Abstract
Understanding the sources of primary production supporting marine food webs is important for conserving critical habitats. However, the importance of allochthonous versus autochthonous production is often unclear. We identify the sources of primary production supporting three species of predatory fish (Lutjanus carponotatus, Lutjanus fulviflamma, and Lethrinus punctulatus) in the Dampier Archipelago, Western Australia. The proportions of mangrove, seagrass, macroalgae, and suspended particulate organic matter (SPOM) assimilated by fish of different life-history stages and from different habitats were determined using stable isotopes and Bayesian mixing models. While contributions of sources varied among species and habitats, we found that SPOM was an important energetic resource for both adults and juveniles of all three fish species. Juvenile L. carponotatus and L. punctulatus inhabiting macroalgal habitats assimilated more seagrass- and macroalgae-derived material than fish from coral or mangrove habitats, while reliance on mangrove carbon was generally limited overall. Assimilation of macrophyte-derived material decreased throughout ontogeny for L. carponotatus, while L. fulviflamma relied on a combination of SPOM and seagrass into adulthood. The contribution of macroalgae and SPOM to the diets of juvenile L. punctulatus and L. carponotatus from macroalgal habitats varied spatially but was unrelated to seascape configuration, habitat quality, or hydrodynamic conditions. While mixing models suggested a modest degree of trophic connectivity, cross-habitat trophic subsidies provided by macrophytes play a minor role in supporting the studied fish of either life-history stage. Instead, microalgal production predominantly fuels the productivity of predatory fish in this turbid, macrotidal system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the sources of primary production supporting marine food webs is important for identifying and conserving critical habitats (Loneragan and others 1997; Polis and others 1997; Abrantes and others 2015; Whitfield 2017; Bearham and others 2023). Within coastal seascapes, macrophytes, such as seagrass, mangroves, and macroalgae, are prominent primary producers that form complex three-dimensional structures that provide shelter for fish (Beck and others 2001; Horinouchi 2007; Whitfield 2017). However, fish seeking refuge in macrophyte habitats do not necessarily derive their nutritional resources from the same habitat (Igulu and others 2013; Vaslet and others 2015). In tropical seascapes, macrophyte habitats often co-occur with coral reefs, forming patch mosaics comprised of multiple habitats that are biologically (e.g., foraging migrations, prey movement) and hydrologically (e.g., tidal transport of detritus) connected (Hyndes and others 2014). This connectivity can result in spatial separation between the habitats occupied by fish and those from which they ultimately derive their nutrition (Connolly and others 2005)
The importance of different sources of primary production to animals is in part dictated by the extent to which habitats are spatially linked and contiguous (structural connectivity) and the degree to which a seascape facilitates or impedes movement between habitats (functional connectivity; Taylor and others 2006; Nagelkerken and others 2008b; Wyatt and others 2012; Abrantes and others 2015). The functional connectivity of a seascape is generally lower for juvenile fish than for their adult counterparts as juveniles are comparatively less mobile (Cummings and others 2010; Green and others 2015; Nash and others 2015). Despite their smaller home ranges, juvenile fish in highly-connected coastal seascapes may still obtain some, or all, of their nutritional resources from neighbouring habitats (Olson and others 2019). Although there is a growing body of research exploring the significance of such cross-habitat trophic subsidies for adult fish (e.g., Connolly and others 2005; Igulu and others 2013; Davis and others 2014), less is known about the relative importance of autochthonous versus allochthonous production for juvenile fish.
An understanding of the sources of production underpinning the diets of fish, and how this may change through ontogeny or be affected by seascape configuration, is important when designing efficient conservation strategies that preserve crucial habitat connections (Connolly and others 2005). In north-western Australia, many species of fish exhibit preferences for coral or macroalgal habitats as juveniles (Wilson and others 2010; Evans and others 2014); however, little is known about the sources of autotrophic production that support their diets and how this varies among habitats. Gaining insight into the trophic ecology of juvenile fish remains challenging due to their small size and high mortality rates. Stable isotope ratios of carbon (13C/12C) and nitrogen (15N/14N) in fish tissue can provide information on the animal’s diet over the preceding days to weeks for fast growing juveniles, or months for older individuals (Herzka 2005; Fry 2006). The isotopic composition of fish tissue can serve as an indirect indicator of the source of primary production at the base of the food chain which can be used to identify trophic linkages between habitats, provided that the isotopic composition of basal sources are sufficiently distinct (Fry and Sherr 1989; Bouillon and others 2008). An isotopic value of an animal that differs from its surrounding habitat may indicate reliance on allochthonous carbon sources or recent immigration to a population (Polis and others 1997; Fry and Ewel 2003; Kimirei and others 2013; Olson and others 2019). For example, several studies have used isotopic techniques to show that juvenile fish inhabiting mangrove habitats relied on carbon derived from seagrass meadows (Vaslet and others 2015; Le and others 2019). This knowledge has important implications for fisheries sustainability and conservation outcomes as both the residence habitat and the source of primary production must be conserved to ensure viable juveniles for population replenishment (Connolly and others 2005; Olds and others 2016).
The Dampier Archipelago, located in northwestern Australia, comprises 42 islands that are fringed by diverse habitats and provide an ideal opportunity to investigate sources of primary production underpinning the diets of fish (Figure 1; Morrison 2004; Moustaka and others 2024). The nearshore benthic habitats of the Archipelago support diverse and abundant assemblages of juvenile fishes (Moustaka and others in review), including lethrinid and lutjanid species that are often targeted by recreational and commercial fishers (Gaughan and others 2019; Newman and others 2019). Although recent research has shown minimal influence of seascape configuration on the composition of fish assemblages (Moustaka and others 2024; in review), seascape effects on the trophic ecology of fish remain unexplored. This study therefore had three objectives: 1) to identify the basal sources underpinning the diets of juveniles and adults of three benthic mesopredatory fish species to assess the extent of allochthonous versus autochthonous carbon use; 2) to examine how the relative importance of basal sources differs among nursery habitats and successive habitats occupied throughout ontogeny; and 3) to evaluate the influence of seascape configuration and hydrodynamic conditions on the assimilation of different basal sources by fish.
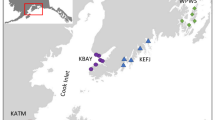
Location of sites (indicated by abbreviated site codes; see Table S1) and habitat patches (pink: coral, orange: macroalgae, green: mangrove) within each site from which samples were collected in the Dampier Archipelago, Western Australia.
Methods
Study Location
The Dampier Archipelago, located on the North West Shelf of Australia (Figure 1), is characterised by macrotidal conditions (maximum 5.1 m tidal range) and a strong cross-shelf turbidity gradient (Pearce and others 2003). The islands of the Archipelago are fringed by patch mosaics of habitats, including coral, macroalgae, mangrove, seagrass, sand, pavement, and rubble (Figure 1).
Thirteen shallow (< 7 m depth) bays were selected as study sites across the Archipelago based on the presence of substantial habitat area in variable configurations (e.g., areal extent of mangrove habitat in each bay ranged from 0 – 0.35 km2) using pre-existing habitat maps (Moustaka and others 2024; Figure1). Sampling was predominantly conducted in February 2021, with additional samples of detrital mangrove leaves collected in July 2021, and filter-feeding bivalves in November 2021 (mangrove habitats) and January 2022 (coral habitats). Samples were collected in each of the main biogenic habitats present in each bay (i.e., coral reef, macroalgal bed, and mangrove fringe), although not all bays contained all habitat types (Table S1).
Sample Collection
Fish collections
Three species of fish were collected for this study: stripy snapper (Lutjanus carponotatus), blackspot sea perch (Lutjanus fulviflamma), and bluespotted emperor (Lethrinus punctulatus). These species were selected based on their relative abundance in the Dampier Archipelago (determined using underwater visual census surveys conducted in January 2021) and their contrasting patterns of habitat use and ontogenetic habitat shift (Fulton and others 2020). Additionally, all three species are of value to commercial and recreational fisheries in Western Australia (Newman and others 2018; Gaughan and others 2019). Lethrinus punctulatus exclusively occupies shallow macroalgal habitats during the juvenile stage before migrating offshore to deeper waters as adults (Taylor and others 2017). In contrast, juvenile L. carponotatus were observed in both coral and macroalgal habitats, while adults were primarily found on coral reefs (Moustaka and others 2024; in review). Adult L. fulviflamma were also observed on coral reefs, whereas juveniles were only recorded in macroalgae and mangrove habitats (Moustaka and others 2024; in review). Globally, L. punctulatus is not regarded as a distinct species but rather a synonym for Lethrinus lentjan (pink ear emperor). However, for the purpose of this study the taxon will be referred to as L. punctulatus and recognised as a distinct species, in line with the classification and management by the Western Australian Department of Primary Industries and Regional Development (Newman and others 2019).
