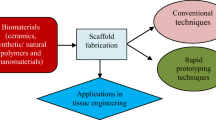
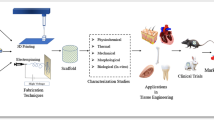
Abstract
Tissue engineering (TE) has made a revolution in repairing, replacing, or regenerating tissues or organs, but it has still a long way ahead. The mechanical properties along with suitable physicochemical and biological characteristics are the initial criteria for scaffolds in TE that should be fulfilled. This research will provide another point of view toward TE challenges concerning the morphological and geometrical aspects of the reconstructed tissue and which parameters may affect it. Based on our survey, there is a high possibility that the final reconstructed tissue may be different in size and shape compared to the original design scaffold. Thereby, the 3D-printed scaffold might not guarantee an accurate tissue reconstruction. The main justification for this is the unpredicted behavior of cells, specifically in the outer layer of the scaffold. It can also be a concern when the scaffold is implanted while cell migration cannot be controlled through the in vivo signaling pathways, which might cause cancer challenges. To sum up, it is concluded that more studies are necessary to focus on the size and geometry of the final reconstructed tissue.
Graphical abstract







Similar content being viewed by others
References
McClelland R, et al. 7—tissue engineering. In: Enderle JD, Blanchard SM, Bronzino JD, editors., et al., Introduction to biomedical engineering. 2nd ed. Boston: Academic Press; 2005. p. 313–402.
Ramos T, Moroni L. Tissue engineering and regenerative medicine 2019: the role of biofabrication—a year in review. Tissue Eng Part C Methods. 2019;26:91–106.
Sun AR, et al. Cartilage tissue engineering for obesity-induced osteoarthritis: physiology, challenges, and future prospects. J Orthopaed Transl. 2020. https://doi.org/10.1016/j.jot.2020.07.004.
Dzobo K, et al. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int. 2018;2018:1–24.
Edgar L, et al. Heterogeneity of scaffold biomaterials in tissue engineering. Materials. 2016;9:332.
Ma PX. Scaffolds for tissue fabrication. Mater Today. 2004;7:30–40.
Vacanti JP, Vacanti CA. Chapter 1—the history and scope of tissue engineering. In: Lanza R, Langer R, Vacanti J, editors. Principles of tissue engineering. 4th ed. Boston: Academic Press; 2014. p. 3–8.
Esmaeili J, et al. Integration of microbubbles with biomaterials in tissue engineering for pharmaceutical purposes. Heliyon. 2020;6:e04189.
Biswal T. Biopolymers for tissue engineering applications: a review. Mater Today Proc. 2021;41:397–402.
Song HHG, et al. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22:340–54.
Swift MR, Weinstein BM. Arterial–venous specification during development. Circ Res. 2009;104:576–88.
Tatara AM, Kontoyiannis DP, Mikos AG. Drug delivery and tissue engineering to promote wound healing in the immunocompromised host: current challenges and future directions. Adv Drug Deliv Rev. 2018;129:319–29.
Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface. 2009;6:S311–24.
Eltom A, Zhong G, Muhammad A. Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv Mater Sci Eng. 2019;2019:3429527.
Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589–601.
Hellman KB. Challenges in tissue engineering and regenerative medicine product commercialization: building an industry. Tissue Eng Part A. 2011;17:1–3.
Rekow D. Informatics challenges in tissue engineering and biomaterials. Adv Dent Res. 2003;17:49–54.
Cobham AE, Mirth CK. The development of body and organ shape. BMC Zool. 2020;5:14.
Thorne CH, Wilkes G. Ear deformities, otoplasty, and ear reconstruction. Plast Reconstr Surg. 2012;129:701e-e716.
Siemionow M, Sonmez E. Face as an organ. Ann Plast Surg. 2008;61:345–52.
Deo KA, et al. Bioprinting 101: design, fabrication, and evaluation of cell-laden 3D bioprinted scaffolds. Tissue Eng Part A. 2020;26:318–38.
