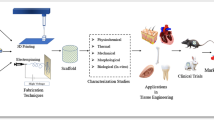
Abstract
Active research in tissue engineering is conducted in concern with different systems of body to regenerate the diseased or injured tissues. Scaffold is the cornerstone in tissue engineering which acts as a template for growing cells. The selection of biomaterials for the preparation of scaffolds is thus an important aspect to take into consideration. Various studies involving the use of different biomaterials (ceramics, natural and synthetic polymers) in scaffolds fabrication are discussed. This review mainly enlists various scaffold manufacturing techniques which are classified as conventional and rapid prototyping methods. Among the different conventional methods discussed in this review, electrospinning was found to be a versatile technique and is powerful in production of 3D nanofibrous structures. The recent advances in rapid prototyping methods lead to the development of 3D printing and bioprinting which are found to be potent scaffold fabricating techniques. The rapid prototyping methods have the potential to overcome the limitations of conventional methods. Few studies have implemented additive manufacturing techniques by combining different methods in preparation of scaffolds and have shown superior results. Even though there are well developed fabrication techniques, the limitations associated with each technique as described in this review should be carefully assessed and more studies to be conducted on modification/improvement of these techniques to exclude the limitations. In future days, the 3D printing and bioprinting have a potential to be the superior scaffold fabrication technique and there will be an enormous increase in usage of 3D printed organs in patients suffering with different pathological conditions.
Lay Summary
Scaffolds are found to be the backbone of tissue engineering. The fabrication of scaffolds thus gained a huge interest in the recent years as there are many techniques available. We, herein, have described various scaffold fabrications techniques with associated challenges and their current applications, hoping that the challenges will be addressed by the researchers in future studies. We also discussed about various biomaterials involved in fabrication of scaffolds and their applications. Recent advances in fabrication of scaffolds are enlisted; 3D printing and bioprinting were found to be the outstanding and newer ones. The pros and cons of different techniques and importance in tissue engineering/regenerative medicine were briefed.

Representation of scaffold fabrication techniques




Similar content being viewed by others
References
O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Materials today. 2011;14:88–95.
Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory’s experience. Acc Chem Res. 2000;33:94–101.
Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–9.
Babensee JE, Anderson JM, McIntire LV, Mikos AG. Host response to tissue engineered devices. Advanced Drug Delivery Reviews. 1998;33:111–39.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–43.
Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regenerative Medicine. 2009;4:65–80.
Laurencin CT, Nair LS. The quest towards limb regeneration: a regenerative engineering approach. Regen Biomater. 2016;3(2):123–5.
Prabhakar O, Arun K. Recent progresses and challenges in graphene based nano materials for advanced therapeutical applications: a comprehensive review. Materials today communications. 2020;22:100823.
Hench LL. Bioceramics. J Am Ceram Soc. 1998;81(7):1705–27.
Oh SH, Kang SG, Kim ES, Cho SH, Lee JH. Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials. 2003;24(22):4011–21.
Liu H, Liu S, Xiao Z, Chen Q, Yang D. Excess molar enthalpies of binary mixtures for (tributylphosphate+methanol/ethanol) at 298.15 K. Journal of Thermal Analysis and Calorimetry. 2006;85(3):541–4.
Gohil SV, Brittain S, Kan HM, Drissi H, Rowe D, Nair LS. Evaluation of enzymatically crosslinked injectable glycol chitosan gel. J Mater Chem B. 2015;3:5511–22.
Kenry WCL, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–50.
Wang SF, Shen L, Zhang WD, Tong YJ. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules. 2005;6(6):3067–72.
Mooney E, Dockery P, Greiser U, Murphy M, Barron V. Carbon nanotubes and mesenchymal stem cells: biocompatibility, proliferation and differentiation. Nano Lett. 2008;8(8):2137–43.
Kam NWS, Jan E, Kotov NA. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2009;9(1):273–8.
Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE. Nanoparticles in tissue engineering: applications, challenges and prospects. Int J Nanomedicine. 2018;13:5637–55. https://doi.org/10.2147/IJN.S153758.
Noh M, Kim S-H, Kim J, Lee J-R, Jeong G-J, Yoon J-K, et al. Graphene oxide reinforced hydrogels for osteogenic differentiation of human adipose-derived stem cells. RSC Adv. 2017;7(34):20779–88.
Sivashankari PR, Prabaharan M. Chitosan/carbon-based nanomaterials as scaffolds for tissue engineering. Biopolymer-Based Composites 2017: 381–97.
