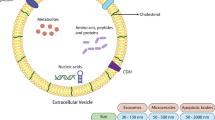
Abstract
This review article aims to address the kinetic of TDEs in cancer cells pre- and post-radiotherapy. Radiotherapy is traditionally used for the treatment of multiple cancer types; however, there is growing evidence to show that radiotherapy exerts NTEs on cells near to the irradiated cells. In tumor mass, irradiated cells can affect non-irradiated cells in different ways. Of note, exosomes are nano-scaled cell particles releasing from tumor cells and play key roles in survival, metastasis, and immunosuppression of tumor cells. Recent evidence indicated that irradiation has the potential to affect the dynamic of different signaling pathways such as exosome biogenesis. Indeed, exosomes act as intercellular mediators in various cell communication through transmitting bio-molecules. Due to their critical roles in cancer biology, exosomes are at the center of attention. TDEs contain an exclusive molecular signature that they may serve as tumor biomarker in the diagnosis of different cancers. Interestingly, radiotherapy and IR could also contribute to altering the dynamic of exosome secretion. Most probably, the content of exosomes in irradiated cells is different compared to exosomes originated from the non-irradiated BCs. Irradiated cells release exosomes with exclusive content that mediate NTEs in BCs. Considering variation in cell type, IR doses, and radio-resistance or radio-sensitivity of different cancers, there is, however, contradictions in the feature and activity of irradiated exosomes on neighboring cells.




Similar content being viewed by others
Abbreviations
- ALIX:
-
ALG2-interacting protein X
- BCs:
-
Bystander cells
- ESCRT:
-
Endosomal sorting complex required for transport
- EMT:
-
Epithelial-to-mesenchymal transition
- ECM:
-
Extracellular matrix
- EVs:
-
Extracellular vesicles
- HSCs:
-
Hematopoietic stem cells
- HRS:
-
Hepatocyte growth factor-regulated tyrosine kinase substrate
- ILVs:
-
Intraluminal vesicles
- IR:
-
Ionizing radiation
- MHC I, II:
-
Major histocompatibility complex class I and class II molecules
- MMPs:
-
Matrix metalloproteinase
- MSCs:
-
Mesenchymal stem cells
- miRNAs:
-
Micro-RNAs
- MVs:
-
Microvesicles
- ABs:
-
Apoptotic bodies
- MVBs:
-
Multivesicular bodies
- NTEs:
-
Non-targeting effects
- PM:
-
Plasma membrane
- ROS:
-
Reactive oxygen species
- SNARs:
-
Soluble NSF attachment protein receptor
- TEM:
-
Transmission electron microscopy
- TDEs:
-
Tumor-derived exosomes
References
Robbins PD, Morelli AE (2014) Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14(3):195
Angelini F, Ionta V, Rossi F, Miraldi F, Messina E, Giacomello A (2016) Foetal bovine serum-derived exosomes affect yield and phenotype of human cardiac progenitor cell culture. BioImpacts 6(1):15–24
Szabó GT, Tarr B, Pálóczi K, Éder K, Lajkó E, Kittel Á, Tóth S, György B, Pásztói M, Németh A (2014) Critical role of extracellular vesicles in modulating the cellular effects of cytokines. Cell Mol Life Sci 71(20):4055–4067
Rafi MA, Omidi Y (2015) A prospective highlight on exosomal nanoshuttles and cancer immunotherapy and vaccination. BioImpacts 5(3):117–122
Sarvar DP, Shamsasenjan K, Akbarzadehlaleh P (2016) Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv Pharm Bull 6 (3):293-299
Azmi AS, Bao B, Sarkar FH (2013) Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 32(3–4):623–642
Wang J-s, Wang H-j, H-l Q (2018) Biological effects of radiation on cancer cells. Mil Med Res 5(1):20
Zhang D, Zhou T, He F, Rong Y, Lee SH, Wu S, Zuo L (2016) Reactive oxygen species formation and bystander effects in gradient irradiation on human breast cancer cells. Oncotarget 7(27):41622
Diamond JM, Vanpouille-Box C, Spada S, Rudqvist N-P, Chapman JR, Ueberheide BM, Pilones KA, Sarfraz Y, Formenti SC, Demaria S (2018) Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol Res 6(8):910–920
Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L (2010) Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78(9):838–848
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12(1):19
Kowal J, Tkach M, Théry C (2014) Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29:116–125
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci 113(8):E968–E977
Théry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 3.22. 21–23.22. 29
Pol E, Coumans F, Grootemaat A, Gardiner C, Sargent I, Harrison P, Sturk A, Leeuwen T, Nieuwland R (2014) Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost 12(7):1182–1192
Hessvik NP, Sandvig K, Llorente A (2013) Exosomal miRNAs as biomarkers for prostate cancer. Front Genet 4:36
Muralidharan-Chari V, Clancy JW, Sedgwick A, D'Souza-Schorey C (2010) Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 123(10):1603–1611