Matai I, et al. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226: 119536.
Rezaei FS, et al. 3D printed chitosan/polycaprolactone scaffold for lung tissue engineering: hope to be useful for COVID-19 studies. RSC Adv. 2021;11:19508–20.
Ramiah P, et al. Hydrogel-based bioinks for 3D bioprinting in tissue regeneration. Front Mater. 2020. https://doi.org/10.3389/fmats.2020.00076.
Bian L. Functional hydrogel bioink, a key challenge of 3D cellular bioprinting. APL Bioeng. 2020;4: 030401.
Fang Q, et al. In vitro and in vivo research on using Antheraea pernyi silk fibroin as tissue engineering tendon scaffolds. Mater Sci Eng C. 2009;29:1527–34.
Cervantes T, et al. Design of composite scaffolds and three-dimensional shape analysis for tissue-engineered ear. J R Soc Interface R Soc. 2013;10:20130413.
Mouriño V, et al. Enhancing biological activity of bioactive glass scaffolds by inorganic ion delivery for bone tissue engineering. Curr Opin Biomed Eng. 2019;10:23–34.
Gurumurthy B, Janorkar AV. Improvements In mechanical properties of collagen-based scaffolds for tissue engineering. Curr Opin Biomed Eng. 2020;17:100253.
Persson M, et al. Osteogenic differentiation of human mesenchymal stem cells in a 3D woven scaffold. Sci Rep. 2018;8:10457–10457.
Dhivya S, et al. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2018;51: e12408.
Wolpert L. One hundred years of positional information. Trends Genet. 1996;12:359–64.
Pina S, et al. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials. 2019;12:1824.
Deng Y, et al. Bioinspired and osteopromotive polydopamine nanoparticle-incorporated fibrous membranes for robust bone regeneration. NPG Asia Mater. 2019;11:39.
Wu Z, Guan K-L. Hippo signaling in embryogenesis and development. Trends Biochem Sci. 2021;46:51–63.
Aihara A, et al. Small molecule LATS kinase inhibitors block the Hippo signaling pathway and promote cell growth under 3D culture conditions. J Biol Chem. 2022;298:101779.
Burrill DR, Silver PA. Making cellular memories. Cell. 2010;140:13–8.
Levin M. The biophysics of regenerative repair suggests new perspectives on biological causation. BioEssays. 2020;42:1900146.
Mekler L. Mechanism of biological memory. Nature. 1967;215:481–4.
Dudas M, et al. Memory encoded throughout our bodies: molecular and cellular basis of tissue regeneration. Pediatr Res. 2008;63:502–12.
Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18.
Pavlovic M, Mayfield J, Balint B. Tissue engineering triangle and its development. In: Handbook of medical and healthcare technologies. New York: Springer; 2013. p. 267–82.
Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176:26S-38S.
Kusuhara H, et al. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009;17:136–46.
Hassanzadeh P, Atyabi F, Dinarvand R. Tissue engineering: still facing a long way ahead. J Control Release. 2018;279:181–97.
Jia L, et al. Regeneration of human-ear-shaped cartilage with acellular cartilage matrix-based biomimetic scaffolds. Appl Mater Today. 2020;20: 100639.
Lin D, et al. A viscoelastic PEGylated poly(glycerol sebacate)-based bilayer scaffold for cartilage regeneration in full-thickness osteochondral defect. Biomaterials. 2020;253: 120095.
Buenzli PR, et al. Cell proliferation and migration explain pore bridging dynamics in 3D printed scaffolds of different pore size. Acta Biomater. 2020;114:285–95.
Calleros EL, et al. Crosslinked, biodegradable polyurethanes for precision-porous biomaterials: synthesis and properties. J Appl Polym Sci. 2020;137:48943.
Chian KS et al. Three-dimensional porous hybrid scaffold and manufacture thereof. 2010, Google Patents.
Bružauskaitė I, et al. Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology. 2016;68:355–69.
Khoshnood N, Zamanian A. A comprehensive review on scaffold-free bioinks for bioprinting. Bioprinting. 2020;19: e00088.