Rezvani Z, Venugopal JR, Urbanska AM, Mills DK, Ramakrishna S, Mozafari M. A bird’s eye view on the use of electrospunnanofibrous scaffolds for bone tissue engineering: current state-of-the-art, emerging directions and future trends. Nanomedicine: Nanotechnology, Biology and Medicine. 2016;12:2181–200.
Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–77. https://doi.org/10.1016/j.trim.2003.12.016.
Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A(7):1541–58.
Boccaccini AR, Blaker JJ. Bioactive composite materials for tissue engineering scaffolds. Expert Rev Med Devices. 2005;2(3):303–17. https://doi.org/10.1586/17434440.2.3.303.
Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17:467–79. https://doi.org/10.1007/s00586-008-0745-3.
Knight RL, Wilcox HE, Korossis SA, Fisher J, Ingham E. The use of acellular matrices for the tissue engineering of cardiac valves. Proc Inst Mech Eng [H]. 2008;222(1):129–43.
Hall S. Axonal regeneration through acellular muscle grafts. J Anat. 1997;190(1):57–71. https://doi.org/10.1046/j.1469-7580.1997.19010057.x.
Borschel GH, Huang YC, Calve S, Arruda EM, Lynch JB, Dow DE, et al. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng2005; 11(5–6) 778–786. https://doi.org/10.1089/ten.2005.11.778.
Yang J, Yamato M, Shimizu T, Sekine H, Ohashi K, Kanzaki M, et al. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007;28(34):5033–43. https://doi.org/10.1016/j.biomaterials.2007.07.052.
Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 2004;351(12): 1187–1196. https://doi.org/10.1056/NEJMoa040455.
Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat Biotechnol. 1996;14(9):1107–11. https://doi.org/10.1038/nbt0996-1107.
Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589–01.
Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19(6):485–02.
Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109–16.
Tsang VL, Bhatia SN (2005) Fabrication of three-dimensional tissues. In: Lee K., Kaplan D. (eds) Tissue Engineering II. Advances in Biochemical Engineering/Biotechnology, vol 103. Springer, Berlin, Heidelberg.
Eltom A, Zhong G, Muhammad A. Scaffold techniques and designs in tissue engineering functions and purposes: a review, Advances in Materials Science and Engineering. 2019; 2019: Article ID 3429527. https://doi.org/10.1155/2019/3429527.
Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, et al. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C. 2017;78:1246–62.
Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9(1):4.
George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan-a review. J Control Release. 2006;114:1–14.
Khan MUA, Al-Thebaiti MA, Hashmi MU, Aftab S, Abd Razak SI, Abu Hassan S, et al. Synthesis of silver-coated bioactive nanocomposite scaffolds based on grafted beta-glucan/hydroxyapatite via freeze-drying method: anti-microbial and biocompatibility evaluation for bone tissue engineering. Materials. 2020;13(4):971.
Govindan R, Gu FL, Karthi S, Girija EK. Effect of phosphate glass reinforcement on the mechanical and biological properties of freeze-dried gelatin composite scaffolds for bone tissue engineering applications. Materials today communications. 2020;22:100765.
Samourides A, Browning L, Hearnden V, Chen B. The effect of porous structure on the cell proliferation, tissue ingrowth and angiogenic properties of poly(glycerol sebacate urethane) scaffolds. Materials Science and Engineering: C. 2020;108:110384.
Preethi GU, Sreekutty J, Unnikrishnan BS, Archana MG, Syama HP, Deepa M, et al. Doxorubicin eluting microporous polysaccharide scaffolds: an implantable device to expunge tumour. Materials Science and Engineering: C. 2020;107:110332.
Tarun G, Onkar S, Saahil A, Murthy R. Scaffold: a novel carrier for cell and drug delivery. Critical reviews in therapeutic drug carrier systems. 2012;29:1–63.
Wu X, Liu Y, Li X, Wen P, Zhang Y, Long Y, et al. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010;6(3):1167–77.
O’Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25(6):1077–86.
Khoramgah MS, Ranjbari J, Abbaszadeh HA, et al. Freeze-dried multiscale porous nanofibrous three dimensional scaffolds for bone regenerations. Bioimpacts. 2020;10(2):73–85. https://doi.org/10.34172/bi.2020.10.
Bruzaukaite I, Bironaite D, Bagdonas E, Bernotiene E. Scaffolds and cells for tissue regeneration: different scaffold pore zies-different cell effects. Cytotechnology. 2016;68(3):355–69.
