González, A., M. C. Pérez Baliero & J. González del Río, 2006. Pediastrum willei nom. et sp. nov. (Chlorophyta, Neochloridales) from the Ebro River, Spain and its relations to P. muticum Kütz. sensu Brunnthaler 1915 pro parte. Algological Studies. 120: 5–13.
CMA (Consejería de Medio Ambiente, Junta de Andalucia), 2005. Caracterización ambiental de humedales en Andalucía. Consejería de Medio Ambiente, Junta de Andalucía. Sevilla, 511 p.
Consejería de Medio Ambiente, Junta de Andalucia, 2002–2007. https://www.juntadeandalucia.es/medioambiente/portal/landing-page//asset_publisher/4V1kD5gLiJkq/content/resultados-de-los-an-c3-a1lisis-fisico-qu-c3-admicos-y-biol-c3-b3gicos-2002-2007-/20151/
Cowardin, L.M., V. Carter, F. C. Golet, E. T. LaRoe, 1979. Classification of ponds and deepwater habitats of the United States, FWS/OBS-79/31, Reprinted 1992. U.S. Fish and Wildlife Service, Washington, DC.
Cumming, B. F., S. E. Wilson., R. I. Hall & J. P. Smol, 1995. Diatoms from British Columbia (Canada) Lakes and their relationship to salinity, nutrients and other limnological variables Bibliotheca Diatomologica 31: 1–207.
Delgado Molina, J. A., P. Carrillo, J. M. Medina Sánchez, M. Villar Argaiz & F. J. Bullejos, 2009. Interactive effects of phosphorus loads and ambient ultraviolet radiation on the algal community in a high mountain lake. Journal of Plankton Research 31(6): 619–634.
Della Bella, V. & L. Mancini, 2009. Freshwater diatom and macroinvertebrate diversity of coastal permanent ponds along a gradient of human impact in a Mediterranean eco-region. Hydrobiologia 634: 25–41. https://doi.org/10.1007/s10750-009-9890-x.
De La Rosa, J. C., 1992. Estudio del fitoplancton de varias lagunas de las provincias de Granada y Málaga, Universidad de Granada, Granada, Memoria de licenciatura:, 215.
EPCN, 2008. The Pond Manifesto. European Pond Conservation Network [available at www.europeanponds.org].
Fanés Treviño, I., A. Comas González & P. M. Sánchez Castillo, 2009. Catálogo de las algas verdes cocales de las aguas continentales de Andalucía. Acta Botanica Malacitana 34: 11–32. https://doi.org/10.24310/abm.v34i0.6892.
Fernández Moreno, D., A. T. Luís, D. Vidakovic, Z. Levkov & P. M. Sánchez Castillo, 2020. Pinnularia baetica sp. Nov. (Bacillariophyceae): comparison with other panduriform species in the Mediterranean area. Phytotaxa 435(2): 85–100.
Fernández-Moreno, D., P. M. Sánchez-Castillo, C. Delgado & S. Almeida, 2022. Diatom Species that Characterize Saline Ponds (Southern Spain) with the Description of a New Navicula Species. Wetlands 42(14): 1–16. https://doi.org/10.1007/s13157-021-01529-z.
Field, C. B., M. J. Behrenfeld, J. T. Randerson & P. G. Falkowski, 1998. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 80(281): 237–240. https://doi.org/10.1126/science.281.5374.237.
Frost, C., J. Tibby & P. Goonan, 2023. Diatom–salinity thresholds in experimental outdoor streams reinforce the need for stricter water quality guidelines in South Australia. Hydrobiologia 850: 2991–3011. https://doi.org/10.1007/s10750-023-05163-0.
Gasse, F., 1986. East African diatoms. Taxonomy, ecological distribution. Bibliotheca Diatomologica 11: 1–202.
Gottschalk, S. & M. Kahlert, 2012. Shifts in taxonomical and guild composition of littoral diatom assemblages along environmental gradients. Hydrobiologia. 694: 41–56.
Hammer, U. T., 1990. The effects of climatic change on the salinity. water levels and the biota of Canadian prairie saline lakes. Verhandlungen Des Internationalen Verein Limnologie. 24: 321–326.