Wang J-Z, et al. Review fantastic medical implications of 3D-printing in liver surgeries, liver regeneration, liver transplantation and drug hepatotoxicity testing: a review. Int J Surg. 2018;56:1–6.
Mabrouk M, Beherei HH, Das DB. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater Sci Eng C. 2020;110: 110716.
Urciuolo A, et al. Intravital three-dimensional bioprinting. Nat Biomed Eng. 2020;4:901–15.
Kim G, et al. A cryogenic direct-plotting system for fabrication of 3D collagen scaffolds for tissue engineering. J Mater Chem. 2009;19:8817–23.
Mavila N, et al. Functional human and murine tissue-engineered liver is generated from adult stem/progenitor cells. Stem Cells Transl Med. 2017;6:238–48.
Gupta P, et al. A novel scaffold-based hybrid multicellular model for pancreatic ductal adenocarcinoma—toward a better mimicry of the in vivo tumor microenvironment. Front Bioeng Biotechnol. 2020. https://doi.org/10.3389/fbioe.2020.00290.
Kenny A. Introduction: the early modern womb. In: Humoral wombs on the Shakespearean stage. New York: Springer; 2019. p. 1–26.
Guariglia L, Rosati P. Embryo-fetal development in the early stages of pregnancy. Radiol Med. 1997;93:586–90.
Murphy CM, et al. Cell-scaffold interactions in the bone tissue engineering triad. Eur Cell Mater. 2013;26:120–32.
Swanson WB, et al. Macropore design of tissue engineering scaffolds regulates mesenchymal stem cell differentiation fate. Biomaterials. 2021;272: 120769.
Godbey WT. Chapter 17—stem cells, tissue engineering, and regenerative medicine. In: Godbey WT, editor. Biotechnology and its applications. 2nd ed. New York: Academic Press; 2022. p. 389–409.
Nantavisai S, et al. Mesenchymal stem cell-based bone tissue engineering for veterinary practice. Heliyon. 2019;5: e02808.
Chen Y, et al. ECM scaffolds mimicking extracellular matrices of endochondral ossification for the regulation of mesenchymal stem cell differentiation. Acta Biomater. 2020;114:158–69.
Chandy T. Chapter 2—tissue repair with natural extracellular matrix (ECM) scaffolds. In: Sharma CP, editor. Regenerated organs. New York: Academic Press; 2021. p. 11–37.
Agarwal T, Maiti TK, Ghosh SK. Decellularized caprine liver-derived biomimetic and pro-angiogenic scaffolds for liver tissue engineering. Mater Sci Eng C. 2019;98:939–48.
Zheng M-H, et al. Liver tissue engineering: promises and prospects of new technology. Cytotherapy. 2010;12:349–60.
Safinsha S, Mubarak Ali M. Composite scaffolds in tissue engineering. Mater Today Proc. 2020;24:2318–29.
Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Willerth SM, Sakiyama-Elbert SE. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. Stem J. 2019;1:1–25.
Gu P, et al. Electrospun polysaccharide scaffolds: wound healing and stem cell differentiation. J Biomater Sci Polymer Ed. 2022;33:858–77.
Chen W, et al. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater Des. 2019;179: 107886.
Fleckman P, et al. Cutaneous and inflammatory response to long-term percutaneous implants of sphere-templated porous/solid poly (HEMA) and silicone in mice. J Biomed Mater Res Part A. 2012;100:1256–68.
Xiao X, et al. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci Rep. 2015;5:1–11.
Madden LR, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci. 2010;107:15211–6.
Hayashi K, Munar ML, Ishikawa K. Effects of macropore size in carbonate apatite honeycomb scaffolds on bone regeneration. Mater Sci Eng C. 2020;111: 110848.
Leong MF, et al. Effect of electrospun poly (d, l-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J Biomed Mater Res Part A. 2009;89:1040–8.
