Abstract
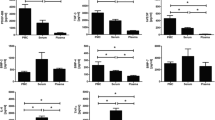
Healthy tendons play an important role in joint movements and subjected to a group of pathologies called tendinopathy due to multiple factors. Tendons have a slowly repairing process due to the low vascularity and cellularity. Treatment options aimed at potentiating the healing response and relieving symptoms. Phototherapy and platelet-rich plasma were novel treatment modalities in tendons based on photobiomodulation and growth factors during healing, and the results were encouraging suggesting calibrating treatment parameters. This study utilizes cell culture to explore the potential effect of light-emitting diode and/or growth factors in the form of platelet-rich plasma (PRP) on the activity of tenocytes isolated from sheep Achilles tendons by measuring the cell metabolism and cell mobility using cell viability and migration assays to proof safety and confirm activity. Results showed that sheep tenocyte-cultured groups treated with 5% platelet-rich plasma alone or combined with 4 J/cm2 light-emitting diode have increased viability significantly when compared to control group after a 48 h, while light-emitting diode treatment has not decreased cell migration significantly when compared with control. Result suggests that using platelet-rich plasma alone or combined with light-emitting diode might have potential to enhance healing response at the conditions applied. PRP could enhance proliferation while LED could enhance migration and proliferation. Further research is needed at longer durations.





Similar content being viewed by others
References
Riley G (2008) Tendinopathy—from basic science to treatment. Nat Rev Rheumatol 4(2):82
Allahverdi A, Sharifi D, Takhtfooladi MA, Hesaraki S, Khansari M, Dorbeh SS (2015) Evaluation of low-level laser therapy, platelet-rich plasma, and their combination on the healing of Achilles tendon in rabbits. Lasers Med Sci 30(4):1305–1313
Maisels MJ, McDonagh AF (2008) Phototherapy for neonatal jaundice. N Engl J Med 358(9):920–928
Steiner R (2011) Basic laser physics. Laser and IPL Technology in Dermatology and Aesthetic Medicine. Springer, In, pp 3–22
Chang M-H, Das D, Varde P, Pecht M (2012) Light emitting diodes reliability review. Microelectron Reliab 52(5):762–782
Opel DR, Hagstrom E, Pace AK, Sisto K, Hirano-ALi SA, Desai S, Swan J (2015) Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol 8(6):36
Seo Y-K, Park J-K, Song C, Kwon S-Y (2014) Comparison of light-emitting diode wavelength on activity and migration of rabbit ACL cells. Lasers Med Sci 29(1):245–255
Xavier M, de Souza RA, Pires VA, Santos AP, Aimbire F, Silva JA, Albertini R, Villaverde AB (2014) Low-level light-emitting diode therapy increases mRNA expressions of IL-10 and type I and III collagens on Achilles tendinitis in rats. Lasers Med Sci 29(1):85–90
Xavier M, David DR, de Souza RA, Arrieiro AN, Miranda H, Santana ET, Silva JA, Salgado MAC, Aimbire F, Albertini R (2010) Anti-inflammatory effects of low-level light emitting diode therapy on achilles tendinitis in rats. Lasers Surg Med 42 (6):553–558
Salate AC, Barbosa G, Gaspar P, Koeke PU, Parizotto NA, Benze BG, Foschiani D (2005) Effect of in-Ga-Al-P diode laser irradiation on angiogenesis in partial ruptures of Achilles tendon in rats. Photomed Laser Ther 23(5):470–475
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, González-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37(4):293–300
Wong-Riley MT, Bai X, Buchmann E, Whelan HT (2001) Light-emitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. Neuroreport 12(14):3033–3037
