Abstract
Background
There are few comparative studies on dual immune checkpoint inhibitors (ICIs) (i.e., IO-IO) and combination therapies comprising ICIs plus tyrosine kinase inhibitors (TKIs) (i.e., IO-TKI) for advanced renal cell carcinoma (RCC), especially in real-world settings.
Methods
We retrospectively evaluated data of 175 patients with IMDC intermediate-risk or poor-risk RCC; as first-line therapy, 103 received IO-IO, and 72 received IO-TKI. An inverse probability of treatment weighting (IPTW) analysis was conducted to balance patients' backgrounds in the IO-IO and IO-TKI groups.
Results
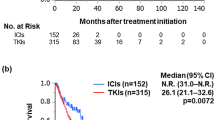
Based on the IPTW analysis, progression-free survival (PFS) was longer in the IO-TKI group than in the IO-IO group (median: 15.6 vs. 8.3 months; p = 0.0386). In contrast, overall survival was not different between groups (median: 46.7 vs. 49.0 months; p = 0.465). Although the IPTW-adjusted objective response rate was not significantly different (51.2% vs. 43.9%; p = 0.359), the progressive disease rate as the best overall response was lower in the IO-TKI group than in the IO-IO group (3.3% vs. 27.4%; p < 0.0001). Regarding the safety profile, the treatment interruption rate was higher in the IO-TKI group than in the IO-IO group (70.3% vs. 49.2%; p = 0.005). In contrast, the IO-IO group had a higher corticosteroid administration rate (43.3% vs. 20.3%; p = 0.001).
Conclusion
IO-TKI therapy exhibited superior effectiveness over IO-IO therapy in terms of PFS improvement and immediate disease progression prevention and was associated with a higher risk of treatment interruption and a lower risk of needing corticosteroids.



Similar content being viewed by others
References
Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378(14):1277–1290. https://doi.org/10.1056/NEJMoa1712126
Rini BI, Plimack ER, Stus V et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1116–1127. https://doi.org/10.1056/NEJMoa1816714
Motzer RJ, Penkov K, Haanen J et al (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1103–1115. https://doi.org/10.1056/NEJMoa1816047
Choueiri TK, Powles T, Burotto M et al (2021) Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384(9):829–841. https://doi.org/10.1056/NEJMoa2026982
Motzer R, Alekseev B, Rha SY et al (2021) Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384(14):1289–1300. https://doi.org/10.1056/NEJMoa2035716
Motzer RJ, Jonasch E, Agarwal N et al (2022) Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20(1):71–90. https://doi.org/10.6004/jnccn.2022.0001
Rathmell WK, Rumble RB, Veldhuizen PJ et al (2022) Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol 40(25):2957–2995. https://doi.org/10.1200/jco.22.00868
Ljungberg B, Albiges L, Abu-Ghanem Y et al (2022) European Association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol 82(4):399–410. https://doi.org/10.1016/j.eururo.2022.03.006
Shah NJ, Sura SD, Shinde R et al (2023) Real-world treatment patterns and clinical outcomes for metastatic renal cell carcinoma in the current treatment era. Eur Urol Open Sci 49:110–118. https://doi.org/10.1016/j.euros.2022.12.015
Stühler V, Herrmann L, Rausch S et al (2022) Real world data on IO-based therapy for metastatic renal cell carcinoma. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-022-04173-0
Ishihara H, Nemoto Y, Nakamura K et al (2022) Changes in real-world outcomes in patients with metastatic renal cell carcinoma from the molecular-targeted therapy era to the immune checkpoint inhibitor era. Target Oncol 17(3):307–319. https://doi.org/10.1007/s11523-022-00879-w
Guida A, Sabbatini R, Gibellini L et al (2021) Finding predictive factors for immunotherapy in metastatic renal-cell carcinoma: what are we looking for? Cancer Treat Rev 94:102157. https://doi.org/10.1016/j.ctrv.2021.102157
Miao D, Margolis CA, Gao W et al (2018) Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359(6377):801–806. https://doi.org/10.1126/science.aan5951