Kaivosoja E, et al. Chemical and physical properties of regenerative medicine materials controlling stem cell fate. Ann Med. 2012;44:635–50.
Zhang Y, et al. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 2010;6:3021–8.
Osathanon T, et al. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials. 2008;29:4091–9.
Xing F, et al. Regulation and directing stem cell fate by tissue engineering functional microenvironments: scaffold physical and chemical cues. Stem Cells Int. 2019;2019:2180925.
Han Y, et al. Effect of pore size on cell behavior using melt electrowritten scaffolds. Front Bioeng Biotechnol. 2021. https://doi.org/10.3389/fbioe.2021.629270.
Matsiko A, Gleeson JP, O’Brien FJ. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng Part A. 2015;21:486–97.
Glaeser JD, et al. Modulation of matrix metalloprotease-2 levels by mechanical loading of three-dimensional mesenchymal stem cell constructs: impact on in vitro tube formation. Tissue Eng Part A. 2010;16:3139–48.
Rossello RA, Kohn DH. Cell communication and tissue engineering. Commun Integr Biol. 2010;3:53–6.
Wang W, et al. The in vitro and in vivo biological effects and osteogenic activity of novel biodegradable porous Mg alloy scaffolds. Mater Des. 2020;189: 108514.
Henning NFC, Jakus AE, Laronda MM. Building organs using tissue-specific microenvironments: perspectives from a bioprosthetic ovary. Trends Biotechnol. 2021;39:824–37.
Stoltz JF, et al. Organ reconstruction: dream or reality for the future. Biomed Mater Eng. 2017;28:S121–7.
Gm C. Signaling molecules and their receptors. In: The cell: a molecular approach. Sunderland: Sinauer Associates; 2000.
Wu Y, et al. Low-intensity pulsed ultrasound regulates proliferation and differentiation of neural stem cells through notch signaling pathway. Biochem Biophys Res Commun. 2020;526:793–8.
Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25–39.
Somaa FA, et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 2017;20:1964–77.
Suderman R, Schauer A, Deeds EJ. Understanding the dynamics of scaffold-mediated signaling. bioRxiv. 2017;9:167205.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY). 2009;324:1029–33.
Zhu J, Thompson CB. Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 2019;20:436–50.
Haschek WM, Rousseaux CG, Wallig MA. Chapter 11—kidney and lower urinary tract. In: Haschek WM, Rousseaux CG, Wallig MA, editors. Fundamentals of toxicologic pathology. 2nd ed. San Diego: Academic Press; 2010. p. 261–318.
Meloche-Dumas L, Mercier F, Lacroix A. Role of unilateral adrenalectomy in bilateral adrenal hyperplasias with Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. 2021;35:101486.
Miyaoka Y, Miyajima A. To divide or not to divide: revisiting liver regeneration. Cell Div. 2013;8:8.
Koong LJ, Ferrell CL. Effects of short term nutritional manipulation on organ size and fasting heat production. Eur J Clin Nutr. 1990;44:73–7.
Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–97.
Arigony ALV, et al. The influence of micronutrients in cell culture: a reflection on viability and genomic stability. Biomed Res Int. 2013;2013:597282–597282.
Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7:a006080.
Aramwit P, Motta A, Kundu SC. Tissue engineering: from basic sciences to clinical perspectives. Biomed Res Int. 2017;2017:8659036.
O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14:88–95.
Fodale V, et al. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J. 2011;17:89–95.
Sung HJ, et al. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25:5735–42.
Li Y, Yang S-T. Effects of three-dimensional scaffolds on cell organization and tissue development. Biotechnol Bioprocess Eng. 2001;6:311–25.
Haswell LE, et al. The development of an in vitro 3D model of goblet cell hyperplasia using MUC5AC expression and repeated whole aerosol exposures. Toxicol Lett. 2021;347:45–57.
Heindl LM, et al. Myofibroblast metaplasia after descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2011;151:1019-1023.e2.
Barad M, et al. Biallelic mutations in LAMA5 disrupts a skeletal noncanonical focal adhesion pathway and produces a distinct bent bone dysplasia. EBioMedicine. 2020;62: 103075.