Hamblin MR, Huang Y (2013) Handbook of photomedicine. Taylor & Francis
Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC (2003) Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci 18(2):95–99
Huang P-J, Huang Y-C, Su M-F, Yang T-Y, Huang J-R, Jiang C-P (2007) In vitro observations on the influence of copper peptide aids for the LED photoirradiation of fibroblast collagen synthesis. Photomed Laser Surg 25(3):183–190
de Mattos LHL, Álvarez LEC, Yamada ALM, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG (2015) Effect of phototherapy with light-emitting diodes (890 nm) on tendon repair: an experimental model in sheep. Lasers Med Sci 30 (1):193–201
Casalechi HL, Nicolau RA, Casalechi VL, Silveira L, De Paula AM, Pacheco MT (2009) The effects of low-level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci 24 (4):659–665
Leal-Junior ECP, Johnson DS, Saltmarche A, Demchak T (2014) Adjunctive use of combination of super-pulsed laser and light-emitting diodes phototherapy on nonspecific knee pain: double-blinded randomized placebo-controlled trial. Lasers Med Sci 29(6):1839–1847
Bjordal JM, Lopes-Martins RA (2013) Lack of adherence to the laser dosage recommendations from the world Association for Laser Therapy in Achilles study. Arch Phys Med Rehabil 94(2):408
Chen M-H, Huang Y-C, Sun J-S, Chao Y-H, Chen M-H (2015) Second messengers mediating the proliferation and collagen synthesis of tenocytes induced by low-level laser irradiation. Lasers Med Sci 30(1):263–272
Enwemeka CS, Reddy GK (2000) The biological effects of laser therapy and other physical modalities on connective tissue repair processes. Laser Ther 12:22–30
James R, Kesturu G, Balian G, Chhabra AB (2008) Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg 33(1):102–112
Anitua E, Andia I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, Nurden P, Nurden AT (2005) Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res 23(2):281–286. https://doi.org/10.1016/j.orthres.2004.08.015
Dragoo JL, Braun HJ, Durham JL, Ridley BA, Odegaard JI, Luong R, Arnoczky SP (2012) Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med 40(6):1274–1281
Kelly BA, Proffen BL, Haslauer CM, Murray MM (2016) Platelets and plasma stimulate sheep rotator cuff tendon tenocytes when cultured in an extracellular matrix scaffold. J Orthop Res 34(4):623–629
Nourissat G, Ornetti P, Berenbaum F, Sellam J, Richette P, Chevalier X (2015) Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine 82(4):230–234
Tumilty S, McDonough S, Hurley DA, Baxter GD (2012) Clinical effectiveness of low-level laser therapy as an adjunct to eccentric exercise for the treatment of Achilles' tendinopathy: a randomized controlled trial. Arch Phys Med Rehabil 93(5):733–739
Freshney RI (2015) Culture of animal cells: a manual of basic technique and specialized applications. John Wiley & Sons
Faber DJ, Mik EG, Aalders MC, van Leeuwen TG (2003) Light absorption of (oxy-) hemoglobin assessed by spectroscopic optical coherence tomography. Opt Lett 28 (16):1436–1438
Alzyoud JA, Khan IM, Rees SG (2017) In vitro studies to evaluate the effect of varying culture conditions and IPL fluencies on tenocyte activities. Lasers Med Sci 32(7):1561–1570
Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T (2005) Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg 63(3):362–369
O'brien J, Wilson I, Orton T, Pognan F (2000) Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. FEBS J 267(17):5421–5426
Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P (2004) Comparison of alamar blue and MTT assays for high through-put screening. Toxicol in Vitro 18(5):703–710
