Abstract
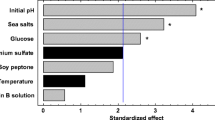
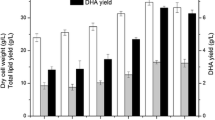
High yield of cell, lipid, and docosahexaenoic acid (DHA) from thraustochytrid strain 12B were achieved without the use of a complex medium and at low NaCl concentration which is detrimental to avoid unnecessary corrosion of steel tank equipment during cultivation. Culture medium that contained only 0.1% NaCl and 1% MgSO4 in an organic base solution containing 8% glucose, 1% yeast extract, and 1% peptone, referred here as NM medium, was found to be as good as or superior to the culture medium prepared from 50%(v/v) seawater with percentage lipid/dry cell weight (DCW) of 66.4%(w/w) and DHA yield up to 43.95 mg/g DCW for the thraustochytrid strain 12B. The NM medium was also applicable to the prominently high DHA-accumulating Schizochytrium limacinum SR21, and therefore this medium could probably be used for other thraustochytrid and other types of microbial strains as well.
Similar content being viewed by others
References
Salem N, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 36: 945–959 (2001)
Raghukumar S. Thraustochytrid marine protists: Production of PUFAs and other emerging technologies. Mar. Biotechnol. 10: 631–640 (2008)
Perveen Z, Ando H, Ueno A, Ito Y, Yamamoto Y, Yamada Y, Takagi T, Kaneko T, Kogame K, Okuyama H. Isolation and characterization of a novel thraustochytrid-like microorganism that efficiently produces docosahexaenoic acid. Biotechnol. Lett. 28: 197–202 (2006)
Nakahara T, Yokochi T, Higashihara T, Tanaka S, Yaguchi T, Honda D. Production of docosahexaenoic and docosapentaenoic acids by Schizochytrium sp. isolated from Yap Islands. J. Am. Oil Chem. Soc. 73: 1421–1426 (1996)
Gupta A, Barrow CJ, Puri M. ω-3 Biotechnology: Thraustochytrids as a novel source of ω-3 oils. Biotechnol. Adv. doi:10.1016/j.biotechadv.2012.02.014: 1–13 (2012)
Hong DD, Anh HTL, Thu NTH. Study on biological characteristics of heterotrophic marine microalga-Schizochytrium mangrovei PQ6 isolated from Phu Quoc island, Kien Giang province, Vietnam. J. Phycol. 47: 944–954 (2011)
Barclay WR. Process for growing thraustochytrium and Schizochytrium using non-chloride salts to produce a microfloral biomass having ω-3 highly unsaturated fatty acids. U.S. Patent 5,340,742 (1994)
Shabala L, McMeekin T, Shabala S. Osmotic adjustment and requirement for sodium in marine protist thraustochytrid. Environ. Microbiol. 11: 1835–1843 (2009)
Wright J, Colling A. Seawater: Its Composition, Properties, and Behavior. 2nd ed. Butterworth-Heinemann, Oxford, UK. pp. 29–31 (1995)
Min KH, Lee HH, Anbu P, Chaulagain BP, Hur BK. The effects of culture condition on the growth property and docosahexaenoic acid production from Thraustochytrium aureum ATCC 34304. Korean J. Chem. Eng. 29: 1211–1215 (2012)
Grundfos. Corrosion resistance chart, aggressive water. Available from: http://nsf.kavi.com/apps/group_public/download.php/2055/Chlorides%20Stainless%20Resistance.pdf. Accessed Aug. 31, 2012.
Unagul P, Assantachai C, Phadungruengluij S, Pongsuteeragul T, Suphantharika M, Verduyn C. Biomass and docosahexaenoic acid formation by Schizochytrium mangrovei Sk-02 at low salt concentrations. Bot. Mar. 49: 182–190 (2006)
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 (1959)
Orikasa Y, Yamada A, Yu R, Ito Y, Nishida T, Yumoto I, Watanabe K, Okuyama H. Characterization of the eicosapentaenoic acid biosynthesis gene cluster from Shewanella sp. strain SCRC-2738. Cell. Mol. Biol. 50: 625–630 (2004)
Okuyama H, Orikasa Y, Nishida T. In vivo conversion of triacylglycerol to docosahexaenoic acid-containing phospholipids in a thraustochytrid-like microorganism, strain 12B. Biotechnol. Lett. 29: 1977–1981 (2007)
Yaguchi T, Tanaka S, Yokochi T, Nakahara T, Higashihara T. Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J. Am. Oil Chem. Soc. 74: 1431–1434 (1997)
Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin. Kidney J. 5(suppl. 1): i3–i14 (2012)
Wolf FI, Cittadini A. Magnesium in cell proliferation and differentiation. Front. Biosci. 4: 607–617 (1999)
Garrill A, Clipson NJW, Jennings DH. Preliminary observations on the monovalent cation relations of Thraustochytrium aureum, a fungus requiring sodium for growth. Mycol. Res. 96: 295–304 (1992)
Wethered JM, Jennings DH. Major solutes contributing to solute potential of Thraustochytrium aureum and T. roseum after growth in media of different salinities. T. Brit. Mycol. Soc. 85: 439–446 (1985)
Zhu L, Zhang X, Ji L, Song X, Kuang C. Changes of lipid content and lipid fatty acid composition of Schizocytrium limacinum in response to different temperatures and salinities. Process Biochem. 42: 210–214 (2007)
Flatman WP. Mechanisms of magnesium transport. Annu. Rev. Physiol. 53: 259–271 (1991)
Flatman PW, Smith LM. Sodium dependent magnesium uptake by ferret red cells. J. Physiol. 443: 217–230 (1991)
Carman GM, Han GS. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem. Sci. 31: 694–699 (2006)
Yen CLE, Stone SJ, Koliwad S, Harris C, Farese RV Jr. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49: 2283–2301 (2008)
Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276: 38870–38876 (2001)
Tsui CKM, Fan KW, Chow RKK, Jones EBG, Vrijmoed LLP. Zoospore production and motility of mangrove thraustochytrids from Hong Kong under various salinities. Mycoscience 53: 1–9 (2012)
Al-hasan RH, Ghannoum MA, Sallal AK, Abu-Elteen KH, Radwan SS. Correlative changes of growth, pigmentation, and lipid composition of Dunaliella salina in response to halostress. J. Gen. Microbiol. 133: 2607–2616 (1987)
Stefanov K, Seizova K, Elenkov I, Kuleva L, Popov S, Dimitrova-Konaklieva S. Lipid composition of the red algae Chondria tenuissima (Good et Wood) Ag. Inhibiting waters with different salinities. Bot. Mar. 37: 445–447 (1994)
Elenkov I, Stefanov K, Dimitrova-Konaklieva S, Popov S. Effect of salinity on lipid composition of Cladophora vagabunda. Photochemistry 42: 39–44 (1996)
Renaud SM, Parry DL. Microalgae for use in tropical aquaculture II: Effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J. Appl. Phycol. 6: 347–356 (1994)
Chaung KC, Chu CY, Su YM, Chen YM. Effect of culture conditions on growth, lipid content, and fatty acid composition of Aurantiochytrium mangrovei strain BL10. AMB Express 2: 1–11 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taha, A.I.B.H.M., Kimoto, T., Kanada, T. et al. Growth optimization of thraustochytrid strain 12B for the commercial production of docosahexaenoic acid. Food Sci Biotechnol 22 (Suppl 1), 53–58 (2013). https://doi.org/10.1007/s10068-013-0048-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-013-0048-2