Liu XH, Smith L, Wei G, Won YJ, Ma PX. Surface engineering of surface engineering of nano-fibrous poly(L-lactic acid) scaffolds via self-assembly technique for bone tissue engineering. J Biomed Nanotechnol. 2005;1:54–60.
Wang Y, Chang HI, Li X, Alpar O, Coombes AG. Delievery of bioactive macromolecules from microporous polymer matrices: release and activity profiles of lysozyme, collagenase and catalase. Eur J Pharm Sci. 2009;37:387–94.
Huang H, Dean D. 3-D printed porous cellulose acetate tissue scaffolds for additive manufacturing. Additive manufacturing. 2020;31:100927.
Haider A, Haider S, Kummara MR, Kamal T, Alghyamah A-AA, et al. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: a technical and statistical review. Journal of Saudi chemical society. 2020;24(2):186–215.
Sanzherrera J, Garciaaznar J, Doblare M. On scaffold designing for bone regeneration: a computational multiscale approach. Acta Biomater. 2009;5(1):219–29.
Sola A, Bertacchini J, D'Avella D, Anselmi L, Maraldi T, Marmiroli S, et al. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater Sci Eng C. 2019;96:153–65.
Xie Y, Lan X-R, Bao R-Y, Lei Y, Cao ZQ, Yang MB, et al. High-performance porous polylactide stereocomplex crystallite scaffolds prepared by solution blending and salt leaching. Mater Sci Eng C. 2018;90:602–9.
Papkov MS, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:187–206.
Liao YH, Jones SA, Forbes B, Martin GP, Brown MB. Hyaluronan: pharmaceutical characterization and drug delivery. Drug Deliv. 2005;12:327–42.
Mao D, Li Q, Li D, Tan Y, Che Q. 3D porous poly(ε-caprolactone)/58S bioactive glass-sodium alginate/gelatin hybrid scaffolds prepared by a modified melt molding method for bone tissue engineering. Mater Des. 2018;160:1–8.
Se Heang Oh, SoungGon Kang, EunSeok Kim, Sang Ho Cho, Jin Ho Le. Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials.2003;24 (22): 4011–4021.
Thadavirul N, Pavasant P, Supaphol P. Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. Journal of Biomedical Materials Research. 2013;102(10):3379–92.
Obayemi JD, Jusu SM, Salifu AA, Ghahremani S, Tadesse M, Uzonwanne VO, et al. Degradable porous drug-loaded polymer scaffolds for localized cancer drug delivery and breast cell/tissue growth. Materials Science & Engineering C. 2020;122:110794.
Nwabor OF, Singh S, Paosen S, Vongkamjan K, Voravuthikunchai SP. Enhancement of food shelf life with polyvinyl alcohol-chitosan nanocomposite films from bioactive Eucalyptus leaf extracts. Food Bioscience. 2020;36:100609.
Jahid MA, Hu J, Thakur S. Novel approach of making porous polyurethane membrane and its properties for apparel application. Applied polymer. 2020;137(15):48566.
Rajzer I, Kurowska A, Jabłoński A, Kwiatkowski R, Piekarczyk W, Hajduga MB, et al. Scaffolds modified with graphene as future implants for nasal cartilage. J Mater Sci. 2020;55:4030–42. https://doi.org/10.1007/s10853-019-04298-7.
Li Z, Xie M-B, Li Y, Ma Y, Li J-S, Dai F-Y. Recent progress in tissue engineering and regenerative medicine. Journal of Biomaterials and Tissue Engineering. 2016;6(10):755–66.
Wang W, Liu D, Li D, du H, Zhang J, You Z, et al. Nanofibrous vascular scaffold prepared from miscible polymer blend with heparin/stromal cell-derived factor-1 alpha for enhancing anticoagulation and endothelialisation. Colloids Surf B: Biointerfaces. 2019;181:963–72.
Rusakov D, Menner A, Bismarck A. High-performance polymer foams by thermally induced phase separation. Macromol Rapid Commun. 2020;41(11):2000110.
Lee CT, Kung PH, Lee YD. Preparation of poly(vinyl alcohol)- chondroitin sulfate hydrogel as matrices in tissue engineering. Carbohydr Polym. 2005;61:348–54.
Benoit DS, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007; 28;28 66–77.
Lu T, Li Y, Chen T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int J Nanomedicine. 2013;8(1):337–50.
Yao Q, Fuglsby KE, Zheng X, Sun H. Nanoclay-functionalized 3D nanofibrous scaffolds promote bone regeneration. J Mater Chem B. 2020;8:3842–51.