Borenfreund E, Babich H, Martin-Alguacil N (1988) Comparisons of two in vitro cytotoxicity assays—the neutral red (NR) and tetrazolium MTT tests. Toxicol in Vitro 2(1):1–6
McCarrel TM, Minas T, Fortier LA (2012) Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. JBJS 94(19):e143
Markopoulou C, Markopoulos P, Dereka X, Pepelassi E, Vrotsos I (2009) Effect of homologous PRP on proliferation of human periodontally affected osteoblasts. In vitro preliminary study. Report of a case. J Musculoskelet Neuronal Interact 9(3):167–172
de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, van Osch GJ (2008) Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med 36 (6):1171–1178
da Costa Santos VB, de Paula Ramos S, Milanez VF, Corrêa JCM, de Andrade Alves RI, Dias IFL, Nakamura FY (2014) LED therapy or cryotherapy between exercise intervals in Wistar rats: anti-inflammatory and ergogenic effects. Lasers Med Sci 29(2):599–605
Whelan HT, Smits RL Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19(6):305–314
Albuquerque-Pontes GM, Casalechi HL, Tomazoni SS, Serra AJ, Ferreira CSB, Brito RBO, de Melo BL, Vanin AA, Monteiro K, Delle H, Frigo L, Marcos RL, de Carvalho PTC, Leal-Junior ECP (2018) Photobiomodulation therapy protects skeletal muscle and improves muscular function of mdx mice in a dose-dependent manner through modulation of dystrophin. Lasers Med Sci 33(4):755–764. https://doi.org/10.1007/s10103-017-2405-5
de Almeida P, Tomazoni SS, Frigo L, de Carvalho Pde T, Vanin AA, Santos LA, Albuquerque-Pontes GM, De Marchi T, Tairova O, Marcos RL, Lopes-Martins RA, Leal-Junior EC (2014) What is the best treatment to decrease pro-inflammatory cytokine release in acute skeletal muscle injury induced by trauma in rats: low-level laser therapy, diclofenac, or cryotherapy? Lasers Med Sci 29(2):653–658. https://doi.org/10.1007/s10103-013-1377-3
de Almeida P, Lopes-Martins RA, Tomazoni SS, Albuquerque-Pontes GM, Santos LA, Vanin AA, Frigo L, Vieira RP, Albertini R, de Carvalho Pde T, Leal-Junior EC (2013) Low-level laser therapy and sodium diclofenac in acute inflammatory response induced by skeletal muscle trauma: effects in muscle morphology and mRNA gene expression of inflammatory markers. Photochem Photobiol 89(2):501–507. https://doi.org/10.1111/j.1751-1097.2012.01232.x
Marcos RL, Leal-Junior EC, Arnold G, Magnenet V, Rahouadj R, Wang X, Demeurie F, Magdalou J, de Carvalho MH, Lopes-Martins RA (2012) Low-level laser therapy in collagenase-induced Achilles tendinitis in rats: analyses of biochemical and biomechanical aspects. J Orthop Res 30(12):1945–1951. https://doi.org/10.1002/jor.22156
Tomazoni SS, Frigo L, Dos Reis Ferreira TC, Casalechi HL, Teixeira S, de Almeida P, Muscara MN, Marcos RL, Serra AJ, de Carvalho PTC, Leal-Junior ECP (2017) Effects of photobiomodulation therapy and topical non-steroidal anti-inflammatory drug on skeletal muscle injury induced by contusion in rats-part 1: morphological and functional aspects. Lasers Med Sci 32(9):2111–2120. https://doi.org/10.1007/s10103-017-2346-z
Tomazoni SS, Leal-Junior EC, Pallotta RC, Teixeira S, de Almeida P, Lopes-Martins RA (2017) Effects of photobiomodulation therapy, pharmacological therapy, and physical exercise as single and/or combined treatment on the inflammatory response induced by experimental osteoarthritis. Lasers Med Sci 32(1):101–108. https://doi.org/10.1007/s10103-016-2091-8
Tomazoni SS, Frigo L, Dos Reis Ferreira TC, Casalechi HL, Teixeira S, de Almeida P, Muscara MN, Marcos RL, Serra AJ, de Carvalho PTC, Leal-Junior ECP (2017) Effects of photobiomodulation therapy and topical non-steroidal anti-inflammatory drug on skeletal muscle injury induced by contusion in rats-part 2: biochemical aspects. Lasers Med Sci 32(8):1879–1887. https://doi.org/10.1007/s10103-017-2299-2