Kusuhara H, et al. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regener. 2009;17:136–46.
Deng Y, et al. Engineering hyaline cartilage from mesenchymal stem cells with low hypertrophy potential via modulation of culture conditions and Wnt/β-catenin pathway. Biomaterials. 2019;192:569–78.
King TC. 1—cell injury, cellular responses to injury, and cell death. In: King TC, editor. Elsevier’s integrated pathology. Philadelphia: Mosby; 2007. p. 1–20.
Huang RJ, et al. Diagnosis and management of gastric intestinal metaplasia: current status and future directions. Gut Liver. 2019;13:596–603.
Esmaeili J, et al. Employing hydrogels in tissue engineering approaches to boost conventional cancer-based research and therapies. RSC Adv. 2021;11:10646–69.
Mastorides S, Maronpot RR. 5—carcinogenesis. In: Haschek WM, Rousseaux CG, Wallig MA, editors. Handbook of toxicologic pathology. 2nd ed. San Diego: Academic Press; 2002. p. 83–122.
Lombardo ME, et al. 3D polymeric supports promote the growth and progression of anaplastic thyroid carcinoma. Biochem Biophys Res Commun. 2020;531:223–7.
Luo Y, et al. Chapter twenty-five—three-dimensional scaffolds. In: Lanza R, Langer R, Vacanti J, editors., et al., Principles of tissue engineering. 3rd ed. Burlington: Academic Press; 2007. p. 359–73.
Hussain R, Ghafoor F, Khattak MA. Chapter 5–3D scaffolds of borate glass and their drug delivery applications. In: Kaur G, editor. Biomedical, therapeutic and clinical applications of bioactive glasses. New York: Woodhead Publishing; 2019. p. 153–73.
Remuzzi A, et al. Effect of the 3D artificial nichoid on the morphology and mechanobiological response of mesenchymal stem cells cultured in vitro. Cells. 2020;9:1873.
Simon Jr CG et al. Morphological changes driven by nanofibrous scaffolds induce marrow stromal cell osteogenesis. 2011.
Shaw AS, Filbert EL. Scaffold proteins and immune-cell signalling. Nat Rev Immunol. 2009;9:47–56.
Mohd Daud N, et al. Degradation and in vitro cell–material interaction studies on hydroxyapatite-coated biodegradable porous iron for hard tissue scaffolds. J Orthopaed Transl. 2014;2:177–84.
Bruzauskaite I, et al. Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology. 2016;68:355–69.
Choi DJ, et al. Effect of the pore size in a 3D bioprinted gelatin scaffold on fibroblast proliferation. J Ind Eng Chem. 2018;67:388–95.
Gupte MJ, et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018;82:1–11.
Zhang Z-Z, et al. Role of scaffold mean pore size in meniscus regeneration. Acta Biomater. 2016;43:314–26.
Chen Z, et al. Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater Sci Eng C. 2020;106: 110289.
Liu Y, et al. Facilitated vascularization and enhanced bone regeneration by manipulation hierarchical pore structure of scaffolds. Mater Sci Eng, C. 2020;110: 110622.
Vijayavenkataraman S, Lu W, Fuh J. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication. 2016;8: 032001.
Albanna M, et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep. 2019;9:1856.
Moncal KK, et al. Intra-operative bioprinting of hard, soft, and hard/soft composite tissues for craniomaxillofacial reconstruction. Adv Func Mater. 2021;31:2010858.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JE contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by JE and LC. The first draft of the manuscript was written by JE and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. AB critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
There are no financial conflicts of interest to disclose.
Ethical approval
This is a review study focusing on the previous studies. The authors confirm that no animal study has been carried out in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esmaeili, J., Barati, A. & Charelli, L.E. Discussing the final size and shape of the reconstructed tissues in tissue engineering. J Artif Organs 26, 95–111 (2023). https://doi.org/10.1007/s10047-022-01360-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-022-01360-1