Hausmann, S., D. F. Charles, J. Gerritsen & T. J. Belton, 2016. A diatom-based biological condition gradient (BCG) approach for assessing impairment and developing nutrient criteria for streams. Science of the Total Environment 562: 914–927.
Inventario Español del Patrimonio Natural y la Biodiversidad; Real Decreto 556/2011 (BOE nº 112, 11 de mayo de 2011).
Krammer, K. & H. Lange-Bertalot, 1986. Bacillariophyceae. Naviculaceae. Süßwasserflora von Mitteleuropa, Vol. 1. - 876 pp. Stuttgart: Gustav Fischer Verlag.
Krammer, K, & H. Lange-Bertalot, 1988. Bacillariophyceae. Bacillariaceae, Epithemiaceae, Surirellacea. Süßwasserflora von Mitteleuropa, Vol. 2 - 596 pp. Stuttgart: Gustav Fischer Verlag.
Krammer, K. & H. Lange-Bertalot, 1991a. Bacillariophyceae. Centrales, Fragilariaceae, Eunoticeae. Süßwasserflora von Mitteleuropa, Vol. 3. - 577 pp. Stuttgart: Gustav Fischer Verlag.
Krammer, K. & H. Lange-Bertalot, 1991b. Bacillariophyceae. Achnanthaceae, Kristische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis. Süßwasserflora von Mitteleuropa, Vol. 4. 437 pp. Stuttgart: Gustav Fischer Verlag.
Lange-Bertalot, H., 2001. Navicula sensu stricto. 10 genera separated from Navicula sensu lato. Frustulia. Diatoms of Europe 2: 1–526.
Lange-Bertalot, H., 2000–2002. Diatoms of Europe. Diatoms of European Inland Waters and Comparable Habitats. Vols. I-IV. (A.R.G. Gantner Verlag K.G: Rugell.).
Lange-Bertalot, H., P. Cavacini, N. Tagliaventi & S. Alfinito., 2003. Diatoms of Sardinia: Rare and 76 New Species in Rock Pools and Other Ephemeral Waters. Vol. 12 In: Lange-Bertalot, H. (Ed.), Iconographia Diatomologica, A.R.G. Gantner Verlag K.G., Ruggell, - 438 pp.
Lange-Bertalot, H., G. Hofmann, M. Werum & M. Cantonati, 2017. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment, Koeltz Botanical Books, Schmitten-Oberreifenberg, English edition with updated taxonomy and added species:, 942.
Legendre, P. & H. J. B. Birks, 2012. From classical to canonical ordination. In: Tracking environmental change using lake sediments. In Birks, H. J. B., A. F. Lotter, S. Juggins & J. P. Smol (eds), Data handling and numerical techniques, Vol. 5. Springer, Dordrecht: 201–248.
Levkov, Z., S. Krstic, T. Nakov & L. Melovski, 2005. Diatom assemblages on Shara and Nidze Mountains, Macedonia. Nova Hedwigia 81: 501–538. https://doi.org/10.1127/0029-5035/2005/0081-0501.
Levkov, Z., 2009. Amphora sensu lato. In: Lange-Bertalot, H. (Ed.) Diatoms of Europe. Diatoms of the European inland waters and comparable habitats 5. Ruggell, A.R.G. Gantner Verlag K.G., 916 pp.
Linares Cuesta, J. E., 2003. Las diatomeas bentónicas de las lagunas del Parque Nacional de Sierra Nevada. Estudio comparado con las colecciones del Herbario de la Universidad de Granada. 324pp., Tesis doctoral. Universidad de Granada.
Mangadze, T., R. J. Wasserman & T. Dalu, 2017. Use of Diatom Communities as Indicators of Conductivity and Ionic Composition in a Small Austral Temperate River System. Water Air Soil Pollut 228: 428. https://doi.org/10.1007/s11270-017-3610-3.
Margalef, R., D. Planas, J. Armengol, A. Vidal, N. Prat, A. Guiset, J. Toja & M. Estrada, 1976. Limnología de los embalses españoles, MOPU, Madrid:, 422.