Tomazoni SS, Leal-Junior EC, Frigo L, Pallotta RC, Teixeira S, de Almeida P, Bjordal JM, Lopes-Martins RA (2016) Isolated and combined effects of photobiomodulation therapy, topical nonsteroidal anti-inflammatory drugs, and physical activity in the treatment of osteoarthritis induced by papain. J Biomed Opt 21(10):108001. https://doi.org/10.1117/1.Jbo.21.10.108001
Marcos RL, Leal Junior EC, Messias Fde M, de Carvalho MH, Pallotta RC, Frigo L, dos Santos RA, Ramos L, Teixeira S, Bjordal JM, Lopes-Martins RA (2011) Infrared (810 nm) low-level laser therapy in rat achilles tendinitis: a consistent alternative to drugs. Photochem Photobiol 87(6):1447–1452. https://doi.org/10.1111/j.1751-1097.2011.00999.x
dos Santos SA, Alves AC, Leal-Junior EC, Albertini R, Vieira RP, Ligeiro AP, Junior JA, de Carvalho Pde T (2014) Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med Sci 29 (3):1051–1058. doi:https://doi.org/10.1007/s10103-013-1467-2
Santos LA, Marcos RL, Tomazoni SS, Vanin AA, Antonialli FC, Grandinetti Vdos S, Albuquerque-Pontes GM, de Paiva PR, Lopes-Martins RA, de Carvalho Pde T, Bjordal JM, Leal-Junior EC (2014) Effects of pre-irradiation of low-level laser therapy with different doses and wavelengths in skeletal muscle performance, fatigue, and skeletal muscle damage induced by tetanic contractions in rats. Lasers Med Sci 29(5):1617–1626. https://doi.org/10.1007/s10103-014-1560-1
da Rosa AS, dos Santos AF, da Silva MM, Facco GG, Perreira DM, Alves AC, Leal Junior EC, de Carvalho Pde T (2012) Effects of low-level laser therapy at wavelengths of 660 and 808 nm in experimental model of osteoarthritis. Photochem Photobiol 88(1):161–166. https://doi.org/10.1111/j.1751-1097.2011.01032.x
Liu Y, Kalén A, Risto O, Wahlström O (2002) Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen 10(5):336–340
Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M (2006) The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 17(2):212–219
Fushimi T, Inui S, Nakajima T, Ogasawara M, Hosokawa K, Itami S (2012) Green light emitting diodes accelerate wound healing: characterization of the effect and its molecular basis in vitro and in vivo. Wound Repair Regen 20(2):226–235
Haker EH, Lundeberg TC (1991) Lateral epicondylalgia: report of noneffective midlaser treatment. Arch Phys Med Rehabil 72(12):984–988
Saunders L (1995) The efficacy of low-level laser therapy in supraspinatus tendinitis. Clin Rehabil 9(2):126–134
Bell L, Madri JA (1989) Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ Res 65(4):1057–1065
Anitua E, Pino A, Orive G (2016) Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J Wound Care 25(11):680–687. https://doi.org/10.12968/jowc.2016.25.11.680
Acknowledgments
J. A. M. Alzyoud is grateful to Hamdi Mango Centre for Scientific Research, The University of Jordan, Amman, Jordan, for their support.
Funding
This research is partially supported by Hamdi Mango Centre for Scientific Research, The University of Jordan, Amman, Jordan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent is needed (in vitro study).
Electronic supplementary material
ESM 1
(DOCX 287 kb)
Rights and permissions
About this article
Cite this article
Alzyoud, J.A.M., Al Najjar, S.A., Talat, S. et al. Effect of light-emitting diodes, platelet-rich plasma, and their combination on the activity of sheep tenocytes. Lasers Med Sci 34, 759–766 (2019). https://doi.org/10.1007/s10103-018-2657-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2657-8