Abstract
Selenoneine, 2-selenyl-Nα, Nα, Nα-trimethyl-L-histidine, is the major organic selenium compound in marine fish. To characterize biological antioxidant function of selenoneine in fish, the accumulation of selenoneine and other selenium compounds, i. e., sodium selenite and selenomethionine, in the muscle and other tissues of red seabream. We reared red seabream by feeding of 1% dry pellet containing of sodium selenite, selenomethionine, or selenoneine of body weight twice a day for 4 weeks. After that, we replaced to 1% of normal commercial dry pellet of body weight twice a day for 1 week from the selenium supplementation, and tissue distribution of total selenium was determined. Selenium supplementation with selenoneine, selenomethionine, and sodium selenite enhanced selenium accumulation in the white muscle, kidney, and hepatopancreas in comparison with the control group. By the dietary intake of selenoneine, total selenium concentrations were increased in the white muscle, heart, kidney, spleen, hepatopancreas, brain, and blood cells in a dose-dependent manner during the trials after 2 weeks. Dietary intake of selenoneine as well as sodium selenite and selenomethionine reduced oxidation–reduction potential (ORP). Selenoneine concentrations in the white muscle and blood cells were accumulated for 4 weeks by the selenoneine intake, whereas selenoneine concentration was not elevated by the intake of selenomethionine and sodium selenite, suggesting that tissue selenoneine levels might be derived from only selenoneine-containing diet. The uptake factor of selenoneine from the artificial feed containing selenoneine was calculated to be 0.0062 in the white muscle and 4.0 in the blood. The half-life of total selenium in the blood cells and white muscle were estimated to be 60 days in the white muscle and 30 days in the blood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium is an essential micronutrient for animals (Combs and Combs 1986; Himeno and Imura 2000; Imura and Naganuma 1991). Selenium has several physiological functions as selenocysteine for the redox enzymes, such as glutathione peroxidase (Brigelius-Flohé 1999; Rotruck et al. 1973), thioredoxin reductase (Mustacich and Powis 2000), thyroid hormone deiodinases (Arthur et al. 1993), and selenoprotein P (Burk and Hill 1994). The antioxidant function of selenium plays protective roles in 50 human diseases, including prostate, lung, and intestine/colon cancers, immunodeficiency and heart diseases (Ghose et al. 2001; Kiremidjian-Schumacher and Roy 2001; Salonen et al. 1982). Selenium deficiency caused myopathy, exudative diathesis, and pancreatic degeneration to animals and birds (Combs and Combs 1986). Muscle dystrophy by selenium deficiency was also reported in carp and rainbow trout (Bell et al. 1984; Combs and Combs 1986; Gatlin and Wilson 1984; Poston et al. 1976). It was also shown that feeding juvenile rainbow trout a plant protein-based diet without added selenium may be detrimental to the antioxidant status of the fish body (Fontagné-Dicharry et al. 2015). Tohfuku et al. (2021) reported that the intake of selenoneine imparted the antioxidant properties of selenium to amberjack. The biological antioxidant functions of selenium-containing compounds are considered to be dependent on the chemical form of molecular species. “Burnt meat” is a problem in aquaculture and fisheries of pelagic ocean fish, such as tuna, yellowtail, and mackerel in Japan (Yamashita 2010; Yamashita and Yamashita 2015). The meat appears “cooked” although raw, and the flesh lacks the typical bright red color of meat. The burnt meat has a more watery, softer texture, and is often seen in fish under stress conditions, for example those caught during the summer spawning period when the water temperature is high. The oxidative stress is caused by selenium deficiency and hypoxia (Tohfuku et al. 2021). After catching, extensive apoptosis and autophagy occur in the white muscle, and hemolysis occurs in fish that contain blood selenium levels of less than 1 µg/g tissue. The antioxidant effects of selenoneine may be essential in enabling fish to adapt and survive in low-oxygen marine environments. In rats, selenium deficiency was shown to induce hemolysis (Rotruck et al. 1972). White muscle disease in selenium-deficient animals shows similar features (Combs and Combs 1986). Selenium levels in the blood of bluefin tuna were closely related to the radical scavenging activity against red blood cells (Yamashita 2010). Therefore, selenium supplementation in cultured fish might reduce the burnt meat problem in aquaculture, and the nutritional selenium requirement of ocean fish species should be evaluated. In aquaculture, since several studies reported Se supplements for fish species using selenium yeast, selenomethionine, Se nanoparticles, and selenite (Ashouri et al. 2015; Cotter et al. 2008, Dawood et al. 2019; Elia et al. 2011; Fz et al. 2009; Han et al. 2011; Hatfield et al. 2006; Ilhan et al. 2016; Shahpar and Johari 2019; Wang et al. 2011), we used selenomethionine and sodium selenite in this study as control diets.