Saha S, Woodbine L, Haines J, Coster M, Ricket N, Barazzuol L, Ainsbury E, Sienkiewicz Z, Jeggo P (2014) Increased apoptosis and DNA double-strand breaks in the embryonic mouse brain in response to very low-dose X-rays but not 50 Hz magnetic fields. J R Soc Interface 11(100):20140783
Sebbagh M, Renvoizé C, Hamelin J, Riché N, Bertoglio J, Bréard J (2001) Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 3(4):346
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654
Ludwig A-K, Giebel B (2012) Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol 44(1):11–15
Raiborg C, Stenmark H (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458(7237):445
Stuffers S, Sem Wegner C, Stenmark H, Brech A (2009) Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10(7):925–937
Hurwitz SN, Nkosi D, Conlon MM, York SB, Liu X, Tremblay DC, Meckes DG (2017) CD63 regulates Epstein-Barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-κB signaling. J Virol 91(5):e02251–e02216
Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zöller M (2010) Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res 0008–5472. CAN-0009-2470
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319(5867):1244–1247
Mazzeo C, Calvo V, Alonso R, Mérida I, Izquierdo M (2016) Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ 23(1):99
Stenmark H, Olkkonen VM (2001) The Rab gtpase family. Genome Biol 2(5):reviews3007.3001
Hsu C, Morohashi Y, S-i Y, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J (2010) Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J Cell Biol 189(2):223–232
Rezaie J, Nejati V, Khaksar M, Oryan A, Aghamohamadzadeh N, Shariatzadeh MA, Rahbarghazi R, Mehranjani MS (2018) Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell Tissue Res 1–11
Bagheri HS, Mousavi M, Rezabakhsh A, Rezaie J, Rasta SH, Nourazarian A, Avci ÇB, Tajalli H, Talebi M, Oryan A (2018) Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling. Lasers Med Sci 1–15
Fevrier B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16(4):415–421
Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, Salles J-P, Bonnerot C, Perret B, Record M (2004) PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett 572(1–3):11–14
Fader CM, Sánchez DG, Mestre MB, Colombo MI (2009) TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta 1793(12):1901–1916
Tiwari N, Wang C-C, Brochetta C, Ke G, Vita F, Qi Z, Rivera J, Soranzo MR, Zabucchi G, Hong W (2008) VAMP-8 segregates mast cell–preformed mediator exocytosis from cytokine trafficking pathways. Blood 111(7):3665–3674
Proux-Gillardeaux V, Raposo G, Irinopoulou T, Galli T (2007) Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol Cell 99(5):261–271
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 81(10):1171–1182
Savina A, Fader CM, Damiani MT, Colombo MI (2005) Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 6(2):131–143
Wajant H, Moosmayer D, Wüest T, Bartke T, Gerlach E, Schönherr U, Peters N, Scheurich P, Pfizenmaier K (2001) Differential activation of TRAIL-R1 and-2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene 20(30):4101
Rana S, Yue S, Stadel D, Zöller M (2012) Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol 44(9):1574–1584
Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, Giorgadze N, Tchkonia T, Kirkland JL, Chari ST (2016) Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 65(7):1165–1174
Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A (2015) Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 6(2):715
Hood JL, Pan H, Lanza GM, Wickline SA (2009) Paracrine induction of endothelium by tumor exosomes. Lab Investig 89(11):1317
Hood JL, San RS, Wickline SA (2011) Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 71(11):3792–3801
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10(5):619
Le MT, Hamar P, Guo C, Basar E, Perdigão-Henriques R, Balaj L, Lieberman J (2014) miR-200–containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest 124(12):5109–5128
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor STF, Chin AR (2014) Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25(4):501–515
You Y, Shan Y, Chen J, Yue H, You B, Shi S, Li X, Cao X (2015) Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci 106(12):1669–1677
Sánchez CA, Andahur EI, Valenzuela R, Castellon EA, Fulla JA, Ramos CG, Triviño JC (2016) Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 7(4):3993
King HW, Michael MZ, Gleadle JM (2012) Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12(1):421
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M (2013) Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci 201220998