Juvenile fish were collected at 13 sites from each of the habitats in which they had been observed (Table S1) using either opera house traps baited with crushed Sardinops spp. or by scuba divers using spearguns with pronged heads. Post-capture, fish were immediately euthanised, placed on ice for transport, and subsequently stored at − 20 °C. Fish were classified as juveniles (Age-0 +) or adults (Age-1 + and older) based on published length-at-age for each species (L. carponotatus = 125 mm fork length [FL; Kritzer 2004], L. fulviflamma = 140 mm FL [Grandcourt and others 2006], and L. punctulatus = 155 mm FL [Wakefield and others unpublished results]). To explore how reliance on carbon sources varied between habitats and throughout ontogeny, adult L. carponotatus and L. fulviflamma were also collected from coral habitats. The aim was to collect 10 individuals per species, per site, per habitat, and per life history stage; however, actual sample sizes varied from 2 to 25 (Table S1). As small sample sizes may not adequately capture variation in isotopic values, spatial comparisons should be interpreted cautiously for sites with low replication.
Source collections
Lutjanus carponotatus, L. fulviflamma, and L. punctulatus are generalist carnivores whose diets predominantly consist of crustaceans (including shrimp, amphipods, crabs) and small fish (typically species of Blenniidae), with echinoderms, cephalopods, molluscs, and annelids occasionally consumed to varying extents (Salini and others 1994; Brewer and others 1995; Connell 1998; Kamukuru and Mgaya 2004; Kulbicki and others 2005; Farmer and Wilson 2011; Piggott 2021). As this study aimed to explore the primary producers underpinning fish diets, and given the wide array of potential prey across the three different habitats, coupled with the uncertainties surrounding the diets of the study species during their early life stages, intermediary prey items were not collected. Instead, sampling effort focused on achieving greater spatial replication of both fish and primary producers, and comprehensive representation of fish across the juvenile size range.
Five samples of common primary producers were collected at each site (where present) including mangroves (Rhizophora mangle or Avicenna marina; whichever was most abundant), detrital mangrove leaves, macroalgae (Sargassum spp.), and seagrass (Halophila ovalis; Table S1). Live mangrove leaves were harvested directly from the trees and detrital leaves were collected from the top layer of sediment under the canopy. Live macroalgae and seagrass samples were collected from macroalgal reefs by hand then rinsed in fresh water to remove any epifauna prior to transport. Corals were not included as a potential basal source as recent work has shown a high dependence on heterotrophic feeding by corals in the Dampier Archipelago (Travaglione and others 2023). Turf algae was also omitted from collections because of its low productivity and nutritional value in this region (Tebbett and others 2023). Mangrove epiphytes were similarly excluded due to their scarcity.
As the fish species targeted in this study are known to feed on invertebrates (Whitfield 1998; Piggott 2021) whose diets can include suspended and deposited particulate matter (e.g., amphipods), common filter-feeding bivalves (a mix of Mytilidae [mussels], Ostreidae [oysters] and Pteriidae [feather oysters]) were collected as a proxy for suspended particulate organic matter (SPOM; Fukumori and others 2008; Christianen and others 2017). SPOM baselines are highly variable and difficult to characterise and as such, invertebrate filter feeders represent a time-integrated proxy for this basal resource (see Statistical analyses section for details of mathematical corrections for trophic enrichment; Post 2002; Richoux and Froneman 2009; Skinner and others 2022). Filter-feeding bivalves can be selective feeders; however, where selection does occur bivalves typically prefer organic and living particles over inorganic and dead particles (Rosa and others 2018). Additionally, isotopic composition of sediment POM at a subset of five sites was provided by Beltran and others (unpublished results; see Text S1 for details of sediment core collection and processing). All samples were placed on ice for transport, and subsequently stored at − 20 °C.
Sample Preparation
In the laboratory, fish samples were defrosted, and the fork length (mm) and weight (g) recorded. Fork length could not be accurately determined for a small number of L. punctulatus (n = 10) and L. fulviflamma (n = 11) samples that were damaged during collection. In these instances, length was estimated based on mean otolith weight using a species-specific polynomial linear regression fitted to a subset of the observed values (i.e., a training dataset) and validated with the remaining observed values (see Text S2, Figure S1, and Table S2 for further details). White muscle tissue samples were collected below the dorsal fin, above the lateral line for fish, and from the foot for bivalves. Muscle tissue was chosen for stable isotope analysis given its slow turnover rate, reflecting the isotopic composition of food consumed over periods of several weeks to months (Fry and Arnold 1982; Herzka 2005).
Mangrove and seagrass leaves were removed from the stems, while macroalgae blades were separated from stipes and holdfasts. All leaves and blades were cleaned of any attached epiphytes using a scalpel. All samples were thoroughly rinsed in deionised water. The sparse epiphytic filamentous algae present on the thali and blades of macroalgae samples were also collected. As epiphytes were not present in large amounts, samples were pooled within sites to ensure sufficient material for SIA yielding one to three replicates per site (Table S1).
Stable Isotope Analyses
All stable isotope samples were oven dried for 48 h at 60 °C, ground to a fine homogenous powder, and weighed into tin capsules. In some instances, this process yielded insufficient dried seagrass material for SIA, and in these cases, the smallest samples from the same site were pooled to provide enough material for a replicate (Table S1). All samples were analysed for δ15N and δ13C (see Text S3 for full details). The combined analytical uncertainty of analyses (1 standard deviation) was 0.10 ‰ for δ15N and δ13C and 0.1 for the ratio of carbon to nitrogen by weight (C:N).
Variation in the lipid content of animal tissues affects δ13C values. Some of the smallest fish samples and several bivalve samples had C:N ratios > 3.5, indicating the presence of excessive lipids (Skinner and others 2016). A mathematical normalisation method was used to correct the δ13C values of these samples (Kiljunen and others 2006; Post and others 2007; Skinner and others 2016; see Text S4 for full details), a procedure that is considered to be an appropriate method for accounting for lipid presence in samples that are analysed for both δ15N and δ13C (Sweeting and others 2006).
Data Analysis
Statistical analyses
Length-isotope and habitat-isotope relationships were initially explored using analysis of variance (ANOVA) tests. Fish length was included as a continuous effect (as opposed to life history stage as a categorical variable) due to substantial variation in the abundance of each life history stage among habitats and sites (of a given habitat type). Separate tests were conducted for δ13C and δ15N, and for each species. Models for L. carponotatus consisted of a fixed effect of habitat, a continuous effect of length, and a random effect of site. Since L. punctulatus was exclusively observed and collected in macroalgal habitats, the model for this species comprised only a continuous effect of length and a random effect of site. As L. fulviflamma were not collected in sufficient numbers for every combination of habitat and site, samples were pooled to habitat and models did not include a random effect of site. Tukey’s Honestly Significant Difference post hoc tests were used to examine differences between groups where there were significant differences for a fixed effect and no interaction with length. Where interactions between categorical independent variables and fish length were significant, effects were investigated by visually inspecting the data. Some of the smallest L. punctulatus and L. carponotatus (< 30 mm, n = 1 & 5, respectively) individuals were markedly more depleted in 13C relative to their conspecifics (Figure 2). These comparatively negative δ13C values likely reflect the larval planktivorous phase in the open ocean before recent settlement to coastal habitats (Fry and Sherr 1989; Herzka and others 2001; Cocheret de la Morinière and others 2003). To prevent any potential influence of these recently settled fish on the assessment of habitat and ontogenetic patterns, ANOVAs were conducted on datasets that both included and excluded them. Significant differences were found between the results obtained from the two datasets (Table S3), and the recently settled fish (represented in Figure 2 by “*”) were therefore excluded from further analysis. Only the results from the ANOVAs that excluded these individuals are discussed herein.
a Size distribution for collected Lutjanus carponotatus, Lutjanus fulviflamma, and Lethrinus punctulatus from coral (pink), macroalgae (orange) and mangrove (green) habitats, and relationship between fish fork length and b δ13C and c δ15N values. Recently settled fish (fork length < 30 mm) that were excluded from analyses are indicated by ‘*’. Vertical lines indicate estimated length at Age-1 + for each species (i.e., the boundary of juvenile to adult).
Between-taxa and between-habitat variations in isotopic values of fish, as well as the proximity of fish from different habitats to potential sources, were examined using biplots of δ13C and δ15N values. Bivalve δ13C and δ15N values (proxy for SPOM [hereon referred to as SPOM*]) were corrected for isotopic discrimination factor to further analysis by a factor of 0.8 for δ13C and 3.6 for δ15N based on the average isotopic discrimination factor reported by Brauns and others (2018) and Ludovisi and others (2022) for marine bivalves (Fukumori and others 2008). To determine whether sources should be grouped prior to inclusion in isotopic mixing models, ANOVAs (including a random effect of site) were used to test for differences in δ13C and δ15N values of basal carbon sources. As mangrove species, live/detrital mangrove leaves, and SPOM* from different habitats could not be distinguished by either their δ13C or δ15N values (pairwise p > 0.05; Table S4), they were each pooled for subsequent analysis. All data were inspected for normality of residuals and homogeneity of variance using Q-Q plots. Analyses were conducted in R (version 4.2.0) using the nlme package (version 3.1–163; R Core Team 2022; Pinheiro and others 2023). Significance for all analyses was p < 0.05 and variation around the mean is presented as standard deviation unless otherwise stated.