Selenoneine, 2-selenyl-Nα, Nα, Nα-trimethyl-L-histidine, is a novel selenium containing compound found in the blood and other tissues of fish (Yamashita and Yamashita 2010; Yamashita et al. 2010, 2011, 2013a) and is a major organic selenium compound in fish as well as selenoproteins (Yamashita et al. 2013a). Selenoneine is taken up into cell by the specific organic cation/carnitine transporter-1 (OCTN1) (Yamashita et al. 2010) and binds to the heme of hemoglobin and myoglobin to prevent autooxidation of iron ions (Yamashita and Yamashita 2010). For industrial application of selenium, selenoneine is produced from fisheries waste and be used for functional foods and feed. Although the antioxidant function of selenoneine is expected, there are few studies on oral administration to fish. Red seabream Pagrus major is one of the most popular marine fish and its farming is active in Japan (Imai 2005). In Japan, wild catch of red sea bream was 9187 t in 2020, and aquaculture production was approximately 62,300 t in 2020. Thus, red sea bream is a popular marine fish species (https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=dataset&toukei=00500208&tstat=000001015664&tclass1=000001036597&tclass2=000001164586&stat_infid=000032187641).
Although the color of the body surface is important for red seabream, cultured red seabream may turn black by oxidative stress such as sunlight. Selenoneine suppresses melanin synthesis by inhibiting tyrosinase (Seko et al. 2019). Hence, selenoneine may reduce oxidative stress conditions and melanin synthesis of cultured red seabream.
In this study, we compared the accumulation and antioxidant function of selenoneine and other selenium compounds, i. e., sodium selenite and selenomethionine, in the muscle and other tissues of red seabream.
Materials and Methods
Materials
Sodium selenite was purchased from Wako Pure Chemical Industries (Osaka, Japan). Seleno-L-methionine was purchased from Sigma-Aldrich Japan (Tokyo, Japan). Selenoneine was purified from internal organs of skipjack tuna and blood of tuna at the National Fisheries University according to methods described previously (Yamashita and Yamashita 2010).
Preparation of Feed The feed was purchased from Hayashikane Sangyo (Yamaguchi, Japan). Sodium selenite, selenomethionine, or selenoneine solution were sprayed on feed and then dried in vacuum. The final selenium concentration of each feed is shown in Table S1.
Fish
Red seabream fry (average body weight of 5.4 g) was obtained from private seed and seedling production company (Oita, Japan) and cultured for seven months up to average body weight of 250 g. Chemical composition of control diet was containing crude protein 50%; crude fiber crude lipid 10.0%; Ca 2.3%; ash 16.0%; and phosphorus 1.5%. Each selenium-added diet was fed twice a day for 4 weeks in an amount of 1% of the average body weight. Body weight of fish in each tank was measured when fish were sampled and amount of diet was calculated according to the body weight of every sampling day. The added selenium concentrations in the diets were set in 0 ppm, 1 ppm, and 2 ppm selenium according to the previous study (Tohfuku et al. 2021). After that, they were fed a commercial diet twice a day for 1 week. The test groups were the control group, the sodium selenite group (1 mg Se/kg feeding group and 2 mg Se/kg feeding group), the selenomethionine group (1 mg Se/kg feeding group and 2 mg Se/kg feeding group), and the selenoneine group (1 mg Se/kg feeding group and 2 mg Se/kg feeding group) were set. The number of fish raised was 15 from the start of rearing in each tank until the 2nd week, 10 from the 3rd to 4th week, and 5 in the 5th week. An FRP circular tank (550 L capacity; 500 L seawater) was used as the tank, and the fish were reared under natural seawater (flow rate: 314 L/h). We reared red seabream by feeding of 1% dry pellet containing of sodium selenite, selenomethionine, or selenoneine of body weight twice a day for 4 weeks. After that, we replaced to 1% of commercial dry pellet of body weight twice a day for 1 week from the selenium supplementation. Average water temperature was 20.9 ℃ (19.0–23.9 ℃) and average dissolved oxygen was 7.41 mg/L. The body weight change of red seabream for 5 weeks is shown in Table S2.