Greening DW, Ji H, Chen M, Robinson BW, Dick IM, Creaney J, Simpson RJ (2016) Secreted primary human malignant mesothelioma exosome signature reflects oncogenic cargo. Sci Rep 6:32643
Svensson KJ, Kucharzewska P, Christianson HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W, Belting M (2011) Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2–mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci 108(32):13147–13152
Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK (2010) Hypoxia modulates tumor microenvironment to enhance angiogenic and metastastic potential by secretion of proteins and exosomes. Mol Cell Proteomics
Cho JA, Park H, Lim EH, Kim KH, Choi JS, Lee JH, Shin JW, Lee KW (2011) Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol 123(2):379–386
Yang J, Liu W, Lu Xa FY, Li L, Luo Y (2015) High expression of small GTPase Rab3D promotes cancer progression and metastasis. Oncotarget 6(13):11125
Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, Montermini L, Kislinger T, Rak J (2012) Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. https://doi.org/10.1074/jbc.M112.401760
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai Y-T, Reagan M, Azab F, Flores LM, Campigotto F, Weller E (2013) BM mesenchymal stromal cell–derived exosomes facilitate multiple myeloma progression. J Clin Invest 123(4):1542–1555
Taylor DD, Gerçel-Taylor Ç, Lyons KS, Stanson J, Whiteside TL (2003) T-cell apoptosis and suppression of T-cell receptor/CD3-ζ by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res 9(14):5113–5119
Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL (2009) Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. https://doi.org/10.4049/jimmunol.0900970
Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M (2011) Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-β1. Haematologica 96(9):1302–1309
Bretz NP, Ridinger J, Rupp A-K, Rimbach K, Keller S, Rupp C, Marmé F, Umansky L, Umansky V, Eigenbrod T (2013) Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via TLR signaling. J Biol Chem. https://doi.org/10.1074/jbc.M113.512806
Whiteside TL (2016) Tumor-derived exosomes and their role in tumor-induced immune suppression. Vaccines 4(4):35
Hong C-S, Sharma P, Yerneni SS, Simms P, Jackson EK, Whiteside TL, Boyiadzis M (2017) Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci Rep 7(1):14684
Cheng L, Wang Y, Huang L (2017) Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther 25(7):1665–1675
Pfeffer SR, Grossmann KF, Cassidy PB, Yang CH, Fan M, Kopelovich L, Leachman SA, Pfeffer LM (2015) Detection of exosomal miRNAs in the plasma of melanoma patients. J Clin Med 4(12):2012–2027
Hummel R, Hussey DJ, Haier J (2010) MicroRNAs: predictors and modifiers of chemo-and radiotherapy in different tumour types. Eur J Cancer 46(2):298–311
Gross JC, Chaudhary V, Bartscherer K, Boutros M (2012) Active Wnt proteins are secreted on exosomes. Nat Cell Biol 14(10):1036
Skog J, Würdinger T, Van Rijn S, Meijer DH, Gainche L, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10(12):1470
Blomme A, Fahmy K, Peulen O, Costanza B, Fontaine M, Struman I, Baiwir D, De Pauw E, Thiry M, Bellahcène A (2016) Myoferlin is a novel exosomal protein and functional regulator of cancer-derived exosomes. Oncotarget 7(50):83669
Jakobsen KR, Paulsen BS, Bæk R, Varming K, Sorensen BS, Jørgensen MM (2015) Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles 4(1):26659
He M, Zeng Y (2016) Microfluidic exosome analysis toward liquid biopsy for cancer. J Lab Autom 21(4):599–608
Konstantinell A, Bruun JA, Olsen R, Aspar A, Škalko-Basnet N, Sveinbjørnsson B, Moens U (2016) Secretomic analysis of extracellular vesicles originating from polyomavirus-negative and polyomavirus-positive Merkel cell carcinoma cell lines. Proteomics 16(19):2587–2591
Roberg-Larsen H, Lund K, Seterdal KE, Solheim S, Vehus T, Solberg N, Krauss S, Lundanes E, Wilson SR (2017) Mass spectrometric detection of 27-hydroxycholesterol in breast cancer exosomes. J Steroid Biochem Mol Biol 169:22–28
Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, Sandvig K, Llorente A (2017) Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer 70:122–132
McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson N-O, Bennet AM, Fornander T, Gigante B, Jensen M-B, Peto R (2011) Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 100(2):167–175
Warters R, Hofer K (1977) Radionuclide toxicity in cultured mammalian cells: elucidation of the primary site for radiation-induced division delay. Radiat Res 69(2):348–358
Leach JK, Van Tuyle G, Lin P-S, Schmidt-Ullrich R, Mikkelsen RB (2001) Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res 61(10):3894–3901
Verheij M, Bartelink H (2000) Radiation-induced apoptosis. Cell Tissue Res 301(1):133–142
