Abstract
Background
Matrix metalloproteinases are catabolic enzymes that play a key role in the articular cartilage degeneration evident in degenerative and inflammatory conditions of articular cartilage. The aim of this study is to assess the ability of pravastatin to modify matrix metalloproteinase (MMP) messenger RNA (mRNA) expression and enzyme activity in a culture of normal human chondrocytes stimulated by interleukin-1β.
Materials and methods
Normal human chondrocytes were stimulated with interleukin (IL)-1β for 6 h to induce MMP expression, simulating a catabolic state, and then treated with pravastatin (1, 5 and 10 μM) for a further 18 h before cell lysates and supernatants were harvested. Cells stimulated with IL-1β but not treated with pravastatin served as controls. Real-time polymerase chain reaction (PCR) was used to assess expression of MMP-3 and MMP-9 mRNA. MMP enzyme activity was assessed using a fluorescent MMP-specific substrate. Statistical analysis was performed using analysis of variance (ANOVA).
Results
MMP-3 and MMP-9 mRNA expression was reduced at all concentrations tested with statistically significant trends in reduction (p = 0.002 and <0.001, respectively). Analysis of culture supernatants revealed that pravastatin treatment led to a reduction in total MMP activity but not to a statistically significant degree (p = 0.07).
Conclusions
Treatment with pravastatin of stimulated human chondrocytes leads to significant down-regulation of selected MMP genes and a non-significant reduction in MMP enzyme activity. Our results provide further evidence that statins may have a role to play in future treatment of disease affecting articular chondrocytes.
Similar content being viewed by others
Introduction
3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or statins, a class of drug initially developed for treatment of hypercholesterolaemia, appear to offer potential in the treatment of inflammatory diseases including those affecting articular cartilage [1, 2]. Statins have been reported to reduce expression of matrix metalloproteinases (MMPs), catabolic enzymes centrally implicated in the pathophysiology of osteoarthritis [3–5]. MMPs are a zinc-containing, calcium-dependent family of enzymes that can cleave matrix components at physiologic pH and temperature [2, 6]. These enzymes possess all the properties necessary to degrade the cartilage matrix and lead to joint degeneration. MMP-3 in particular has been shown to be a sensitive predictor of joint inflammation and cartilage catabolism in diseased joints [7, 8].
To date, no study has examined the effect of pravastatin, a commonly used statin, on MMP production by human articular chondrocytes. In this study, we aimed to evaluate the effect of pravastatin on MMP expression and MMP activity in a simple in vitro model of articular cartilage catabolism.
Materials and methods
Reagents
IL-1β (R&D Systems, USA) was obtained in a lyophilized form and reconstituted with sterile phosphate-buffered saline containing 0.1 % bovine serum albumin. Pravastatin sodium salt (Sigma, UK) was obtained in a lyophilized form and reconstituted with sterile water.
Cell culture and treatment
Normal human chondrocytes were obtained commercially (Promocell, Germany). The cells were obtained from the normal articular cartilage of a femoral head and cryopreserved. The chondrocytes were cultured in Chondrocyte Growth Media (Promocell, Germany) at 37 °C in a humidified atmosphere of 95 % air and 5 % CO2. At passage 4, before cell dedifferentiation, cells were seeded into six-well plates at density of 1 × 105 cells/well and cultured to approximately 80 % confluence prior to treatment.
To investigate the effect of pravastatin treatment, cells were pre-treated with IL-1β for 6 h, and then treated with pravastatin for 18 h at 1, 5 and 10 μM. After a total of 24 h incubation, culture supernatants were removed and stored and cell lysates were collected. Cells cultured in the presence of IL-1β, but without pravastatin, served as experimental controls. All treatments were performed in triplicate.
Stimulation with IL-1β was performed to simulate the pathophysiology of osteoarthritis in which IL-1β is considered a key catabolic stimulant for the production of matrix metalloproteinases [9]. Previous studies focussing on the in vitro response of articular chondrocytes to statins have also used IL-1β as a stimulant of catabolic gene expression and enzyme production, and the treatment times used in this study were also based on these previous similar bodies of work [10–13].
Cell proliferation analysis
The effect of pravastatin treatment on chondrocyte proliferation was determined using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, UK). Cells were seeded into 96-well plates at density of 1 × 104 cells/well. At approximately 80 % confluence, cells were treated for 24 h with varying concentrations of pravastatin (1, 5 and 10 μM). Cells cultured in the presence of media, and an equivalent volume of sterile water, served as experimental controls. Following 24 h of treatment, 20 μl phenazine methosulphate solution was added to each well, and this was incubated for 4 h at 37 °C. The absorbance of the culture medium at 490 nm was recorded using a spectrometer. The cell proliferation of treatment cultures was reported as a percentage of control cultures.