Red seabream was anesthetized by adding 2 mL of anesthetic (1/100 volume of 2-methylquinoline dissolved in ethanol) to 1 L of seawater. A small amount of an anticoagulant (heparin sodium dissolved in physiological saline; 800 units/mL) was placed in a syringe in advance, and blood was collected from the caudal peduncle of each fish. The collected blood was centrifuged at 5000 rpm for 5 min (4 °C), divided into a plasma fraction and a precipitated fraction, and stored at − 50 °C. The precipitated fraction was used for analysis as the blood cell fraction.
As shown in Table S1, we used commercially available dry pellet as the control diet, and dry pellets containing selenium compounds, such as sodium selenite, selenomethionine, and selenoneine at 1 mg Se/kg or 2 mg Se/kg for feeding experiments.
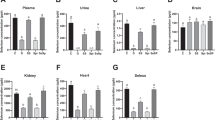
We measured total selenium concentration in the tissues, such as, white muscle, heart, kidney, spleen, hepatopancreas, brain, and blood cell (Table 1) for each feeding group.
Selenium Concentration Measurement
Total selenium concentration was determined by fluorometric assay with 2,3-diamino-naphthalene, after digestion in 1.5 mL of nitric acid and perchloric acid (1:2 in volume) at 200–220 ℃ (Watkinson 1966; Yamashita and Yamashita 2010).
Selenoneine Concentration Measurement
Selenoneine was separated by chromatography using a Shodex GF-310 column (4.5 mm × 150 mm; Showa Denko, Tokyo, Japan) equilibrated with 100 mM ammonium formate buffer containing 0.1% (w/v) Igepal CA-630 (MP Biomedicals, CA, USA). The injection volume was at 10 μL. The mobile phase delivered at 0.5 mL/min isocratically. 82Se was detected with high performance liquid chromatography inductively coupled plasma mass spectrometry (HPLC-ICP-MS; Agilent 1100 Series and Agilent 7500 Series, Agilent Technology) (Yamashita and Yamashita 2010). During separation, selenoneine was eluted at a retention time of 200 s, and the selenium concentration was determined using selenoneine as a standard.
Oxidation–reduction Potential (ORP) Measurement
ORP was measured with ORP electrode (9300, Horiba, Kyoto, Tokyo). The electrode was pierced into muscle and pressed gently until the potential stabilized for 60 s.
Glutathione Peroxidase (GPx) Activity
GPx activity in the hepatopancreas of red seabream in each group 4 weeks after feeding with selenium supplementation diet was examined as a biochemical marker for selenium supplementation by monitoring the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) in the presence of glutathione reductase, which catalyzes the reduction of oxidized glutathione formed by GPx (Rotruck et al. 1973; Yamashita et al. 2012). The activity was measured at 37 ℃ in a solution containing 50 mM sodium phosphate buffer (pH 7.0), 2.5 mM EDTA, 1 mM sodium azide, 0.5 mM reduced glutathione, 0.005 U/mol glutathione reductase, 0.4 mM hydrogen peroxide, and 0.15 mM NADPH. Solution fluorescence was measured with a fluorometer at 460 nm with an excitation wavelength of 355 nm. One enzyme unit of GPx activity is defined as 1 µmol of NADPH oxidized per minute at 37 ℃.