Asadian M, Chan KV, Norouzi M, Grande S, Cools P, Morent R, et al. Fabrication and plasma modification of nanofibrous tissue engineering scaffolds. Nanomaterials. 2020;10:119.
Liu X, Ma PX. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30(25):4094–03.
Chen P, Zhou Z, Liu W, Zhao Y, Huang T, Li X, et al. Preparation and characterization of poly(L-lactide-co-glycolide-co-ε-caprolactone) scaffolds by thermally induced phase separation. Journal of macromolecular science, Part B. 2020;59(7):427–39.
Soundarya SP, Menon AH, Chandran SV, Selvamurugan N. Bone tissue engineering: scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int J Biol Macromol. 2018;119:1228–39.
Ceccaldi C, Bushkalova R, Cussac D, Duployer B, Tenailleau C, Bourin P, et al. Elaboration and evaluation of alginate foam scaffolds for soft tissue engineering. Int J Pharm. 2017;524:433–42.
Liao X, Zhang H, He T. Preparation of porous biodegradable polymer and its nanocomposites by supercritical CO2 foaming for tissue engineering. J Nanomater. 2012;6:1–12.
Nam YS, Yoon JJ, Park TG. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. Journal of Biomedical Materials Research banner. 2000;53(1):1–7.
Kim TK, Yoon JJ, Lee DS, Park TG. Gas foamed open porous biodegradable polymeric microspheres. Biomaterials. 2006;27(2):152–9.
Kazimierczak P, Benko A, Palka K, Canal C, Kolodynska D, Przekora A. Novel synthesis method combining a foaming agent with freeze-drying to obtain hybrid highly macroporous bone scaffolds. Journal of Materials Science and amp; Technology. 2020;43:52–63. https://doi.org/10.1016/j.jmst.2020.01.006.
Januariyasa IK, Yusuf Y. Porous carbonated hydroxyapatite-based scaffold using simple gas foaming method. Journal of Asian Ceramic Societies. 2020. https://doi.org/10.1080/21870764.2020.1770938.
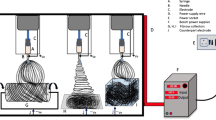
Sarkar K, Gomez C, Zambrano S, Ramirez M, de Hoyos E, Vasquez H. Electrospinning to forcespinning™. Mater Today. 2010;13(11):12–4.
Jia W, Li M, Kang L, Gu G, Guo Z, Chen Z. Fabrication and comprehensive characterization of biomimetic extracellular matrix electrospun scaffold for vascular tissue engineering applications. J Mater Sci. 2019;54:10871–83. https://doi.org/10.1007/s10853-019-03667-6.
Rodoplu D, Mutlu M. Effects of electrospinning setup and process parameters on nanofiber morphology intended for the modification of quartz crystal microbalance surfaces. J EngFiber Fabr. 2012;2:118–23.
Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Ghayour H, Ismail AF, Nur H, et al. Electrospun nano-fibers for biomedical and tissue engineering applications: a comprehensive review. Materials. 2020;13:2153.
Belgheisi G, Nazarpak MH, Hashjin MS. Bone tissue engineering electrospun scaffolds based on layered double hydroxides with the ability to release vitamin D3: fabrication, characterization and in vitro study. Applied Clay Science. 2020;185:105434.
Sun B, Long YZ, Zhang HD, Li MM, Duvail JL, Jiang XY, et al. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog Polym Sci. 2014;39(5):862–90.
Alhosseini SN, Moztarzadeh F, Mozafari M, Asgari S, Dodel M, Samadikuchaksaraei A, et al. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Nanomedicine. 2012;7:25–34.
Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22(7):354–62.
Chung HJ, Park TG. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007;59:249–59.
Ma Z, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue engineering. 2005;11:7–8.
Venugopal JR, Low S, Choon AT, Kumar AB, Ramakrishna S. Nanobioengineeredelectrospun composite nanofibers and osteoblasts for bone regeneration. Artif Organs. 2008;32(5):388–97.
Jun I, Han HS, Edwards JR, Jeon H. Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci. 2018;19(3):745. https://doi.org/10.3390/ijms19030745.
Chen H, Peng Y, Wu S, Tan LP. Electrospun 3D fibrous scaffolds for chronic wound repair. Materials. 2016;9(4):272. https://doi.org/10.3390/ma9040272.
Koski A, Yim K, Shivkumar S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater Lett. 2004;58:493–7. https://doi.org/10.1016/S0167-577X(03)00532-9.