RNA extraction and reverse transcription
Total RNA was extracted from chondrocytes treated with IL-1β or pravastatin using the TRI reagent according to the manufacturer’s instructions (Sigma, Ireland). Contaminating genomic DNA was removed from RNA samples using a DNA-free™ kit (Applied Biosystems, UK), and the resulting RNA was then converted to complementary DNA (cDNA) using Enhanced Avian Reverse Transcriptase (Sigma, Ireland).
Real-time polymerase chain reaction
cDNA served as template for real-time PCR, which was conducted using a QuantiTect SYBR Green PCR kit (Qiagen, UK). Using gene-specific primer pairs, mmp3 and mmp9 gene products were measured using absolute quantification and are reported as a function of crossing time (Ct), the cycle number at which PCR amplification becomes linear. mRNA expression was normalised to control and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression resulting in mean fold change values. Primers were custom made by Sigma Genosys (nucleotide sequences are presented in Table 1).
Total MMP activity assay
The total MMP activity of culture supernatants was evaluated using the MMP-substrate Mca-Arg-Pro-Lys-Pro-Tyr-Ala-Nva-Trp-Met-Lys(Dnp)-NH2 (Bachem, UK) with a quencher and a highly fluorescent end. Activity assays were carried out by incubating 100 μl of substrate (3 μM) with 10 μl of culture supernatant in 0.05 M Tris–HCl, 0.01 M CaCl2, 0.15 M NaCl, NaN3 (0.02 %), at pH 7.5 for 1 h at 37 °C. The reaction was stopped by addition of 20 μl stopping solution [100 nM ethylenediamine tetraacetic acid (EDTA), NaN3 (0.02 %), pH 8.0]. Following cleavage of the quenching moiety, fluorescence was measured at 325 nm excitation and 393 nm emission [14, 15].
Statistical analysis
All data collected were compiled in an Excel database. Results for mRNA expression and cell viability are demonstrated as a proportion of control (assumed to equal 1). Results from the MMP activity assay are reported as the values recorded. Analysis of variance (ANOVA) testing was used to test for statistically significant trends in the data. Statistical significance was accepted if p < 0.05.
Results
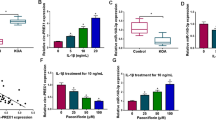
Pravastatin treatment resulted in a reduction in MMP-3 and MMP-9 mRNA expression. MMP-3 mRNA expression was reduced at all concentrations tested (Fig. 1). ANOVA testing confirmed a statistically significant trend in this data (p = 0.002). A statistically significant trend in reduction of MMP-9 expression was also observed (p < 0.001) (Fig. 2). Mean values for MMP-3 and MMP-9 mRNA expression are presented in Table 1.
Bar graph illustrating the effect of pravastatin concentration (x) on MMP-3 expression (y) by normal human chondrocytes after initial stimulation with IL-1β. Gene expression is presented as fold change relative to control. MMP expression was normalised to GAPDH. Error bars represent one standard deviation. *p < 0.05
Bar graph illustrating the effect of pravastatin concentration (x) on MMP-9 expression (y) by normal human chondrocytes after initial stimulation with IL-1β. Gene expression is presented as fold change relative to control. MMP expression was normalised to GAPDH. Error bars represent one standard deviation. *p < 0.05
This reduction in MMP mRNA expression was accompanied by a decrease in MMP activity. Analysis of culture supernatants revealed that pravastatin treatment led to a reduction in total MMP activity (Fig. 3). However, this reduction just failed to achieve statistical significance (p = 0.07). Data from the fluorescence assay are presented in Table 2.
Bar graph illustrating the effect of pravastatin concentration (x) on MMP activity (y) by normal human chondrocytes after initial stimulation with IL-1β. Cells cultured in the presence of IL-1β only served as an experimental control. The MMP activity of culture supernatants was investigated by cleavage of a fluorescent MMP specific substrate. Data are presented as mean ± standard deviation. *p < 0.05
An analysis of chondrocyte cell proliferation (CellTiter 96® AQueous One Solution Cell Proliferation Assay) revealed that cells treated with pravastatin were found to have a similar rate of proliferation when compared with control cells. Cell viability at 1, 5 and 10 μM concentration was 0.92 (SD 0.03), 0.97 (0.05) and 0.88 (0.02), respectively.