Isotopic mixing models
The relative contribution of different carbon sources to fish was assessed using Bayesian stable isotope mixing models (SIMM) using the MixSIAR R package (Stock and others 2018). Mixing models were run separately for each species and life history stage (excluding adult L. carponotatus and L. punctulatus in macroalgae due to low sample replication). To avoid uninformative outputs from the mixing models, the number of sources included was limited to four (mangroves, seagrass, macroalgae, and SPOM*; Phillips and others 2014). Sediment POM was not included as a source as it was isotopically indistinguishable from SPOM* and was only collected at a subset of sites (Table S1 & S3). Epiphytic algae were also excluded from mixing models due to low replication and low biomass. As not all sources were able to be collected from all sites (Table S1), average isotopic values (± standard deviation) of sources across the Archipelago were used in mixing models. It should be noted that the necessary use of source averages and standard deviations in mixing models may lead to a loss of information regarding the variability within each source (Hyndes and others 2013; Phillips and others 2014). Additionally, as this study was replicated spatially but not temporally, potential seasonal variation in isotopic values was not considered (Olin and others 2012; Hyndes and others 2013). Similarly, temporal variation in the isotopic composition of primary producers may have influenced the results of this study as detrital mangrove leaves and some bivalves were collected 9—11 months after fish.
Corrections for isotopic discrimination were applied when modelling feasible mixtures (Peterson and Fry 1987). The degree of isotopic discrimination between trophic levels (TL) varies considerably between species, tissues, diets, temperatures, and growth rates, and is generally higher for 15N (~2.0–3.5 ‰ per trophic level) than 13C (~0–1.5 ‰ per trophic level; Minagawa and Wada 1984; Zanden and Rasmussen 2001; Vanderklift and Ponsard 2003; Caut and others 2009). This variation introduces some uncertainty regarding the isotopic discrimination factor for 15N. Indeed, the δ15N values of fish in this study demonstrated considerable variation between species, life history stage, and habitat, as would be expected based on relationships between gape size and prey size, and probable differences in prey assemblages in each habitat (Kamukuru and Mgaya 2004; Dunic and Baum 2017; Fraser and others 2020; Nguyen and others 2020). Therefore, using a single TL to estimate total isotopic discrimination was deemed inappropriate. Instead, the average TL was calculated for each combination of species, life history stage, and habitat following Post (2002; see Text S5 for full methods and Table S5 for calculated TLs). An isotopic discrimination factor of 1.76 ± 0.2 for δ13C and 2.54 ± 0.2 for δ15N per trophic level was used for both TL calculations and mixing models (Caut and others 2009). Biplots of δ13C and δ15N values of sources and consumers (corrected for isotopic discrimination) were inspected prior to running mixing models to ensure that all consumer values fell within the mixing polygon.
Multiplicative (residual * process) error was specified in the mixing models as the sampled animals consume sources via predation events and to account for variation related to the sampling procedures (Stock and others 2018). Site was included as a random effect in all models, and habitat as a fixed effect in the L. carponotatus and L. fulviflamma models. Models used uninformative priors and were run for 3,000,000 iterations with the first 1,500,000 discarded as burn in. Model convergence was assessed using the trace plots and the Gelman–Rubin and Geweke diagnostic tests.
Drivers of Spatial Variation in Assimilation of Basal Sources by Fish
A series of mixing models incorporating different covariates were run to explore the potential drivers of spatial variation in source assimilation by juvenile L. carponotatus and L. punctulatus from macroalgal habitats. Lutjanus fulviflamma was excluded from the analysis due to the limited number of sites where fish were collected (3 and 5 for macroalgae and mangrove habitats, respectively). Four models containing different continuous covariates were run for each species to assess the influence of local habitat quality (average percent cover of macroalgae), seascape configuration (proportional area of macroalgae within a 500 m radius buffer), and hydrodynamic conditions (average significant wave height and water residence time). These variables were selected as the spatial variation in source assimilation by fish predominantly reflected differences in the assimilation of macroalgae and SPOM* (Table S6). For detailed methods regarding predictor variable data collection and ecological justification for inclusion see Table S7 and Text S6. A model containing a random effect of site was included in the suite of candidate models for each species as the null model. Models were compared using LOOIC (approximate leave-one-out cross-validation information criterion) and model weights. LOOIC was used to compare models as it is a more robust information criterion than WAIC (widely applicable information criterion) in cases of weak predictors and influential observations (Vehtari and others 2017).
Results
Stable Isotope Values of Fish
A total of 523 fish (L. carponotatus n = 292, L. fulviflamma n = 85, and L. punctulatus n = 146) ranging from 21 – 317 mm in length (Figure 2a; Table S1) were collected across 13 sites in the Dampier Archipelago. δ13C values of fish ranged from -16.7 to -9.2 ‰ while δ15N values ranged from 7.6 to 11.8 ‰ (Figure 2c; Table S1; Table S5). δ15N increased with fish length for all species, indicating a shift towards higher trophic level prey throughout ontogeny (Figure 2c; Table S3). Lutjanus fulviflamma adults collected in coral habitats were both larger and more enriched in 15N than those from macroalgae and mangrove habitats (post hoc p < 0.001; Table S3). Juvenile L. fulviflamma from macroalgal habitats were more 15N enriched than juveniles collected in mangrove habitats (post hoc p < 0.001; Figure 2c; Table S3; Table S5). Variation in the δ15N − length trends for L. carponotatus indicated there were differences in the trophic ecology of this species among habitats, and δ15N values were generally lower for fish collected in macroalgal than coral habitats (Figure 2c; Table S3).
Among all species and habitats, L. fulviflamma from mangrove and coral habitats exhibited the widest range of δ13C values (from -14.6 to -9.2 ‰ and from -15.3 to -10.1 ‰, respectively), with mangrove L. fulviflamma exhibiting the highest values (Figure 2b). In contrast, the δ13C values of L. fulviflamma collected in macroalgal habitats were more consistent and were depleted in 13C (from -14.3 to -11.4 ‰) relative to those collected in mangrove and coral habitats, which did not differ from each other (Figure 2b; Table S3). There was no relationship between length and δ13C values for L. fulviflamma nor L. punctulatus. Lutjanus carponotatus became increasingly enriched in 13C as fish size increased in both macroalgal and coral habitats; however, this relationship was weaker in fish from coral habitats (Figure 2; Table S3).
Stable Isotope Values of Basal Sources
Primary producers exhibited lower δ15N values than fish (ranging from -2.7 to 6.8 ‰) and a broad range of δ13C values (from -30.5 to -6.1 ‰; Figure 3, Table S1). Live and detrital mangrove leaves were the most depleted in 13C of the primary producers, followed by macroalgae and sediment POM, while seagrass and epiphytes were the most enriched (Figure 3). Bivalves collected in mangrove and coral habitats were indistinguishable based on either δ13C or δ15N, implying isotopically similar SPOM composition across both habitats (Table S4). The isotopic value of SPOM* was indistinguishable from sediment POM and was close to that of macroalgae (Figure 3; Table S4).
Average (± standard deviation; SD) δ13C and δ15N values of basal sources, juvenile (Age-0) fish, and adult (Age-1 +) fish samples collected from coral (pink), macroalgae (orange) and mangrove (green) habitats in the Dampier Archipelago Western Australia. Recently settled fish (fork length < 30 mm) were excluded. Colours indicate sample type for sources and collection habitat for fish. MD mangrove detritus, M live mangrove, SP* suspended particulate organic matter proxy (bivalve δ13C and δ15N values corrected for isotopic discrimination), SD sediment particulate organic matter, MA macroalgae, E epiphytes, SG seagrass, LC Lutjanus carponotatus, LF Lutjanus fulviflamma, LP Lethrinus punctulatus.
Relative Contributions of Basal Sources to Fish
Isotopic mixing models indicated that SPOM* contributed substantially to the diets of both adults and juveniles of the three studied fish species across coral, macroalgae, and mangrove habitats (Figure 4; Table 1). Assimilation of macroalgal and seagrass carbon was more variable among species, habitats, and life history stages, while reliance on mangrove carbon was consistently limited (Figure 4; Table 1). SPOM* was the dominant source of nutrients assimilated by both juvenile (mean ± SD; 45 ± 11%) and adult L. carponotatus (61 ± 15% for adults) in coral habitats followed by mangrove (21 ± 6% for juveniles and 18 ± 4% for adults; Figure 4; Table 1; Table S6). Variations in source contributions were more pronounced among different habitats than across ontogeny for L. carponotatus, with juveniles from macroalgal habitats exhibiting comparable reliance on carbon derived from mangroves (21 ± 6%), but higher dependence on macroalgae (34 ± 12%) and SPOM* (32 ± 16%) than their conspecifics from coral habitats (Figure 4; Table 1; Table S6). Both juvenile and adult L. carponotatus from coral habitats exhibited similar patterns of source assimilation across sites (Table S6). In contrast, juvenile L. carponotatus from macroalgal habitats demonstrated more variable source reliance among sites (e.g., macroalgal assimilation ranging from 17 ± 12% to 56 ± 11% and SPOM* assimilation ranging from 10 ± 6% to 45 ± 21%; Table S6).