Lombardi P, Filetti M, Falcone R et al (2022) New first-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Cancer Treat Rev 106:102377. https://doi.org/10.1016/j.ctrv.2022.102377
Nocera L, Karakiewicz PI, Wenzel M et al (2022) Clinical outcomes and adverse events after first-line treatment in metastatic renal cell carcinoma: a systematic review and network meta-analysis. J Urol 207(1):16–24. https://doi.org/10.1097/ju.0000000000002252
Quhal F, Mori K, Bruchbacher A et al (2021) First-line Immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol 4(5):755–765. https://doi.org/10.1016/j.euo.2021.03.001
Bosma NA, Warkentin MT, Gan CL et al (2022) Efficacy and safety of first-line systemic therapy for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Open Sci 37:14–26. https://doi.org/10.1016/j.euros.2021.12.007
Dudani S, Graham J, Wells JC et al (2019) First-line immuno-oncology combination therapies in metastatic renal-cell carcinoma: results from the international metastatic renal-cell carcinoma database consortium. Eur Urol 76(6):861–867. https://doi.org/10.1016/j.eururo.2019.07.048
Shah NJ, Sura SD, Shinde R et al (2023) Real-world clinical outcomes of patients with metastatic renal cell carcinoma receiving pembrolizumab + axitinib vs. ipilimumab + nivolumab. Urol Oncol. https://doi.org/10.1016/j.urolonc.2023.08.009
Zarrabi KK, Handorf E, Miron B et al (2023) Comparative effectiveness of front-line ipilimumab and nivolumab or axitinib and pembrolizumab in metastatic clear cell renal cell carcinoma. Oncologist 28(2):157–164. https://doi.org/10.1093/oncolo/oyac195
Santoni M, Massari F, Myint ZW et al (2023) Global real-world outcomes of patients receiving immuno-oncology combinations for advanced renal cell carcinoma: the ARON-1 study. Target Oncol 18(4):559–570. https://doi.org/10.1007/s11523-023-00978-2
Santoni M, Buti S, Myint ZW et al (2023) Real-world outcome of patients with advanced renal cell carcinoma and intermediate- or poor-risk international metastatic renal cell carcinoma database consortium criteria treated by immune-oncology combinations: differential effectiveness by risk group? Eur Urol Oncol. https://doi.org/10.1016/j.euo.2023.07.003
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 11). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v.4 (http://evs.nci.nih.gov/ftp1/CTCAE/About.html).
Ishihara H, Nemoto Y, Nakamura K et al (2023) Comparison of outcomes between therapeutic combinations based on immune checkpoint inhibitors or tyrosine kinase inhibitor monotherapy for first-line therapy of patients with advanced renal cell carcinoma outside of clinical trials: a real-world retrospective multi-institutional study. Target Oncol 18(2):209–220. https://doi.org/10.1007/s11523-023-00956-8
Numakura K, Sekine Y, Hatakeyama S et al (2023) Primary resistance to nivolumab plus ipilimumab therapy in patients with metastatic renal cell carcinoma. Cancer Med 12(16):16837–16845. https://doi.org/10.1002/cam4.6306
Michot JM, Bigenwald C, Champiat S et al (2016) Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54:139–148. https://doi.org/10.1016/j.ejca.2015.11.016
Acknowledgements
The authors thank Ms. Nobuko Hata (Department of Urology, Tokyo Women’s Medical University) for her secretarial work.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Toshio Takagi received honoraria from Bristol-Myers Squibb and Ono Pharmaceutical. Tsunenori Kondo received honoraria from Pfizer, Novartis, Bristol-Myers Squibb, and Ono Pharmaceutical. Kenji Omae received honoraria from Pfizer and Ono Pharmaceutical outside the submitted work. No other disclosures were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ishihara, H., Omae, K., Nemoto, Y. et al. First-line dual immune checkpoint inhibitor therapies versus combination therapies comprising immune checkpoint inhibitors and tyrosine kinase inhibitors for advanced renal cell carcinoma: a comparative analysis of the effectiveness using real-world data. Int J Clin Oncol 29, 473–480 (2024). https://doi.org/10.1007/s10147-024-02471-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02471-w