Montes, C., E. González-Capitel, F. Molina, J.M. Moreira, J.C. Rubio, P.L Lomas, F. Borja, M. Manzano, & M. Florín-Beltrán (2004) Plan Andaluz de Humedales. https://doi.org/10.13140/2.1.4839.5521.
Nicolet, P., J. Biggs, G. Fox, M. J. Hodson, C. Reynolds, M. Withfield & P. Williams, 2004. The wetland plant and macroinvertebrate assemblages of temporary ponds in England and Wales. Biological Conservation 120: 265–282.
Oksanen J., F.G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P.R. Minchin, R.B. O’Hara, G.L. Simpson, P. Solymos, M.H.H. Stevens, E. Szoecs & H.Wagner, 2015. Vegan: community ecology package. R package version 2.4–0. http://CRAN.R-proje ct.org/package=vegan
Pérez-Martínez, C. & P. M. Sánchez-Castillo, 1990. Dinámica de la comunidad fitoplanctónica de una laguna somera (Padul, Granada). Scientia Gerundensis 16(2): 99–112.
Pienitz, R., J. Smol & H. Birks, 1995. Assessment of freshwater diatoms as quantitative indicators of past climatic change in the Yukon and Northwest territories, Canada. Journal of Paleolimnology 13(1): 21–49. https://doi.org/10.1007/BF00678109.
Pimm, S. L., G. J. Russell, J. L. Gittlemann & T. M. Brooks, 1995. The future of biodiversity. Science 269: 347–350.
Polge, N., A. Sukatar, E. N. Soylu & A. Gönülol, 2010. Epipelic algal flora in the Küçükçekmece Lagoon. Turkish Journal of Fisheries and Aquatic Sciences 10(1): 39–45. https://doi.org/10.4194/trjfas.2010.0106.
Potapova, M. & D. F. Charles, 2003. Distribution of benthic diatoms in U.S. rivers in relation to conductivity and ionic composition. Freshwater Biology 48: 1311–1328.
Ramsar Convention Secretariat, 2016. An Introduction to the Convention on Wetlands (previously The Ramsar Convention Manual) (Ramsar HandBook 5th edition). Ramsar Convention Secretariat, Gland.
RD, 2015. Real Decreto 817/2015, de 11 de septiembre, por el que se establecen los criterios de seguimiento y evaluación del estado de las aguas superficiales y las normas de calidad ambiental. Boletín Oficial Del Estado 219: 8058280669.
Reed, J. M., 1998. A diatom–conductivity transfer function for Spanish salt lakes. Journal Paleolimnology 19: 399–416.
Ribeiro, L., 2010. Intertidal benthic diatoms of the Tagus estuary: taxonomic composition and spatial-temporal variation. PhD thesis, Universidade de Lisboa. Faculdade de Ciências, Lisboa.
Rodríguez-Alcalá, O., S. Blanco, J. García-Girón, E. Jeppesen, K. Irvine, P. Nõges, T. Nõges, E. M. Gross & E. Bécares, 2020. Large-scale geographical and environmental drivers of shallow lake diatom metacommunities across Europe. Sci Total Environ. 10(707): 135887. https://doi.org/10.1016/j.scitotenv.2019.135887.
Round, F. E., 1953. An investigation of two benthic algal communities in Malharm Tarn, Yorkshire. Journal of Ecology 41: 97–174.
Sánchez-Castillo, P. M., 1983. Clorofitas de la ciudad de Granada. Trabajos Del Departamento De Botánica De La Universidad De Granada 3: 63–79.
Sánchez-Castillo, P. M., 1986. Estudio de las comunidades fitoplanctónicas de las lagunas de alta montaña de Sierra Nevada, Universidad de Granada, Granada, Tesis doctoral:, 246.
Sánchez-Castillo, P. M., 1987. Influencia de la salinidad sobre las poblaciones algales de tres lagunas litorales (Albuferas de Adra, Almería). Limnetica 3: 47–53.
Sánchez Castillo, P. M., L. Cruz Pizarro & P. Carrillo, 1989. Caracterización del fitoplancton de las lagunas de alta montaña de Sierra Nevada (Granada, España) en relación con las características fisicoquímicas del medio. Limnetica 5: 37–50.