Casper CL, Stephens JS. Controlling surface morphology of electrospun polysterene fibers: effect of humidity and molecular weight in electrospinning process. Macromolecules. 2004;37:573–8.
Beachley V, Wen X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater Sci Eng C. 2009;29:663–8. https://doi.org/10.1016/j.msec.2008.10.037.
McKee MG, Wilkes GL, Colby RH, Long TE. Correlations of solution rheology with electrospun fiber formation of linear and branched polyesters. Macromolecules. 2004;37:1760–7. https://doi.org/10.1021/ma035689h.
Luo CJ, Nangrejo M, Edirisinghe M. A novel method of selecting solvents for polymer electrospinning. Polymer (Guildf). 2010;51:1654–62. https://doi.org/10.1016/j.polymer.2010.01.031.
Nie H, He A, Zheng J, Xu S, Li J, Han CC. Effects of chain conformation and entanglement on the electrospinning of pure alginate. Biomacromolecules. 2008;9:1362–5. https://doi.org/10.1021/bm701349j.
Munir MM, Suryamas AB, Iskandar F, Okuyama K. Scaling law on particle-to-fiber formation during electrospinning. Polymer (Guildf). 2009;50:4935–43. https://doi.org/10.1016/j.polymer.2009.08.011.
Xu F, Gough I, Dorogin J, Sheardown H, Hoare T. Nanostructured degradable macroporous hydrogel scaffolds with controllable internal morphologies via reactive electrospinning. Acta Biomater. 2020;104:135–46. https://doi.org/10.1016/j.actbio.2019.12.038.
Tan H-L, Kai D, Pasbakhsh P, Teow S-Y, Lim Y-Y, Pushpamalar J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: a potential scaffold for tissue engineering. Colloids and surfaces B:Biointerfaces. 2020;188:110713.
Mina H, Hajir Bahrami S, Ranjbar-Mohammadi M, Milan PB. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Materials science and engineering:C. 2019;103:109768.
Yeong W-Y, Chua C-K, Leong K-F, Chandrasekaran M. Rapid prototyping in tissue engineering: challenges and potential. Trends in biotechnology. 2004;22(12):643–52.
Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121–30.
Melchels FPW, Bertoldi K, Gabbrielli R, Velders AH, Feijen J, Grijpma DW. Mathematically defined tissue engineering scaffold architectures prepared by stereolithography. Biomaterials. 2010;31(27):6909–16.
Landers R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromolecular Materials & Engineering. 2015;282(1):17–21.
Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng. 2014;16(1):247–76.
Ralf S, Matthias L, Andreas H, Martin David P, Hoerstrup Simon P, Potapov Evgenij V, et al. Application of stereolithography for scaffold fabrication for tissue engineered heart valves. ASAIO. 2002;48(1):12–6.
Rider P, Kačarević ŽP, Alkildani S, Retnasingh S, Schnettler R, Barbeck M. Additive manufacturing for guided bone regeneration: a perspective for alveolar ridge augmentation. Int J Mol Sci. 2018;19(11). https://doi.org/10.3390/ijms19113308.
Du D, Asaoka T, Shinohara M, Kageyama T, Ushida T, Furukawa KS. Microstereolithography based fabrication of anatomically shaped beta-tricalcium phosphate scaffolds for bone tissue engineering. Biomed Res Int. 2015;2015:859456–9. https://doi.org/10.1155/2015/859456.
Le Guéhennec L, Van Hede D, Plougonven E, Nolens G, Verlée B, De Pauw M-C, et al. In vitro and in vivo biocompatibility of calcium-phosphate scaffolds three-dimensional printed by stereolithography for bone regeneration. J Biomed Mater Res A. 2020;108(3):412–25.
Rimell JT, Marquis PM. Selective laser sintering of ultra high molecular weight polyethylene for clinical applications. J Biomed Mater Res. 2015;53(4):414–20.
Bin Duan, Min Wang, Wen You Zhou, Wai Lam Cheung, Zhao Yang Li, William W. Lu. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. 2010;6(12):4495–4505.
Tan KH, Chua CK, Leong KF, Cheah CM, Cheang P, Abu Bakar MS, et al. Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials. 2003;24(18):3115–23.
Higa CF, Gradowski T, Elifio-Esposito S, de Oliveira MF, Inforçatti P, da Silva JVL, et al. Influence of selective laser sintering process parameters on microstructure and physicochemical properties of poly(vinyl alcohol) for the production of scaffolds. Rapid Prototyp J. 2020;26(6):1155–64.