Posterior distributions from the isotopic mixing models showing the relative proportional contribution of sources to Lutjanus carponotatus and Lutjanus fulviflamma, and Lethrinus punctulatus a adults in coral habitats and juveniles in b coral, c macroalgal, and d mangrove habitats. Sources included in the model included a proxy for suspended particulate organic matter (SPOM*; bivalve isotopic values corrected for isotopic discrimination, macroalgae, mangrove, and seagrass. Recently settled fish (fork length < 30 mm) were excluded.
SPOM* was also the main basal source assimilated by L. fulviflamma across all three habitats (average contribution of 39 ± 13%, 49 ± 14%, and 60 ± 17%), followed by seagrass (30 ± 10%, 24 ± 9%, and 20 ± 8% in coral, macroalgal and mangrove habitats, respectively; Figure 4; Table 1; Table S6). Lutjanus fulviflamma assimilated limited macroalgae (10 ± 10%, 10 ± 10%, and 8 ± 13%) or mangrove (21 ± 7%, 17 ± 6%, and 11 ± 5% in coral, macroalgal and mangrove habitats, respectively) material in any habitat (Figure 4; Table 1; Table S6). Basal source contributions differed more clearly among habitats than across ontogeny for L. fulviflamma, with juveniles exhibiting considerable variation in source assimilation between macroalgae and mangrove habitats (Figure 4; Table 1).
Similar to juvenile L. carponotatus from macroalgal habitats, juvenile L. punctulatus assimilated comparable proportions of nutrients derived from SPOM* (34 ± 9%), seagrass (29 ± 8%), and macroalgae (25 ± 1%; Figure 4; Table 1; Table S6). However, L. punctulatus exhibited considerable spatial variation in assimilation of SPOM* and macroalgae among sites, with reliance on SPOM* varying inversely with macroalgae and ranging from 7 ± 5% to 61 ± 1%, (Table S6).
Drivers of Spatial Variation in Relative Contributions of Basal Sources to Fish
The considerable spatial variation in source assimilation exhibited by juvenile L. carponotatus and L. punctulatus from macroalgal habitats (Table S6) did not appear to be linked to measures of local habitat quality, seascape configuration, or hydrodynamic conditions. For both species, the best model was the null (random effect of site only) as indicated by the lowest LOOic value and highest model weight (Table 2).
Discussion
Using dual stable isotopes and Bayesian mixing models we show that SPOM (as indicated by a bivalve-derived proxy; SPOM*) was a key basal source underpinning the secondary production of three benthic mesopredatory fish species in the Dampier Archipelago, Western Australia. SPOM was consistently important for both adult and juvenile fishes across coral, macroalgal, and mangrove habitats. While juvenile fish collected from macroalgal habitats also incorporated carbon from benthic macrophytes (macroalgae/seagrass), mixing models demonstrated that SPOM assimilation remained substantial. Reliance on benthic macrophytes decreased throughout ontogeny for L. carponotatus, while L. fulviflamma continued to assimilate a combination of SPOM and seagrass into adulthood. Little mangrove carbon was assimilated by fish of either life history stage (including those collected from mangrove habitats), aligning with a growing body of evidence demonstrating that fringing mangroves primarily provide refuge rather than direct energetic resources to fish (Nagelkerken and others 2008a; Vaslet and others 2012; Igulu and others 2013; Abrantes and others 2015). Spatial variation in proportional source assimilation by fish was unrelated to differences in local habitat condition (cover of macroalgae), hydrodynamic conditions (SHW and WRT), or seascape configuration (PA macroalgae) at the scale (sites spanning ~30 km) of this study. Although mixing models suggested a modest degree of trophic connectivity among benthic habitats, cross-habitat trophic subsidies from macrophytes (e.g., mangroves and macroalgae) typically represented < 30% of resources assimilated by predatory fish in this system. The ubiquitous significance of SPOM-based trophic pathways evident in this system may be a function of the relatively high nutritional value of microphotoautotrophs compared to other primary producers (Mann 1988; Atkinson and Falter 2020) and the frequent resuspension and mixing of POM driven by strong tidal currents (Abrantes and others 2015). While similar reliance on SPOM-based food chains by predatory fish (Wyatt and others 2012; McMahon and others 2016; Skinner and others 2021) and invertebrates (Kang and others 2003; Bearham and others 2023) has been observed elsewhere, it has rarely been reported for juvenile fish (but see Wells and others 2008). Overall, our results demonstrate that SPOM is the primary source underpinning secondary productivity of predatory fish in this macrotidal system, regardless of seascape configuration or prevailing hydrodynamic conditions.
SPOM was an important energetic resource for both adults and juveniles of all three fish species, regardless of collection habitat. As SPOM is composed of a variable mixture of plankton, detritus, bacteria and exudates (Volkman and Tanoue 2002; Briand and others 2015; Bearham and others 2023), determining the ultimate source of primary production fuelling SPOM-based food chains can be challenging. In macrotidal systems, such as the Dampier Archipelago, resuspended benthic microalgae can also make a significant contribution to the organic carbon in the water column (Kang and others 2003; Hernández Fariñas and others 2017). Indeed, the overlapping isotopic values of SPOM* and sediment POM observed here likely indicate a high degree of bentho-pelagic coupling in this system (Hernández Fariñas and others 2017). The average δ13C value of SPOM* in this study (-17.5 ± 0.6 ‰) fell between that of macroalgae (-15.7 ± 1.9 ‰) and the value for phytoplankton reported elsewhere in north-western Australia (-24 ± 0.1 ‰; Wyatt and others 2013). Although we did not observe or collect benthic microalgae, their isotopic value is likely similar to that of SPOM* as they are, on average, –5 ‰ more enriched relative to phytoplankton in marine systems (Doi and others 2010). The isotopic composition of SPOM* observed here is therefore indicative of macro- and microalgal material. Particulate material in water samples collected throughout the Archipelago had C:N ratios (average of 5.2 ± 0.9; Table S8; Text S7) close to those typical of phytoplankton and benthic microalgae (– 6.6; that is, the Redfield Ratio; Atkinson and Smith, 1983) and far lower than those observed for macrophytes in this study (average of 75.1 ± 43.4, 47.2 ± 16.6, and 19.2 ± 5.3 for mangroves, macroalgae, and seagrass, respectively; Table S1). Collectively, these data suggest that microalgae constitute the bulk of the SPOM, and thus provide the majority of energetic resources to fish, while macrophytes primarily serve as refugia. It should however be noted that a dual-tracer approach was used in this study which limited the number of sources that could be included in mixing models (Phillips and others 2014). Consequently, several sources of primary production (e.g., turf algae and other macroalgal taxa) and microbial organisms associated with decomposition were not considered. Future work would benefit from more advanced isotopic approaches (e.g., compound-specific SIA) or the use of additional tracers (e.g., sulphur; δ34S) to confirm these findings and to clarify the importance of pelagic versus benthic microalgal production (McMahon and others 2011; Connolly and others 2014).
Our results add to the growing body of evidence demonstrating significant regional variation in the proportional contribution of primary producers to the diets of predatory fish. While several studies have reported similar reliance on microalgal resources by demersal fish (Yeager and Layman 2011; McMahon and others 2016; Skinner and others 2019; Zgliczynski and others 2019), others have found that seagrass (Connolly and others 2005; Davis and others 2014; Vaslet and others 2015; Le and others 2018) or macroalgal (Kieckbusch and others 2004; Wells and others 2008; Olson and others 2019) productivity underpinned food chains. The importance of microalgal productivity to coastal food webs observed in this study and others could be a consequence of the greater nutritional value (more N relative to C) of microalgae compared to macrophytes (Mann 1988; Atkinson and Falter 2020). Although the focal fish species in this study are generalist carnivores and do not consume microalgae directly, their diets include a range of pelagic and demersal invertebrate (predominantly crustaceans) and piscine (e.g., cryptobenthic teleosts) prey (Connell 1998; Kamukuru and Mgaya 2004; Kulbicki and others 2005; Farmer and Wilson 2011) that may selectively feed on organic matter of higher nutritional value and palatability (Cruz-Rivera and Hay 2000; Park and others 2013; Kang and others 2015; Brandl and others 2018). Alternatively, it has been suggested that the contribution of primary producers to food webs in coastal seascapes depends on their relative availability (Abrantes and others 2015). Microalgae have lower light requirements compared to benthic macrophytes (Herbert 1999; Lee and others 2007), which may lead to higher net community productivity of both pelagic and benthic microalgae, potentially explaining the importance of the SPOM-based trophic pathway in this turbid, macrotidal system.