Discussion
In this study, we investigated the ability of pravastatin to reduce MMP expression and activity by IL-1β-stimulated chondrocytes. The principle finding of this study is that pravastatin treatment led to a reduction in both MMP gene expression and MMP activity, although the MMP activity reduction failed to reach a statistically significant level. The development of agents capable of reducing or inhibiting the expression of MMPs could potentially lead to a slowing down or inhibition of articular cartilage destruction [2, 3].
Statins were originally developed to combat hypercholesterolaemia. However, it has been noted that these drugs also have anti-inflammatory properties, and efforts have been made to apply this potential to the treatment of osteoarthritis (OA). In recent years, a small number of in vitro and animal studies have reported the ability of statins to reduce the gene expression and protein expression of catabolic enzymes implicated in the pathogenesis of osteoarthritis [10, 16]. The treatment of osteoarthritic chondrocytes with simvastatin and mevastatin has shown a reduction in the expression of MMP-3 [10, 12]. Treatment with atorvastatin has been shown to reduce both mRNA and protein expression of MMP-13 [13]. Pravastatin has previously been suggested as a potential treatment based on reduction of selected inflammatory cytokines in a collagen-induced arthritis model in mice [17]. As non-steroidal anti-inflammatory drugs (NSAIDs), the preferred pharmacological agent of patients suffering from osteoarthritis, confer significant gastrointestinal, cardiovascular and renal side-effects, unearthing a more acceptable drug is warranted [18–20].
Pravastatin has been shown to reduce serum levels of C-reactive protein (CRP) [21]. However, it has not yet been tested for its ability to down-regulate MMP gene expression by human chondrocytes nor for its ability to attenuate MMP activity. In this study we found that treatment with pravastatin of IL-1β-stimulated chondrocytes resulted in down-regulation of gene expression of MMP-3 and MMP-9. Each of these MMPs is able to cleave different components of the cartilage matrix. The down-regulation of these MMP genes by pravastatin is consistent with previous research assessing the efficacy of different statins. Lazzerini et al. reported on the ability of simvastatin to attenuate MMP-3 protein expression in cultured osteoarthritic chondrocytes [12]. Simopolou et al. found that treatment with atorvastatin of osteoarthritic chondrocytes resulted in decreases MMP-13 expression at both gene and protein levels [13]. In an animal model of osteoarthritis, Akasaki et al. have shown that intra-articular mevastatin can attenuate histological degradation, and this may be a future therapeutic route of administration [10, 16]. Alternatively they may be used as an oral augment, as has been successfully shown in rheumatoid arthritis, although the pathophysiology of this disease obviously differs [22].
We did notice that a clear dose response was seen in the down-regulation of MMP-9 expression, but we failed to detect a similar dose response with MMP-3. Although we do not have a good explanation for this, it may be related to the massive up-regulation in MMP-3 expression in catabolically stimulated normal articular chondrocytes compared with the significantly lesser up-regulation of other matrix metalloproteinases [23]. We also note that the reduction in mRNA production did not necessarily result in a corresponding decrease in MMP activity. As we used a non-specific measure of MMP activity, it is plausible that other enzymes from the MMP class are not suppressed and remain active. Further work can clarify which other MMP members are inhibited by pravastatin.
We acknowledge that this is an in vitro assessment and that in vitro results can be readily criticised for not accurately replicating the in vivo process. However, monolayer culture remains a reasonable starting point for assessment of new treatment options and the best possible method available. We must also acknowledge that the pathophysiology of various articular cartilage diseases differs and IL-1β is not the only driver of catabolic activity. The use of other stimulants [such as tumour necrosis factor (TNF)-α for example] may be of assistance, but this would also add an extra layer of complexity to the analysis when study of statins as a potential treatment is still just being explored.
In summary, using an in vitro model, we have found that treatment with pravastatin results in a reduction of MMP-3 and MMP-9 mRNA expression and MMP enzyme activity. These findings provide further support for use of statins as a potential treatment of diseases of articular cartilage in which there is an inflammatory or catabolic component to the disease process. Although further work is clearly warranted, this may represent an exciting juncture in the development of new treatments.