Sánchez Castillo, P. M., 1993. Amphora margalefii Tomas var. lacustris P. Sanchez var. nova, a new brackish water diatom. Hydrobiologia 269/270: 81–86.
Saros, J. & S. Fritz, 2000. Nutrients as a link between ionic concentration/composition and diatom distributions in saline lakes. Journal of Paleolimnology 23: 449–453. https://doi.org/10.1023/A:1008186431492.
Sarthou, G., K. R. Timmermans, S. Blain & P. Tréguer, 2005. Growth physiology and fate of diatoms in the ocean: a review. J Sea Res 53: 25–42. https://doi.org/10.1016/j.seares.2004.01.007.
Stepanek, J. G. & J. P. Kociolek, 2015. Three new species of the diatom genus Halamphora (Bacillariophyta) from the prairie pothole lakes region of North Dakota, USA. Phytotaxa 197(1): 27–36.
Stenger-Kovács, C., E. Lengyel, K. Buczkó, F. M. Tóth, L. O. Crossetti, A. Pellinger, Z. Z. Doma & J. Padisák, 2014. Vanishing world: alkaline, saline lakes in Central Europe and their diatom assemblages. Inland Waters 4: 383–396.
Stenger-Kovács, C., K. Körmendi, E. Lengyel, A. Abonyi, É. Hajnal, B. Szabó, K. Buczkó & J. Padisák, 2018. Expanding the trait-based concept of benthic diatoms: development of trait- and species-based indices for conductivity as the master variable of ecological status in continental saline lakes. Ecological Indicators 95: 63–74.
Stenger-Kovács, C., V. B. Béres, K. Buczkó, K. Tapolczai, J. Padisák, G. B. Selmeczy & E. Lengyel, 2023. Diatom community response to inland water salinization: a review. Hydrobiologia 850: 4627–4663. https://doi.org/10.1007/s10750-023-05167-w.
Stevenson, J., 2014. Ecological assessments with algae: a review and synthesis. J. Phycol. 50: 437–461.
Stewart, P. M., J. T. Butcher & T. O. Swinford, 2000. Land use, habitat, and water quality effects on macroinvertebrate communities in three watersheds of a Lake Michigan associated marsh system. Aquatic Ecosystem Health and Management 3: 179–189.
Soeprobowati, T. R., 2016. The minimum number of valves for diatoms identification in Rawapening Lake, Central Java. Biotropia 23(2): 97–100.
Ollis, D. J., J. L. Ewart-Smith, J. A. Day, N. M. Job, D. M. Macfarlane, C. D. Snaddon, E. J. J. Sieben, J. A. Dini & N. Mbona, 2015. The development of a classification system for inland aquatic ecosystems in South Africa. Water SA 41: 727–745.
Ubierna León, M. A. & P. M. Sánchez-Castillo, 1992. Diatom flora de varias lagunas de aguas mineralizadas de las provincias de Málaga y Granada. Anales Del Jardín Botánico De Madrid 49: 171–185.
Urrea, G. & S. Sabater, 2009. Epilithic diatom assemblages and their relationship to environmental characteristics in an agricultural watershed (Guadiana River, SW Spain). Ecological Indicators 9: 693–703.
Wallis, C., N. Seon-Massin., F. Martini & M. Schouppe, 2011. Implementation of the Water Framework Directive: When Ecosystem services come into play. Brussels.
Williamson, C. E., J. E. Saros, W. F. Vincent & J. P. Smol, 2009. Lakes and reservoirs as sentinels, integrators, and regulators of climate change. Limnology and Oceanography 54(6part2): 2273–2282.
Witkowski, A., H. Lange-Bertalot & D. Metzeltin, 2000. Diatom Flora of Marine Coasts I Iconographia Diatomologica 7: 1–925.
Acknowledgements
The authors thank to Jose María Conde-Porcuna and Manuel Jesús López-Rodríguez for statistical advice.
Funding
Funding for open access publishing: Universidad de Granada/CBUA. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Judit Padisák
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Moreno, D., Delgado, C., González-Paz, L. et al. Exploring epipelic diatom species composition across wetlands conductivity gradients in southern Spain. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05566-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05566-7