Although reliance on SPOM remained consistently high, mixing models indicated that proportional contributions of benthic macrophytes to fish differed among habitats. However, this variation appeared to predominantly reflect increased reliance on local sources, rather than trophic subsidies provided by adjacent macrophyte habitats. For example, fish inhabiting macroalgal habitats assimilated a greater proportion of carbon derived from macroalgae than those from coral and mangrove habitats. Despite observing transport of detrital mangrove leaves and macroalgal wrack into adjacent habitats by currents, we found little evidence that this transported material was a significant energetic resource for fish in recipient habitats. These results contrast with a number of past studies conducted in coastal seascapes (e.g., Connolly and others 2005; Davis and others 2014; Vaslet and others 2015; Le and others 2019; Cartraud and others 2021). However, it should be noted that we cannot exclude the possibility of cross-habitat trophic subsidies in the form of benthic microalgae, given that sediment POM was isotopically indistinguishable from SPOM. On a similar note, our mixing models revealed that assimilation of seagrass was higher than anticipated, considering its relative sparsity in the Archipelago (Moustaka and others 2024). As seagrass frequently occurs in small patches, interspersed among other habitats, we cannot determine if this represents an allochthonous carbon source, or instead, an unquantified autochthonous source within coral, macroalgal, and mangrove habitats.
We found considerable variation in source assimilation throughout ontogeny for L. carponotatus. However, as both juvenile and adult L. carponotatus from coral habitats exhibited lower reliance on macroalgal production than juveniles from macroalgae habitats, this variation is likely a function of residence habitat, rather than life history stage. Comparable patterns of decreased reliance on planktonic resources in back-reef habitats have been observed elsewhere in Western Australia (Wyatt and others 2012) and in the Red Sea (McMahon and others 2016). This variation in basal source assimilation among habitats may simply reflect the greater availability of macroalgal (or benthic microalgal) and seagrass carbon in macroalgal habitats, both in terms of benthic cover and SPOM contribution (Litvin and Weinstein 2003; Abrantes and others 2015; Claudino and others 2015; Gruber and others 2018), alongside a concurrent decrease in availability of planktonic production to food webs (Wyatt and others 2012; Morais and Bellwood 2019). Alternatively, as the studied fish species are generalist carnivores (Farmer and Wilson 2011), these patterns may be a product of adaptive foraging on the different types of prey available in each habitat (Yeager and others 2014; Hempson and others 2017; Martínez-Juárez and others 2024). Indeed, δ15N values increased with length for all three fish species, indicating a gradual shift to feeding on higher trophic level prey throughout ontogeny, a pattern commonly observed in many carnivorous species (e.g., Cocheret de la Morinière and others 2003; Vaslet and others 2015) as a consequence of increasing gape size (Dunic and Baum 2017). Macroalgal habitats typically support greater diversity and productivity of epifaunal invertebrates (an important food source for juvenile fishes) compared to coral reefs, seagrass meadows, and mangroves, which may account for the higher reliance on benthic carbon exhibited by fish in these habitats (Cocheret de la Morinière and others 2003; Cowles and others 2009; Tano and others 2016; Chen and others 2021; Chen 2022). The composition of mobile invertebrate assemblages also varies considerably among coastal habitats (Nagelkerken and Faunce 2008; Stella and others 2010; Chen and others 2020). Given that mobile invertebrates serve as an important conduit for transferring benthic production to higher trophic levels, differences in the composition of invertebrate assemblages (and their associated dietary strategies) among habitats are likely to influence the extent to which carbon sources contribute to the diets of predatory fish.
Lutjanus carponotatus collected in coral habitats exhibited relatively consistent reliance on SPOM across all sites. In contrast, assimilation of SPOM and macroalgae by juvenile L. carponotatus and L. punctulatus from macroalgal habitats varied significantly among sites, but was unrelated to local habitat quality, seascape configuration, or hydrodynamic conditions. These findings diverge from other studies that reported differences in proportional contributions of primary producers to fish diets as a function of shelf position (Wyatt and others 2012; McMahon and others 2016), hydrodynamics (Bergamino and others 2011), and seascape configuration (Davis and others 2014; Gorman and others 2023). The lower spatial variability in source assimilation demonstrated by fish from coral habitats, compared to those in macroalgal habitats, may reflect differences in the relative availability of high-quality (i.e., microalgae) nutritional resources among habitats. Intense feeding on SPOM resources by coral reef communities of different sizes, combined with variation in hydrodynamic conditions, may result in considerably lower pelagic resource availability in back-reef habitats at some sites compared to others (Hamner and others 2007; Bergamino and others 2011; Skinner and others 2022). Exploring how composition of SPOM changes among habitats within a site, and comparing the magnitude of such differences among sites, may provide insights into the mechanisms underlying these patterns.
This study sheds light on the sources of primary production underpinning the productivity of three commercially and recreationally valuable fish species in north-western Australia. Our findings emphasise the importance of SPOM-based food chains for both juvenile and adult fishes across various habitats within a coastal seascape. Whilst the contribution of SPOM to fish diets varied spatially, these differences did not relate to local habitat quality, seascape configuration, water residence time, or significant wave height. Our mixing models suggest that assimilation of cross-habitat trophic subsidies derived from mangroves and macroalgae by mesopredatory fish is relatively limited in the Dampier Archipelago. Instead, our findings indicate that microalgal production plays a key role in supporting the secondary production of predatory fish species in macrotidal environments.
Data Availability
Data supporting this research have been archived in the University of Western Australia data repository (https://doi.org/https://doi.org/10.26182/k2a5-1a71)
References
Abrantes KG, Barnett A, Baker R, Sheaves M. 2015. Habitat-specific food webs and trophic interactions supporting coastal-dependent fishery species: an Australian case study. Reviews in Fish Biology and Fisheries 25:337–63. https://doi.org/10.1007/s11160-015-9385-y
Atkinson MJ, Falter JL. 2020. Coral reefs. In: Black K, Shimmield G, editors. Biogeochemistry of Marine Systems. Boca Raton, Florida: CRC Press. pp 40–64. https://doi.org/10.1201/9780367812423-2
Atkinson M, Smith S. 1983. C:N:P ratios of benthic marine plants. Limnology and Oceanography 28:568–74. https://doi.org/10.4319/lo.1983.28.3.0568
Bearham D, Gorman D, McLaughlin MJ, Liu D, Keesing J. 2023. The importance of blue carbon as a food source to oysters (Saccostrea cucullata) inside a tropical pristine Australian estuary. Estuarine, Coastal and Shelf Science 283:108269. https://doi.org/10.1016/j.ecss.2023.108269.
Beck MW, Heck KLH, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MP. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633–41. https://doi.org/10.1641/0006-3568%282001%29051%5B0633%3ATICAMO%5D2.0.CO%3B2
Bergamino L, Lercari D, Defeo O. 2011. Food web structure of sandy beaches: Temporal and spatial variation using stable isotope analysis. Estuarine, Coastal and Shelf Science 91:536–543. https://doi.org/10.1016/j.ecss.2010.12.007.
Bouillon S, Connolly RM, Lee SY. 2008. Organic matter exchange and cycling in mangrove ecosystems: Recent insights from stable isotope studies. Journal of Sea Research 59:44–58. https://linkinghub.elsevier.com/retrieve/pii/S1385110107000469
Brandl SJ, Goatley CHR, Bellwood DR, Tornabene L. 2018. The hidden half: ecology and evolution of cryptobenthic fishes on coral reefs. Biological Reviews 93:1846–1873. https://doi.org/10.1111/brv.12423
Brauns M, Boëchat IG, de Carvalho APC, Graeber D, Gücker B, Mehner T, von Schiller D. 2018. Consumer-resource stoichiometry as a predictor of trophic discrimination (Δ13C, Δ15N) in aquatic invertebrates. Freshwater Biology 63:1240–1249.
Brewer DT, Blaber SJM, Salini JP, Farmer MJ. 1995. Feeding ecology of predatory fishes from Groote eylandt in the Gulf of Carpentaria, Australia, with special reference to predation on penaeid prawns. Estuarine, Coastal and Shelf Science 40:577–600. https://linkinghub.elsevier.com/retrieve/pii/S027277148570039X
Briand MJ, Bonnet X, Goiran C, Guillou G, Letourneur Y. 2015. Major sources of organic matter in a complex coral reef lagoon: Identification from isotopic signatures (δ13C and δ15N). PLoS ONE 10:1–21.
Cartraud AE, Lavery PS, Rae CM, Hyndes GA. 2021. Pathways to spatial subsidies by kelp in seagrass meadows. Estuaries and Coasts 44:468–480.
Caut S, Angulo E, Courchamp F. 2009. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46:443–453.
Chen YY, Cooper P, Fulton CJ. 2020. Sargassum epifaunal communities vary with canopy size, predator biomass and seascape setting within a fringing coral reef ecosystem. Marine Ecology Progress Series 640:17–30.