Valentin J (2005) Low-dose extrapolation of radiation-related cancer risk. Ann ICRP 35(4):1–140
Fleenor CJ, Marusyk A, DeGregori J (2010) Ionizing radiation and hematopoietic malignancies: altering the adaptive landscape. Cell Cycle 9(15):3077–3083
Mutschelknaus L, Azimzadeh O, Heider T, Winkler K, Vetter M, Kell R, Tapio S, Merl-Pham J, Huber SM, Edalat L (2017) Radiation alters the cargo of exosomes released from squamous head and neck cancer cells to promote migration of recipient cells. Sci Rep 7(1):12423
Wilson KD, Sun N, Huang M, Zhang WY, Lee AS, Li Z, Wang SX, Wu JC (2010) Effects of ionizing radiation on self-renewal and pluripotency of human embryonic stem cells. Cancer Res 0008–5472. CAN-0009-4238
Kato K, Kuwabara M, Kashiwakura I (2011) The influence of gender-and age-related differences in the radiosensitivity of hematopoietic progenitor cells detected in steady-state human peripheral blood. J Radiat Res 52(3):293–299
Masuda S, Hisamatsu T, Seko D, Urata Y, Goto S, Li TS, Ono Y (2015) Time-and dose-dependent effects of total-body ionizing radiation on muscle stem cells. Physiol Rep 3(4)
Shao C, Folkard M, Michael BD, Prise KM (2005) Bystander signaling between glioma cells and fibroblasts targeted with counted particles. Int J Cancer 116(1):45–51
Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J (2010) Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One 5(12):e15353
Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D (2015) The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 41(6):503–510
Blyth BJ, Sykes PJ (2011) Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res 176(2):139–157
Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S (2009) Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti–CTLA-4 antibody. Clin Cancer Res 1078–0432. CCR-1009-0265
Arscott WT, Tandle AT, Zhao S, Shabason JE, Gordon IK, Schlaff CD, Zhang G, Tofilon PJ, Camphausen KA (2013) Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Transl Oncol 6(6):638–IN636
Yu X, Harris SL, Levine AJ (2006) The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 66(9):4795–4801
Gallet R, Dawkins J, Valle J, Simsolo E, De Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR (2016) Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38(3):201–211
Jelonek K, Wojakowska A, Marczak L, Muer A, Tinhofer-Keilholz I, Lysek-Gladysinska M, Widlak P, Pietrowska M (2015) Ionizing radiation affects protein composition of exosomes secreted in vitro from head and neck squamous cell carcinoma. Acta Biochim Pol 62(2)
Hazawa M, Tomiyama K, Saotome-Nakamura A, Obara C, Yasuda T, Gotoh T, Tanaka I, Yakumaru H, Ishihara H, Tajima K (2014) Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem Biophys Res Commun 446(4):1165–1171
Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA (2012) Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat Res 177(5):539–545
Mutschelknaus L, Peters C, Winkler K, Yentrapalli R, Heider T, Atkinson MJ, Moertl S (2016) Exosomes derived from squamous head and neck cancer promote cell survival after ionizing radiation. PLoS One 11(3):e0152213
Al-Mayah A, Bright S, Chapman K, Irons S, Luo P, Carter D, Goodwin E, Kadhim M (2015) The non-targeted effects of radiation are perpetuated by exosomes. Mut Res Fundam Mol Mech Mutagen 772:38–45
Kumar Jella K, Rani S, O'driscoll L, McClean B, Byrne H, Lyng F (2014) Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat Res 181(2):138–145
Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, Zhou G, Wang J (2014) MiR-21 is involved in radiation-induced bystander effects. RNA Biol 11(9):1161–1170
Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, Hua J, Wei W, Zhu Q (2015) Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol 12(12):1355–1363
Song M, Wang Y, Shang Z-F, Liu X-D, Xie D-F, Wang Q, Guan H, Zhou P-K (2016) Bystander autophagy mediated by radiation-induced exosomal miR-7-5p in non-targeted human bronchial epithelial cells. Sci Rep 6:30165
Cicero AL, Delevoye C, Gilles-Marsens F, Loew D, Dingli F, Guéré C, André N, Vié K, Van Niel G, Raposo G (2015) Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat Commun 6:7506
Acknowledgments
The authors thank the staffs of Solid Tumor Research Center, Urmia University of Medical Sciences.
Funding
This study was supported by a grant from Urmia University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jabbari, N., Karimipour, M., Khaksar, M. et al. Tumor-derived extracellular vesicles: insights into bystander effects of exosomes after irradiation. Lasers Med Sci 35, 531–545 (2020). https://doi.org/10.1007/s10103-019-02880-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02880-8