Uptake Factor
Uptake factor is defined as a ratio of selenoneine concentration in muscle or blood cells to those in feed, and is the same as the biomagnification factor (Yamada et al. 1994). Thus, uptake factor is an indicator showing the degree of accumulation of selenoneine in muscle or blood cells by dietary intake of feed. The uptake factor was calculated by the following equation on the equilibrium period of the dietary exposure experiments:
Uptake factor = ((Cen − Cb) / CF) × 10–3where Cen is selenoneine concentration in muscle or blood cells of fish fed with selenoneine-contained feed (ng/kg), Cb is selenoneine concentration in muscle or blood cells of the control fish (ng/kg wet), and CF is the selenoneine concentration in the contained feed (μg/kg).
The elimination half-life in muscle and blood cells was calculated using the total selenium concentration data of the 4th and 5th weeks after replaced to 1% of normal commercial dry pellet in Table 1 by the method described previously (Nomura et al. 2011).
Statistical Analysis
The results are expressed as means ± standard error. Data were analyzed by Dunnett’s multiple comparison test to identify any significant differences (P < 0.05). For the Pearson correlation coefficient analysis, the GraphPad Prism 9 software (GraphPad Software, San Diego, CA, USA) was used. Statistical significance was set at P < 0.05.
Results
Total Selenium Concentration
In the group of the sodium selenite at 1 mg Se/kg and 2 mg Se/kg, total selenium was accumulated in white muscle, kidney, and hepatopancreas in compared with the control group. In the group of the selenomethionine at 1 mg Se/kg and 2 mg Se/kg, total selenium was accumulated in white muscle and heart. In the group of the selenoneine at 1 mg Se/kg, total selenium was accumulated in white muscle, kidney, spleen, hepatopancreas, brain, and blood cell. In the group of the selenoneine at 2 mg Se/kg, total selenium was accumulated in white muscle, heart, kidney, spleen, hepatopancreas, brain, and blood cell.
Selenoneine Concentrations
Selenoneine concentrations were determined in the white muscle and blood cell by monitoring 82Se levels by HPLC-ICP-MS (Table 2). In the control group, selenoneine levels were 0.018 ± 0.001 mg Se/kg in the white muscle and 2.38 ± 0.71 mg Se/kg in the blood cells. In the fish fed with selenoneine at 1 mg Se/kg and 2 mg Se/kg, selenoneine was accumulated in both white muscle and blood cell. Selenonine levels were 4.72 ± 1.06 mg Se/kg in the blood cells and 0.025 ± 0.003 mg Se/kg in the white muscle of fish fed with 1 mg Se/kg, and 10.29 ± 3.14 mg Se/kg in the blood cells and 0.030 ± 0.007 mg Se/kg of fish fed with 2 mg Se/kg. These selenoneine concentrations accounted for more than 63% of total selenium in blood cell and for 4.6–7.0% of selenium in white muscle. In contrast, the fish fed with other selenium compounds, sodium selenite, and selenomethionine contained small amount of selenoneine similar to the control fish.
We evaluated the uptake factor and the half-life from the concentrations of selenoneine in the feed, white muscle, and blood of the fish cultured for 4 weeks and after replaced from selenoneine-feed to control feed for additional 1 week. The uptake factor of selenoneine from the artificial feed containing selenoneine at 0.1–2 mg Se/kg was calculated to be 0.0062 in the white muscle and 4.0 in the blood, indicating that the uptake in the blood cells was higher than that in white muscle (Table 3). In addition, the elimination half-life of selenium in blood cells and white muscle was estimated from Table 1. The half-life of total selenium in the blood cells and white muscle were estimated to be 60 days in the white muscle and 30 days in the blood (Table 1).
ORP
ORP in muscle was analyzed to evaluate the redox status in fish (Fig. 1). All of selenium administration groups decrease ORP at 2 weeks compared to control, but not 4 weeks and 5 weeks. Correlation coefficient between total selenium content and ORP in white muscle was − 0.46 (Fig. 2), and the t-test showed a significant (P = 0.032).
GPx Activity
GPx activity in hepatopancreas of the fish at 4 weeks were compared with the activities of the selenium supplemented groups (Fig. 3). There was no apparent difference in the activities among the different groups.