Chen YY, Cooper P, Fulton CJ, Fox RJ. 2021. Quantifying epifaunal secondary production within tropical macroalgal meadows: Seasonality and sensitivity to canopy structure. Limnology and Oceanography 66:4267–4284.
Chen YY. 2022. The nature and significance of ‘macroalgae - epifauna - invertivorous fish’ trophic links within a macroalgal-dominated reef ecosystem.
Christianen MJA, Middelburg JJ, Holthuijsen SJ, Jouta J, Compton TJ, van der Heide T, Piersma T, Sinninghe Damsté JS, van der Veer HW, Schouten S, Olff H. 2017. Benthic primary producers are key to sustain the Wadden Sea food web: stable carbon isotope analysis at landscape scale. Ecology 98:1498–512. https://doi.org/10.1002/ecy.1837
Claudino MC, Pessanha ALM, Araújo FG, Garcia AM. 2015. Trophic connectivity and basal food sources sustaining tropical aquatic consumers along a mangrove to ocean gradient. Estuarine, Coastal and Shelf Science 167:45–55.
Cocheret de la Morinière E, Pollux BJA, Nagelkerken I, Hemminga MA, Huiskes AHL, Van der Velde G. 2003. Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: stable isotopes and gut-content analysis. Marine Ecology Progress Series 246:279–289.
Connell SD. 1998. Patterns of pisciviory by resident predatory reef fish at One Tree Reef, Great Barrier Reef. Marine and Freshwater Research 49:25. http://www.publish.csiro.au/?paper=MF97034
Connolly RM, Hindell JS, Gorman D. 2005. Seagrass and epiphytic algae support nutrition of a fisheries species, Sillago schomburgkii, in adjacent intertidal habitats. Marine Ecology Progress Series 286:69–79.
Connolly RM, Melville MA, Guest AJ, Oakes JM. 2014. Sulfur stable isotopes separate producers in marine food-web analysis. Oecologia 138:161–167.
Cowles A, Hewitt JE, Taylor RB. 2009. Density, biomass and productivity of small mobile invertebrates in a wide range of coastal habitats. Marine Ecology Progress Series 384:175–185.
Cruz-Rivera E, Hay ME. 2000. Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology 81:201–219.
Cummings DO, Booth DJ, Lee RW, Simpson SJ, Pile AJ. 2010. Ontogenetic diet shifts in the reef fish Pseudanthias rubrizonatus from isolated populations on the North-West Shelf of Australia. Marine Ecology Progress Series 419:211–222.
Davis JP, Pitt KA, Fry B, Olds AD, Connolly RM. 2014. Seascape-scale trophic links for fish on inshore coral reefs. Coral Reefs 33:897–907.
Gaughan DJ, Molony BW, Santoro K, editors. 2019. Status Reports of the Fisheries and Aquatic Resources of Western Australia 2017/18: The State of the Fisheries. Hillarys, Western Australia: Department of Primary Industries and Regional Development
Doi H, Kikuchi E, Shikano S, Takagi S. 2010. Differences in nitrogen and carbon stable isotopes between planktonic and benthic microalgae. Limnology 11:185–192.
Dunic JC, Baum JK. 2017. Size structuring and allometric scaling relationships in coral reef fishes. Journal of Animal Ecology 86:577–589.
Evans RD, Wilson SK, Field SN, Moore JAY. 2014. Importance of macroalgal fields as coral reef fish nursery habitat in north-west Australia. Marine Biology 161:599–607.
Farmer BM, Wilson SK. 2011. Diet of finfish targeted by fishers in North West Australia and the implications for trophic cascades. Environmental Biology of Fishes 91:71–85. https://doi.org/10.1007/s10641-010-9761-3
Fraser KM, Stuart-Smith RD, Ling SD, Heather FJ, Edgar GJ. 2020. Taxonomic composition of mobile epifaunal invertebrate assemblages on diverse benthic microhabitats from temperate to tropical reefs. Marine Ecology Progress Series 640:31–43.
Fry B, Sherr EB. 1989. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. In: Rundel P, Ehleringer J, Nagy K, editors. Stable Isotopes in Ecological Research. Ecological Studies. Vol. 68. Springer New York. https://doi.org/10.1007/978-1-4612-3498-2_12
Fry B, Arnold C. 1982. Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 54:200–204.
Fry B, Ewel KC. 2003. Using stable isotopes in mangrove fisheries research - A review and outlook. Isotopes in Environmental and Health Studies 39:191–196.
Fry B. 2006. Stable Isotope Ecology. New York, NY: Springer New York https://doi.org/10.1007/0-387-33745-8
Fukumori K, Oi M, Doi H, Takahashi D, Okuda N, Miller TW, Kuwae M, Miyasaka H, Genkai-Kato M, Koizumi Y, Omori K, Takeoka H. 2008. Bivalve tissue as a carbon and nitrogen isotope baseline indicator in coastal ecosystems. Estuarine, Coastal and Shelf Science 79:45–50.
Fulton CJ, Berkström C, Wilson SK, Abesamis RA, Bradley M, Åkerlund C, Barrett LT, Bucol AA, Chacin DH, Chong-Seng KM, Coker DJ, Depczynski M, Eggertsen L, Eggertsen M, Ellis D, Evans RD, Graham NAJJ, Hoey AS, Holmes TH, Kulbicki M, Leung PTYY, Lam PKSS, Lier J, Matis PA, Noble MM, Pérez-Matus A, Piggott C, Radford BT, Tano S, Tinkler P. 2020. Macroalgal meadow habitats support fish and fisheries in diverse tropical seascapes. Fish and Fisheries 21:700–17. https://doi.org/10.1111/faf.12455
Gorman D, Beale DJ, Crosswell J, Stephenson SA, Shah RM, Hillyer KE, Steven ADL. 2023. Multiple-biomarkers show the importance of blue carbon to commercially important fishery species. Science of the Total Environment 881:163162. https://doi.org/10.1016/j.scitotenv.2023.163162.
Grandcourt EM, Al Abdessalaam TZ, Francis F. 2006. Age, growth, mortality and reproduction of the blackspot snapper, Lutjanus fulviflamma (Forsskål, 1775), in the southern Arabian Gulf. Fisheries Research 78:203–210.
Green AL, Maypa AP, Almany GR, Rhodes KL, Weeks R, Abesamis RA, Gleason MG, Mumby PJ, White AT. 2015. Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biological Reviews 90:1215–1247.
Gruber RK, Lowe RJ, Falter JL. 2018. Benthic uptake of phytoplankton and ocean-reef exchange of particulate nutrients on a tide-dominated reef. Limnology and Oceanography 63:1545–1561.
Hamner WM, Colin PL, Hamner pp. 2007. Export-import dynamics of zooplankton on a coral reef in Palau. Marine Ecology Progress Series 334:83–92.
Hempson TN, Graham NAJ, MacNeil MA, Williamson DH, Jones GP, Almany GR. 2017. Coral reef mesopredators switch prey, shortening food chains, in response to habitat degradation. Ecology and Evolution 7:2626–2635.
Herbert RA. 1999. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiology Reviews 23:563–590.
Hernández Fariñas T, Ribeiro L, Soudant D, Belin C, Bacher C, Lampert L, Barillé L. 2017. Contribution of benthic microalgae to the temporal variation in phytoplankton assemblages in a macrotidal system. Journal of Phycology 53:1020–1034.
Herzka SZ. 2005. Assessing connectivity of estuarine fishes based on stable isotope ratio analysis. Estuarine, Coastal and Shelf Science 64:58–69.
Herzka SZ, Holt SA, Holt GJ. 2001. Documenting the settlement history of individual fish larvae using stable isotope ratios: Model development and validation. Journal of Experimental Marine Biology and Ecology 265:49–74.
Horinouchi M. 2007. Review of the effects of within-patch scale structural complexity on seagrass fishes. Journal of Experimental Marine Biology and Ecology 350:111–129.
Hyndes GA, Hanson CE, Vanderklift MA. 2013. The magnitude of spatial and temporal variation in δ15N and δ13C differs between taxonomic groups: Implications for food web studies. Estuarine, Coastal and Shelf Science 119:176–187. https://doi.org/10.1016/j.ecss.2012.12.015.
Hyndes GA, Nagelkerken I, Mcleod RJ, Connolly RM, Lavery PS, Vanderklift MA. 2014. Mechanisms and ecological role of carbon transfer within coastal seascapes. Biological Reviews 89:232–254.
Igulu MM, Nagelkerken I, van der Velde G, Mgaya YD. 2013. Mangrove fish production is largely fuelled by external food sources: a stable isotope analysis of fishes at the individual, species, and community levels from across the globe. Ecosystems 16:1336–1352.