References
Baker JF, Walsh P, Mulhall KJ (2011) Statins: a potential role in the management of osteoarthritis? Jt Bone Spine 78:31–34. doi:10.1016/j.jbspin.2010.02.035
Wu YS, Hu YY, Yang RF, Wang Z, Wei YY (2007) The matrix metalloproteinases as pharmacological target in osteoarthritis: statins may be of therapeutic benefit. Med Hypotheses 69:557–559. doi:10.1016/j.mehy.2007.01.042
Tetlow LC, Adlam DJ, Woolley DE (2001) Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum 44:585–594. doi:10.1002/1529-0131(200103)44:3<585:AID-ANR107>3.0.CO;2-C
Murphy G, Knauper V, Atkinson S, Butler G, English W, Hutton M, Stracke J, Clark I (2002) Matrix metalloproteinases in arthritic disease. Arthritis Res 4(Suppl 3):S39–S49
Iannone F, Lapadula G (2003) The pathophysiology of osteoarthritis. Aging Clin Exp Res 15:364–372
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Lohmander LS, Hoerrner LA, Lark MW (1993) Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum 36:181–189
Taylor DJ, Cheung NT, Dawes PT (1994) Increased serum proMMP-3 in inflammatory arthritides: a potential indicator of synovial inflammatory monokine activity. Ann Rheum Dis 53:768–772
Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB (2008) Current concepts in the pathogenesis of osteoarthritis. Osteoarthr Cartil 16(Suppl 3):S1–S3. doi:10.1016/j.joca.2008.06.025
Akasaki Y, Matsuda S, Nakayama K, Fukagawa S, Miura H, Iwamoto Y (2009) Mevastatin reduces cartilage degradation in rabbit experimental osteoarthritis through inhibition of synovial inflammation. Osteoarthr Cartil 17:235–243. doi:10.1016/j.joca.2008.06.012
Dombrecht EJ, Van Offel JF, Bridts CH, Ebo DG, Seynhaeve V, Schuerwegh AJ, Stevens WJ, De Clerck LS (2007) Influence of simvastatin on the production of pro-inflammatory cytokines and nitric oxide by activated human chondrocytes. Clin Exp Rheumatol 25:534–539
Lazzerini PE, Capecchi PL, Nerucci F, Fioravanti A, Chellini F, Piccini M, Bisogno S, Marcolongo R, Laghi Pasini F (2004) Simvastatin reduces MMP-3 level in interleukin 1beta stimulated human chondrocyte culture. Ann Rheum Dis 63:867–869. doi:10.1136/ard.2003.009746
Simopoulou T, Malizos KN, Poultsides L, Tsezou A (2010) Protective effect of atorvastatin in cultured osteoarthritic chondrocytes. J Orthop Res 28:110–115. doi:10.1002/jor.20953
Sondergaard BC, Schultz N, Madsen SH, Bay-Jensen AC, Kassem M, Karsdal MA (2010) MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation–divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthr Cartil 18:279–288. doi:10.1016/j.joca.2009.11.005
Nagase H, Fields CG, Fields GB (1994) Design and characterization of a fluorogenic substrate selectively hydrolyzed by stromelysin 1 (matrix metalloproteinase-3). J Biol Chem 269:20952–20957
Akasaki Y, Matsuda S, Iwamoto Y (2009) Progress of research in osteoarthritis. The anti-inflammatory effects of intra-articular injected statin on experimental osteoarthritis. Clin Calcium 19:1653–1662
Yamagata T, Kinoshita K, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M (2007) Effects of pravastatin in murine collagen-induced arthritis. Rheumatol Int 27:631–639. doi:10.1007/s00296-006-0270-9
Cooper JW, Burfield AH (2003) Assessment and management of chronic pain in the older adult. J Am Pharm Assoc 50:e89–e99. doi:10.1331/JAPhA.2010.10028 quiz e100–101
Bijlsma JW, Bijlsma JW (2010) Patient benefit-risk in arthritis—a rheumatologist’s perspective. Rheumatology (Oxford) 49(Suppl 2):ii11–ii17. doi:10.1093/rheumatology/keq057
Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN (2010) Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev 18:204–212. doi:10.1097/CRD.0b013e3181ce1521
Albert MA, Danielson E, Rifai N, Ridker PM (2001) Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 286:64–70
McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N (2004) Trial of atorvastatin in rheumatoid arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 363:2015–2021. doi:10.1016/S0140-6736(04)16449-0
Fan Z, Bau B, Yang H, Soeder S, Aigner T (2005) Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheum 52:136–143. doi:10.1002/art.20725
Conflict of interest
None of the authors are aware of any conflict of interest in preparation of this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Baker, J.F., Walsh, P.M., Byrne, D.P. et al. Pravastatin suppresses matrix metalloproteinase expression and activity in human articular chondrocytes stimulated by interleukin-1β. J Orthopaed Traumatol 13, 119–123 (2012). https://doi.org/10.1007/s10195-012-0200-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10195-012-0200-4