Discussion
A feeding experiment of red seabream with feeds containing of sodium selenite, selenomethionine, or selenoneine was carried out for 4 weeks. After selenium supplementation, we replaced to normal commercial dry pellet for 1 week from the selenium supplementation, and tissue distribution of total selenium was determined. Selenium supplementation with selenoneine, selenomethionine, and sodium selenite enhanced selenium accumulation in the white muscle, kidney, and hepatopancreas in comparison with the control group. By the dietary intake of selenoneine, total selenium concentrations were increased in the white muscle, heart, kidney, spleen, hepatopancreas, brain, and blood in a dose-dependent manner during the trials after 2 weeks. Dietary intake of selenoneine as well as sodium selenite and selenomethionine reduced ORP.
Levels of selenium were determined in white muscle, heart, kidney, spleen, hepatopancreas, brain, and blood cell at 2 weeks, 4 weeks, and 5 weeks. Levels of selenoneine were determined in white muscle and blood cell at 2 weeks. Dietary intake of sodium selenite or selenomethionine resulted in accumulation selenium in some tissue while dietary intake of selenoneine resulted in accumulation selenium in all tissue we measured in this study. Especially, high levels of selenoneine accumulated in blood cell (Table 2). It is suggested that selenium accumulation mechanism differs for each selenium compound and selenoneine accumulates in each tissue through blood cell. We found that selenoneine was not produced from other selenium compounds, such as sodium selenite and selenomethionine in the red seabream (Table 2), which suggest that selenoneine might be accumulated by dietary intake from feeds but not biosynthesis from other selenium source. We also measured the elimination half-life of total selenium in the blood cells and white muscle were estimated to be 60 days in the white muscle and 30 days in the blood (Table 1), indicating that accumulated selenoneine in the blood cells and white muscle was eliminated gradually.
Selenoneine has antioxidant function (Yamashita and Yamashita 2010) and methylmercury (MeHg) detoxification (Yamashita et.al. 2013b). In a zebrafish toxicity assay, selenoneine demethylated MeHg and then the demethylated MeHg is taken up into secretory extracellur lysosomal vesicles and discharged through OCTN1 (Yamashita et.al. 2013b). Yamashita et.al. also reported red blood cells express the OCTN1 and accumulated selenoneine (Yamashita et.al. 2013a). Therefore, the reason why selenium accumulates in blood cells at a high level is that it exerts an antioxidant and mercury detoxifying functions on blood cell which have a high risk of oxidation and contain a large amount of methylmercury.
The ratio of selenium used in selenoneine in white muscle of wild red seabream was about 10% (Yamashita et al. 2011). In this study, that of the control and selenoneine 2 ppm administration groups were about 5% and about 7.0%, respectively. This result indicates that dietary intake of selenoneine increase the ratio of selenoneine and enhance nutritional status.
Selenoneine administration groups reduced ORP as well as sodium selenite and selenomethionine at 2 weeks. ORP in the control group at 4 and 5 weeks decreased, but it may be due to selenium originally in the commercial dry pellet. Our unpublished research showed that dietary intake of selenoneine in yellowtail reduced ORP and approached the ORP of wild fish. Measurement of ORP can be used for evaluation of antioxidant activity in the tissues by dietary intake of selenium in vivo.