Li J, Zhao Z, Yan R, Yang Y. Mechanical properties of graded scaffolds developed by curve interference coupled with selective laser sintering. Materials Science and Engineering: C. 2020;116:111181.
Lin K, Liu J, Wu J-M, Sun Y, Li F, Zhou Y, et al. Selective laser sintered nano-HA/PDLLA composite microspheres for bone scaffolds applications. Rapid Prototyp J. 2020;26(6):1131–43.
Xiong Z, Yan Y, Wang S, Zhang R, Zhang C. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr Mater. 2002;46(11):771–6.
Lu W, Gramlich WM, Gardner DJ. Improving the impact strength of poly(lactic acid) (PLA) in fused layer modeling (FLM). Polymer. 2017;2017(114):242–8.
Zein I, Hutmacher DW, Tan KC, Teoh SH. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002;23(4):1169–85.
Sanz-Horta R, Elvira C, Gallardo A, Reinecke H, Rodríguez-Hernández J. Fabrication of 3D-printed biodegradable porous scaffolds combining multi-material fused deposition modeling and supercritical CO2 techniques. Nanomaterials. 2020;10:1080.
Sachs E, Cima M, Cornie J, Brancazio D, Cornie J. Three-dimensional printing: rapid tooling and prototypes directly from a CAD model. CIRP Ann. 1990;39(1):201–4.
Wang M, Favi P, Cheng X, Golshan NH, Ziemer KS, Keidar M, et al. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016;46:256–65.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85.
Oliveira SM, Reis RL, Mano JF. Towards the design of 3D multiscale instructive tissue engineering constructs: current approaches and trends. Biotech Adv. 2015;33(6):842–55.
Mironov V, Kasyanov V, Markwald RR. Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotech. 2011;22:667–73.
Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–74.
An J, Teoh JEM, Suntornnond R, Chua CK. 3D printing-review design and 3D printing of scaffolds and tissues. Engineering. 2016;1(2):261–8.
Holzl K et al., Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016; 8 (3).
He Y, Yang FF, Zhao HM, Gao Q, Xia B, Fu JZ. Research on the printability of hydrogels in 3D bioprinting. Sci Rep. 2016;6:29977.
Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60(3):691–9.
Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016;102:20–42.
Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioactive materials. 2018;3:144–56.
Dababneh AB, Ozbolat IT. Bioprinting technology: a current state-of-the-art review. J Manuf Sci Eng. 2014;136:061016.
Billiet T, Vandenhaute M, Schelfhout J, van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33(26):6020–41.
Hopp B et al., Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt. Eng. 2012;51 (1).
Pati F, Song T-H, Rijal G, Jang J, Kim SW, Cho D-W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 2015;37:230–41.
Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2011;35(13):4026–34.
Wan Z, Zhang P, Liu Y, Lv L, Zhou Y. Four-dimensional bioprinting: current developments and applications in bone tissue engineering. Acta Biomater. 2020;101:26–42.
Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536.
Datta S, Jana S, Das A, Chakraborty A, Chowdhury AR, Datta P. Bioprinting of radiopaque constructs for tissue engineering and understanding degradation behavior by use of micro-CT. Bioactive Materials. 2020;5(3):569–76.
Rees A, Powell LC, Chinga-Carrasco G, Gethin DT, Syverud K, Hill KE, et al. 3D bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. Biomed Res Int. 2015;2015:925757–7. https://doi.org/10.1155/2015/925757.
Wu ZJ, Su X, Xu Y, Kong B, Sun W, Mi S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016;6:1–10.
Gao G, Schilling AF, Hubbell K, Yonezawa T, Truong D, Hong Y, et al. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnology letters. 2015;37(11):2349–55.
Lee JW, Choi Y-J, Yong W-J, Pati F, Shim J-H, Kang KS, et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 2016;8(1):015007.
Catros S, Fricain J-C, Guillotin B, Pippenger B, Bareille R, Remy M, et al. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication. 2011;3(2):025001.
Keriquel V, Oliveira H, Rémy M, Ziane S, Delmond S, Rousseau B, et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Scientific reports. 2017;7:1178.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koyyada, A., Orsu, P. Recent Advancements and Associated Challenges of Scaffold Fabrication Techniques in Tissue Engineering Applications. Regen. Eng. Transl. Med. 7, 147–159 (2021). https://doi.org/10.1007/s40883-020-00166-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-020-00166-y