Kamukuru AT, Mgaya YD. 2004. The food and feeding habits of blackspot snapper, Lutjanus fulviflamma (Pisces: Lutjanidae) in shallow waters of Mafia Island, Tanzania. African Journal of Ecology 42:49–58. https://doi.org/10.1111/j.0141-6707.2004.00487.x
Kang CK, Kim JB, Lee KS, Bin Kim J, Lee PY, Hong JS. 2003. Trophic importance of benthic microalgae to macrozoobenthos in coastal bay systems in Korea: Dual stable C and N isotope analyses. Marine Ecology Progress Series 259:79–92.
Kang CK, Park HJ, Choy EJ, Choi KS, Hwang K, Bin Kim J. 2015. Linking intertidal and subtidal food webs: Consumer-mediated transport of intertidal benthic microalgal carbon. PLoS ONE 10:1–26.
Kieckbusch DK, Koch MS, Serafy JE, Anderson WT. 2004. Trophic linkages among primary producers and consumers in fringing mangroves of subtropical lagoons. Bulletin of Marine Science 74:271–285.
Kiljunen M, Grey J, Sinisalo T, Harrod C, Immonen H, Jones RI. 2006. A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. Journal of Applied Ecology 43:1213–22. https://doi.org/10.1111/j.1365-2664.2006.01224.x
Kimirei IA, Nagelkerken I, Mgaya YD, Huijbers CM. 2013. The mangrove nursery paradigm revisited: otolith stable isotopes support nursery-to-reef movements by Indo-Pacific fishes. Goldstien SJ, editor. PLoS ONE 8:e66320. https://doi.org/10.1371/journal.pone.0066320
Kritzer JP. 2004. Sex-specific growth and mortality, spawning season, and female maturation of the stripey bass (Lutjanus carponotatus) on the Great Barrier Reef. Fishery Bulletin 102:94–107.
Kulbicki M, Bozec Y, Labrosse P, Letourneur Y, Mou-Tham G, Wantiez L. 2005. Diet composition of carnivorous fishes from coral reef lagoons of New Caledonia. Aquatic Living Resources 18:231–50. https://doi.org/10.1051/alr:2005029
Le DQ, Tanaka K, Hii YS, Sano Y, Nanjo K, Shirai K, Quang D, Tanaka K, Siang Y, Sano Y, Nanjo K, Shirai K. 2018. Importance of seagrass-mangrove continuum as feeding grounds for juvenile pink ear emperor Lethrinus lentjan in Setiu Lagoon, Malaysia: Stable isotope approach. Journal of Sea Research 135:1–10. https://doi.org/10.1016/j.seares.2018.02.004.
Le DQ, Fui SY, Tanaka K, Suratman S, Sano Y, Shirai K. 2019. Feeding habitats of juvenile reef fishes in a tropical mangrove-seagrass continuum along a Malaysian shallow-water coastal lagoon. Bulletin of Marine Science 96:1–18.
Lee KS, Park SR, Kim YK. 2007. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. Journal of Experimental Marine Biology and Ecology 350:144–175.
Litvin SY, Weinstein MP. 2003. Life history strategies of estuarine nekton: the role of marsh macrophytes, benthic microalgae, and phytoplankton in the trophic spectrum. Estuaries 26:552–62. https://www.jstor.org/stable/1353368%0A=
Loneragan NR, Bunn SE, Kellaway DM. 1997. Are mangroves and seagrasses sources of organic carbon for penaeid prawns in a tropical Australian estuary? A multiple stable-isotope study. Marine Biology 130:289–300.
Ludovisi A, Goretti E, Pallottini M, Lucentini L, Pizzirani C, Vizzini S, Mancinelli G. 2022. Stable isotope analysis reveals trophic segregation between the invasive zebra mussel Dreissena polymorpha and the native duck mussel Anodonta anatina in Lake Trasimeno (Italy). Hydrobiologia 849:2091–2108. https://doi.org/10.1007/s10750-022-04846-4.
Mann KH. 1988. Production and use of detritus in various freshwater, estuarine, and coastal marine ecosystems. Limnology and Oceanography 33:910–930.
Martínez-Juárez LF, Schmitter-Soto JJ, Mercado-Silva N, Cabanillas-Terán N. 2024. Trophic position and prey preferences of snappers (Teleostei: Lutjanidae) in a connected bay-to-reef Caribbean system. Estuarine, Coastal and Shelf Science 299:108688. https://doi.org/10.1016/j.ijbiomac.2023.125944.
McMahon KW, Fogel ML, Johnson BJ, Houghton LA, Thorrold SR. 2011. A new method to reconstruct fish diet and movement patterns from δ 13C values in otolith amino acids. Canadian Journal of Fisheries and Aquatic Sciences 68:1330–1340.
McMahon KW, Thorrold SR, Houghton LA, Berumen ML. 2016. Tracing carbon flow through coral reef food webs using a compound-specific stable isotope approach. Oecologia 180:809–821.
Minagawa M, Wada E. 1984. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochimica Et Cosmochimica Acta 48:1135–1140.
Morais RA, Bellwood DR. 2019. Pelagic subsidies underpin fish productivity on a degraded coral reef. Current Biology 29:1521–1527. https://doi.org/10.1016/j.cub.2019.03.044.
Morrison PF. 2004. A general description of the subtidal habitats of the Dampier Archipelago, Western Australia. Records of the Western Australian Museum, Supplement 66:51.
Moustaka M, Evans RD, Kendrick GA, Hyndes GA, Cuttler MVW, Bassett TJ, O’Leary MJ, Wilson SK. 2024. Local habitat composition and complexity outweigh seascape effects on fish distributions across a tropical seascape. Landscape Ecology 39:28. https://doi.org/10.1007/s10980-024-01814-2.
Nagelkerken I, Faunce CH. 2008. What makes mangroves attractive to fish? Use of artificial units to test the influence of water depth, cross-shelf location, and presence of root structure. Estuarine, Coastal and Shelf Science 79:559–565.
Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM, Sasekumar A, Somerfield PJ. 2008a. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquatic Botany 89:155–185.
Nagelkerken I, Bothwell J, Nemeth RS, Pitt JM, Van Der Velde G. 2008b. Interlinkage between Caribbean coral reefs and seagrass beds through feeding migrations by grunts (Haemulidae) depends on habitat accessibility. Marine Ecology Progress Series 368:155–164.
Nash KL, Welsh JQ, Graham NAJ, Bellwood DR. 2015. Home-range allometry in coral reef fishes: comparison to other vertebrates, methodological issues and management implications. Oecologia 177:73–83.
Newman S, Wakefield C, Skepper C, Boddington D, Jones R, Smith E. 2018. North coast demersal resource status report 2017. Perth, Western Australia
Newman S, Wakefield C, Skepper C, Boddington D, Blay N. 2019. North coast demersal resource status report 2019. (Gaughan D, Santoro K, editors.). Perth, Western Australia: The Department of Primary Industries and Regional Development
Nguyen BN, Shen EW, Seemann J, Correa AMS, O’Donnell JL, Altieri AH, Knowlton N, Crandall KA, Egan SP, McMillan WO, Leray M. 2020. Environmental DNA survey captures patterns of fish and invertebrate diversity across a tropical seascape. Scientific Reports 10:1–14.
Olds AD, Connolly RM, Pitt KA, Pittman SJ, Maxwell PS, Huijbers CM, Moore BR, Albert S, Rissik D, Babcock RC, Schlacher TA. 2016. Quantifying the conservation value of seascape connectivity: A global synthesis. Global Ecology and Biogeography 25:3–15.
Olin JA, Rush SA, MacNeil MA, Fisk AT. 2012. Isotopic ratios reveal mixed seasonal variation among fishes from two subtropical estuarine systems. Estuaries and Coasts 35:811–820.
Olson AM, Hessing-Lewis M, Haggarty D, Juanes F. 2019. Nearshore seascape connectivity enhances seagrass meadow nursery function. Ecological Applications 29:1–14.
Park HJ, Choy EJ, Lee KS, Kang CK. 2013. Trophic transfer between coastal habitats in a seagrass-dominated macrotidal embayment system as determined by stable isotope and fatty acid signatures. Marine and Freshwater Research 64:1169–1183.
Pearce AF, Buchan S, Chiffings T, D’Adamo N, Fandry CB, Fearns PRCS, Mills DJ, Phillips RC, Simpson C. 2003. A review of the oceanography of the Dampier Archipelago, Western Australia. In: The Marine flora and fauna of Dampier, Western Australia: proceedings of the Eleventh International Marine Biological Workshop. Perth, Western Australia: Western Australian Museum. pp 13–50.
Peterson BJ, Fry B. 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18:293–320.
Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ. 2014. Best practices for use of stable isotope mixing models in food-web studies. Canadian Journal of Zoology 92:823–835.
Piggott C. 2021. Patterns in the distribution and ecology of juvenile fishes in a macrotidal tropical ecosystem.
Pinheiro J, Bates D, R Core Team. 2023. nlme: Linear and Nonlinear Mixed Effects Models.
Polis GA, Anderson WB, Holt RD. 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28:289–316. https://doi.org/10.1146/annurev.ecolsys.28.1.289
Post DM. 2002. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83:703–718.
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG. 2007. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189.