In conclusion, we showed that selenoneine accumulated in the body and enhanced antioxidant function by dietary intake of selenoneine. To utilize selenoneine in food and aquaculture, we should demonstrate biological function in vivo and required amount of selenoneine for each fish species.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ORP:
-
Oxidation-reduction potential
- HPLC-ICP-MS:
-
High performance liquid chromatography inductively coupled plasma mass spectrometry
- GPx:
-
Glutathione peroxidase
- GPC:
-
Gel permeation chromatography
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- OCTN1:
-
Organic cations/carnitine transporter-1
- Se:
-
Selenium
- MeHg:
-
Methylmercury
References
Arthur JR, Nicol F, Beckett GJ (1993) Selenium deficiency, thyroid hormone metabolism, and thyroid hormone deiodinases. Am J Clin Nutr 57:236S-239S
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29
Bell JG, Cowey CB, Youngson A (1984) Rainbow trout liver microsomal lipid peroxidation. The effect of purified glutathione peroxidase, glutathione S-transferase and other factors. Biochim Biophys Acta 795:91–99
Brigelius-Flohé R (1999) Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med 27:951–965
Burk RF, Hill KE (1994) Selenoprotein P. A Selenium-Rich Extracellular Glycoprotein J Nutr 124:1891–1897
Combs GF, Combs SB (1986) The role of selenium in nutrition. Academic Press, New York
Cotter PA, Craig SR, Mclean E (2008) Hyper accumulation of selenium in hybrid striped bass: a functional food for aquaculture? Aquac Nutr 14:215–222
Dawood MAO, Koshio S, Zaineldin AI, Van Doan H, Ahmed HA, Elsabagh M, Abdel-Daim MM (2019) An evaluation of dietary selenium nanoparticles for red sea bream (Pagrus major) aquaculture: growth, tissue bioaccumulation, and antioxidative responses. Environ Sci Pollut Res Int 30:30876–30884
Elia AC, Prearo M, Pacini N, Dörr A, AJM, Abete MC (2011) Effects of selenium diets on growth, accumulation and antioxidant response in juvenile carp. Ecotoxicol Environ Safety 74:166–173
Fontagné-Dicharry S, Godin S, Liu H, Antony Jesu Prabhu P, Bouyssière B, Bueno M, Tacon P, Médale F, Kaushik SJ (2015) Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br J Nutr 113:1876–1887
Fz K, Yazlak H, Karaca I, Sahin N, Tuzcu M, Mn C, Sahin K (2009) The effects of dietary organic or inorganic selenium in rainbow trout (Oncorhynchus mykiss) under crowding conditions. Aquac Nutr 15:569–576
Gatlin DM 3rd, Wilson RP (1984) Dietary selenium requirement of fingerling channel catfish. J Nutr 114:627–633
Ghose A, Fleming J, Harrison PR (2001) Selenium and signal transduction: roads to cell death and anti-tumour activity. BioFactors 14:127–133
Han D, Xie S, Liu M, Xiao X, Liu H, Zhu X, Yang Y (2011) The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio) Aquac Nutr 17:e741-e749
Hatfield DL, Berry MJ, Gladyshev VN (2006) Selenium: its molecular biology and role in human health, 2nd edn. Springer, New York
Himeno S, Imura N (2000) New aspects of physiological and pharmacological roles of selenium. J Health Sci 46:393–398
Ilham I, Siddik MAB, Fotedar R (2016) Effects of organic selenium supplementation on growth, accumulation, haematology and histopathology of juvenile barramundi (Lates calcarifer) fed high soybean meal diets. Biol Trace Elem Res 174:436–447
Imai T (2005) Sea farming of red sea bream pagrus major (Temmick et Schlegel) in waters off Kanagawa Prefecture, Japan with special reference to stock enhancement effect. Bull Kanagawa Pref Fish Res Inst 10:65–71
Imura N, Naganuma A (1991) Advances in mercury toxicology. In: Suzuki T, Imura N, Clarkson TW (eds), Plenum Press, New York
Kiremidjian-Schumacher L, Roy M (2001) Effect of selenium on the immunocompetence of patients with head and neck cancer and on adoptive immunotherapy of early and established lesions. BioFactors 14:161–168
Mustacich D, Powis G (2000) Thioredoxin reductase. Biochem J 346:1–8
Nomura H, Ogiso M, Yamashita M, Takaku H, Kimura A, Chikasou M, Nakamura Y, Fujii S, Watai M, Yamada H (2011) Uptake by dietary exposure and elimination of aflatoxins in muscle and liver of rainbow trout (Oncorhynchus mykiss). J Agric Food Chem 59:5150–5158
Poston HA, Combs GF Jr, Leibovitz L (1976) Vitamin E and selenium interrelations in the diet of Atlantic salmon (Salmo salar): gross, histological and biochemical deficiency signs. J Nutr 106:892–904