R Core Team. 2022. R: A Language and Environment for Statistical Computing.
Richoux NB, Froneman PW. 2009. Plankton trophodynamics at the subtropical convergence, Southern Ocean. Journal of Plankton Research 31:1059–1073.
Rosa M, Ward JE, Shumway SE. 2018. Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: a review. Journal of Shellfish Research 37:727–746.
Salini J, Blaber S, Brewer D. 1994. Diets of trawled predatory fish of the Gulf of Carpentaria, Australia, with particular reference to predation on prawns. Marine and Freshwater Research 45:397.
Skinner MM, Martin AA, Moore BC. 2016. Is lipid correction necessary in the stable isotope analysis of fish tissues? Rapid Communications in Mass Spectrometry 30:881–889.
Skinner C, Newman SP, Mill AC, Newton J, Polunin NVC. 2019. Prevalence of pelagic dependence among coral reef predators across an atoll seascape. Harrod C, editor. Journal of Animal Ecology 88:1564–74. https://doi.org/10.1111/1365-2656.13056
Skinner C, Mill AC, Fox MD, Newman SP, Zhu Y, Kuhl A, Polunin NVCC. 2021. Offshore pelagic subsidies dominate carbon inputs to coral reef predators. Science Advances 7.
Skinner C, Pei Y, Morimoto N, Miyajima T, Wyatt ASJ. 2022. Stable isotopes elucidate body-size and seasonal fluctuations in the feeding strategies of planktivorous fishes across a semi-enclosed tropical embayment. Frontiers in Ecology and Evolution:1–18.
Stella JS, Jones GP, Pratchett MS. 2010. Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral Reefs 29:957–973.
Stock BC, Jackson AL, Ward EJ, Parnell AC, Phillips DL, Semmens BX. 2018. Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 2018.
Sweeting CJ, Polunin NVC, Jennings S. 2006. Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Communications in Mass Spectrometry 20:595–601. https://doi.org/10.1002/rcm.2347
Tano S, Eggertsen M, Wikström SA, Berkström C, Buriyo AS, Halling C. 2016. Tropical seaweed beds are important habitats for mobile invertebrate epifauna. Estuarine, Coastal and Shelf Science 183:1–12.
Taylor PD, Fahrig L, With KA. 2006. Landscape connectivity: a return to the basics. In: Crooks KR, Sanjayan ME, Eds. Connectivity Conservation, . Conservation Biology: Cambridge University Press. pp 29–43.
Taylor M, McLean D, Candland L, Piggott C, Wakefield C, Langlois T. 2017. Ontogenetic shifts in commercially important Lethrinus punctulatus a short-lived endemic species of the Pilbara, Western Australia. In: Babcock R, Donovan A, Collin S, C O, editors. Pilbara Marine Conservation Partnership – Final Report. Brisbane. pp 757–71.
Tebbett SB, Bellwood DR, Bassett T, Cuttler MVW, Moustaka M, Wilson SK, Yan HF, Evans RD. 2023. The limited role of herbivorous fishes and turf-based trophic pathways in the functioning of turbid coral reefs. Reviews in Fish Biology and Fisheries 34:439–460. https://doi.org/10.1007/s11160-023-09823-1.
Travaglione N, Evans R, Moustaka M, Cuttler M, Thomson DP, Tweedley J, Wilson S. 2023. Scleractinian corals rely on heterotrophy in highly turbid environments. Coral Reefs 42:997–1010. https://doi.org/10.1007/s00338-023-02407-2.
Zanden MJ Vander, Rasmussen JB. 2001. Variation in δ 15 N and δ 13 C trophic fractionation: Implications for aquatic food web studies. Limnology and Oceanography 46:2061–6. https://doi.org/10.4319/lo.2001.46.8.2061
Vanderklift MA, Ponsard S. 2003. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia 136:169–182.
Vaslet A, Phillips DL, France C, Feller IC, Baldwin CC. 2012. The relative importance of mangroves and seagrass beds as feeding areas for resident and transient fishes among different mangrove habitats in Florida and Belize: Evidence from dietary and stable-isotope analyses. Journal of Experimental Marine Biology and Ecology 434:81–93. https://doi.org/10.1016/j.jembe.2012.07.024.
Vaslet A, Phillips DL, France CAM, Feller IC, Baldwin CC. 2015. Trophic behaviour of juvenile reef fishes inhabiting interlinked mangrove-seagrass habitats in offshore mangrove islets. Journal of Fish Biology 87:256–273.
Vehtari A, Gelman A, Gabry J. 2017. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Statistics and Computing 27:1413–1432.
Volkman JK, Tanoue E. 2002. Chemical and biological studies of particulate organic matter in the ocean. Journal of Oceanography 58:265–279.
Wells RJD, Cowan JH, Fry B. 2008. Feeding ecology of red snapper Lutjanus campechanus in the northern Gulf of Mexico. Marine Ecology Progress Series 361:213–225.
Whitfield AK. 2017. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Reviews in Fish Biology and Fisheries 27:75–110.
Whitfield A. 1998. Biology and ecology of fishes in southern African estuaries. Grahamstown, South Africa: J.L.B. Smith Institute of Ichthyology
Wilson SK, Depczynski M, Fisher R, Holmes TH, O’Leary RA, Tinkler P. 2010. Habitat associations of juvenile fish at Ningaloo Reef, Western Australia: the importance of coral and algae. PLoS ONE 5:e15185. https://doi.org/10.1371/journal.pone.0015185
Wyatt ASJ, Waite AM, Humphries S. 2012. Stable isotope analysis reveals community-level variation in fish trophodynamics across a fringing coral reef. Coral Reefs 31:1029–44. https://doi.org/10.1007/s00338-012-0923-y
Wyatt ASJ, Lowe RJ, Humphries S, Waite AM. 2013. Particulate nutrient fluxes over a fringing coral reef: Source-sink dynamics inferred from carbon to nitrogen ratios and stable isotopes. Limnology and Oceanography 58:409–27. https://doi.org/10.4319/lo.2013.58.1.0409
Yeager LA, Layman CA. 2011. Energy flow to two abundant consumers in a subtropical oyster reef food web. Aquatic Ecology 45:267–277.
Yeager LA, Layman CA, Hammerschlag-Peyer CM. 2014. Diet variation of a generalist fish predator, grey snapper Lutjanus griseus, across an estuarine gradient: Trade-offs of quantity for quality? Journal of Fish Biology 85:264–277.
Zgliczynski BJ, Williams GJ, Hamilton SL, Cordner EG, Fox MD, Eynaud Y, Michener RH, Kaufman LS, Sandin SA. 2019. Foraging consistency of coral reef fishes across environmental gradients in the central Pacific. Oecologia 191:433–445. https://doi.org/10.1007/s00442-019-04496-9.
Acknowledgements
The authors would like to thank Dr Will Robbins, Miss Andrea Asunsolo, and Dr Johanna Zimmerhackel for their efforts in the field and assistance with sample preparation. We thank Dr Grzegorz Skrzypek and Mr Douglas Ford at the West Australian Biogeochemistry Centre for assistance with the stable isotope analyses. We also thank Dr Lauren Peel for her constructive feedback on this manuscript.
Additional data for the hydrodynamic modelling was provided by Rio Tinto and Pilbara Ports Authority. We recognise and acknowledge the Murujuga People, the traditional custodians of the land and sea country on which this study was conducted.
This research was conducted in accordance with the Department of Biodiversity, Conservation and Attractions animal ethics permit #2020-16A and Department of Primary Industries and Regional Development exemption #3671.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was funded by the Kieran McNamara World Heritage PhD Top-Up Scholarship, the Australian Government Research Training Program Scholarship, the Holsworth Wildlife Research Endowment – Equity Trustees Charitable Foundation & the Ecological Society of Australia, the Australian Coral Reef Society Student Research Award, the Woodside-operated Pluto Project’s State Environmental Offset Program “D” administered by the Department of Biodiversity, Conservation and Attractions. The hydrodynamic modelling work was supported by resources provided by the Pawsey Supercomputing Research Centre with funding from the Australian Government and the Government of Western Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Author contributions MM: Conceived of or designed study, performed research, analysed data, wrote the paper. TJB: Performed research, wrote the paper. LB: Performed research. MVWC: Analysed data, contributed new methods or models, wrote the paper. RDE: Conceived of or designed study, performed research, wrote the paper. DG: Conceived of or designed study, analysed data, wrote the paper. CMG: Analysed data, contributed new methods or models, wrote the paper. RKG: Performed research, wrote the paper. GAH: Conceived of or designed study, wrote the paper. GAK: Conceived of or designed study, wrote the paper. NT: Performed research, wrote the paper. SKW: Conceived of or designed study, wrote the paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moustaka, M., Bassett, T.J., Beltran, L. et al. Suspended Particulate Organic Matter Supports Mesopredatory Fish Across a Tropical Seascape. Ecosystems (2024). https://doi.org/10.1007/s10021-024-00929-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10021-024-00929-6