Rotruck JT, Pope AL, Ganther HE, Hoekstra WG (1972) Prevention of oxidative damage to rat erythrocytes by dietary selenium. J Nutr 102:689–696
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P (1982) Association between cardiovascular death and myocardial infarction and serum selenium in matched-pair longitudinal study. Lancet 2:175–179
Seko T, Imamura S, Ishihara K, Yamashita Y, Yamashita M (2019) Selenoneine suppresses melanin synthesis by inhibiting tyrosinase in murine B16 melanoma cells and 3D-cultured human melanocytes. Fish Sci 86:171–179
Shahpar Z, Johari SA (2019) Effects of dietary organic, inorganic, and nanoparticulate zinc on rainbow trout, Oncorhynchus mykiss larvae. Biol Trace Elem Res 190:535–540
Tohfuku T, Ando H, Morishita N, Yamashita M, Kondo M (2021) Dietary intake of selenoneine enhances antioxidant activity in the muscles of the amberjack Seriola dumerili grown in aquaculture. Marine Biotechnol 23:847–853
Wang YX, Zhan XA, Zhang RW, Yua D (2011) Comparison of different forms of dietary selenium supplementation on growth performance, meat quality, selenium deposition, and antioxidant property in broilers. Biol Trace Elem Res 143:261–273
Watkinson JH (1966) Fluorometric determination of selenium in biological material with 2,3-diaminonaphthalene. Anal Chem 38:92–97
Yamada H, Tateishi M, Takayanagi K (1994) Bioaccumulation of organotin compounds in the red sea bream (Pagrus major) by two uptake pathways: dietary uptake and direct uptake from water. Environ Toxicol Chem 13:1415–1422
Yamashita M (2010) Stress Responses of Fish during Capture. Quality control of tuna meat through optimization of fishing and handling. (Konno K., Ochiaki Y, and Fukuda Y. eds) Kouseisha Kouseikaku, Tokyo 81–94
Yamashita M, Yamashita Y (2015) Selenoneine in marine organisms. Springer Handbook of Marine Biotechnology (ed. by S.-K. Kim). Springer-Verlag, Berlin, Heidelberg, 1059–1069
Yamashita M, Yamashita Y, Ando T, Wakamiya J, Akiba S (2013a) Identification and determination of selenoneine, 2-selenyl-Nα, Nα, Nα-trimethyl-l-histidine, as the major organic selenium in blood cells in a fish-eating population on remote Japanese islands. Biol Trace Elem Res 156:36–44
Yamashita M, Yamashita Y, Suzuki T, Kani Y, Mizusawa N, Imamura S, Takemoto K, Hara T, Hossain A, Yabu T, Touhata K (2013b) Selenoneine, a novel selenium-containing compound, mediates detoxification mechanisms against methylmercury accumulation and toxicity in zebrafish embryo. Mar Biotechnol 15:559–570
Yamashita Y, Yamashita M (2010) Identification of a novel selenium-containing compound, selenoneine, as the predominant chemical form of organic selenium in the blood of bluefin tuna. J Biol Chem 285:18134–18138
Yamashita Y, Yabu T, Yamashita M (2010) Discovery of the strong antioxidant selenoneine in tuna and selenium redox metabolism. World J Biol Chem 1:144–150
Yamashita Y, Amlund H, Suzuki T, Hara T, Hossain AM, Yabu T, Touhata K, Yamashita M (2011) Selenoneine, total selenium, and total mercury content in the muscle of fishes. Fish Sci 77:679–686
Yamashita Y, Yabu T, Touhata K, Yamashita M (2012) Purification characterization of glutathione peroxidase 1 in the red muscle of Pacific bluefin tuna Thunnus orienalis. Fish Sci 78:407–413
Author information
Authors and Affiliations
Contributions
Y. Shimokawa, K. Abe, M. Ohura, M. Yamamoto, H. Ando, and T. Tohfuku prepared fish feed, reared fish, and assayed selenium and other chemical components. M. Kondo supervised rearing the fish. Y. Shimokawa and M. Yamashita wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was according to the animal experimentation regulations concerning animal care, storage, and pain relief in the Japan Fisheries Research and Education Agency. No specific permission was required for the collection of fish.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue - JSMB Marine Biotechnology 2022 Conference
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimokawa, Y., Abe, K., Ohura, M. et al. Nutritional Supplementation and Enhanced Antioxidant Function by Dietary Intake of Selenoneine and Other Selenium Compounds in Red Seabream Pagrus major. Mar Biotechnol 25, 683–690 (2023). https://doi.org/10.1007/s10126-023-10215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-023-10215-6