Abstract
Macroalgae and phytoplankton support the base of highly productive nearshore ecosystems in cold-temperate regions. To better understand their relative importance to nearshore food webs, this study considered four regions in the northern Gulf of Alaska where three indicator consumers were collected, filter-feeding mussels (Mytilus trossulus), pelagic-feeding Black Rockfish (Sebastes melanops), and benthic-feeding Kelp Greenling (Hexagrammos decagrammus). The study objectives were to (1) estimate the proportional contributions of macroalgal and phytoplankton organic matter using carbon and nitrogen stable isotopes, (2) determine if macroalgal use affected consumer growth using annual growth rings in shells or otoliths, and (3) describe changes in organic matter use and growth during the Pacific Marine Heatwave (PMH; 2014–2016) in one consumer, mussels. Macroalgae were the major organic matter source (> 60%) to the diet for all three consumers. The relationships between macroalgal contribution and growth were neutral for both fish species and significantly positive for mussels. During the PMH, mussels had a drop (> 10%) in macroalgal contributions and grew 45% less than in other time periods. Simultaneously, the relationship between macroalgal contribution and mussel growth was strongest during the PMH, explaining 48% variation compared to 3–12% before or after the PMH. Collectively, the results suggest that macroalgae is likely more important to cold-temperate nearshore food webs than phytoplankton. Management actions aimed at conserving and expanding macroalgae are likely to benefit nearshore food webs under all climate scenarios and especially during marine heatwaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nearshore marine environment provides important ecosystem services, including serving as nursery habitats for commercial, sport, and subsistence fisheries species, acting as a carbon sink in the global carbon cycle, and providing recreational areas for tourism (Chen and Borges 2009; Johnson et al. 2010; Evans and Mathis 2013). These services rely on the maintenance of functioning food webs to support energy flow to higher trophic levels. Temperate and higher-latitude nearshore systems are often highly productive, with phytoplankton, macroalgae, and terrestrial primary production contributing to the base of nearshore food webs (Duggins et al. 1989; Tallis 2009; von Biela et al. 2013, 2016). Understanding the relative importance of these primary producers to nearshore food webs can inform future ecosystem projections and contribute to decision-making (e.g., conservation, mariculture, human infrastructure development).
Phytoplankton are often regarded as a preferred and higher quality source of primary production when compared to alternatives, such as macroalgae (Bracken et al. 2012; Guo et al. 2016). Macroalgae are considered a less valuable food source because fresh macroalgae are difficult to digest due to more complex multicellular structures than single-celled phytoplankton (Santelices and Correa 1985) and secondary metabolites that act in chemical defense against grazers (Amsler et al. 2005). Those metabolites, however, are generally lower in North Pacific species, likely because the ecosystem has very few consumers that graze on fresh macroalgae (Steinberg 1988; Estes and Steinberg 1988). Across all macroalgae species, senescence and decomposition dismantle their complex structure and remove secondary metabolites that result in increases to their digestibility and create labile particulate organic matter (POM) and dissolved organic matter (DOM; Valiela et al. 1997; Duggins and Eckman 1997; Fredriksen 2003). Indeed, detrital pathways of decomposed macroalgae are likely the main route into cold-temperate food webs (Duggins et al. 1989). Moreover, many long-lived macroalgae also provide a more consistent food input into the nearshore system throughout the year as compared to short-duration phytoplankton blooms (Simenstad and Wissmar 1985; Duggins and Eckman 1997; Renaud et al. 2015).
Climate variations can cause changes in primary producers in terms of abundance and altered timing of peak abundance (Batten et al. 2016; Rogers-Bennett and Catton 2019; Weitzman et al. 2021; Wyatt et al. 2022; Arteaga and Rousseaux 2023). Such changes in climate and primary producers are expected to reverberate throughout the nearshore ecosystem (Weitzman et al. 2021; Arteaga and Rousseaux 2023). This especially applies to high-latitude systems (e.g., Jones and Driscoll 2022) where climate alterations are particularly pronounced. One of the climate events garnering recent concern worldwide is the increasing frequency and magnitude of marine heatwaves (Wernberg et al. 2021) and their influences on food webs (Piatt et al. 2020; Arimitsu et al. 2021). Marine heatwaves in coastal systems are driven by the long-term changes in sea-surface temperature and increased greenhouse gas emissions that cause strong perturbations to the productivity of coastal systems (Oliver et al. 2019; Marin et al. 2021). For example, the Pacific Marine Heatwave (PMH; 2014–2016) resulted in severe macroalgal declines (Rogers-Bennett and Catton 2019; Weitzman et al. 2021) and earlier peak abundance of phytoplankton (Batten et al. 2016; Wyatt et al. 2022; Arteaga and Rousseaux 2023). Estimates of the use and value of primary producers to consumers across a range of climatic conditions will improve understanding of future nearshore food webs.
Consumer growth is a useful performance metric to gain insight into the relative value of phytoplankton and macroalgae to consumers because growth, and potential changes in growth, may occur due to marine heatwaves. For example, in mussels (Mytilus spp.), both the quality of the organic matter source (Grant and Bacher 1998), as well as food quantity (Smaal and van Stralen 1990), are important determinants of growth. If phytoplankton were indeed a higher-quality resource for consumers (Guo et al. 2016), the growth of individuals should be faster in locations and years with a greater reliance on phytoplankton. Growth rates are also dependent on water temperature, another main driver of growth in marine invertebrates (Hoegh-Guldberg and Pearse 1995). Warmer temperatures are often associated with higher growth rates due to increased metabolic rates, given adequate energy sources to sustain growth (Shelley and Johnson 2022). On the other hand, if temperatures exceed the thermal tolerance of a species, for example during marine heatwaves, growth rates can decline, as individuals will allocate more energy towards maintenance or die if temperatures exceed the lethal threshold (Steeves et al. 2018; Traiger et al. 2022).
Three focal species, representing various feeding strategies and trophic levels, were chosen to determine use of phytoplankton- and macroalgal-based trophic pathways and their relationships to growth within the nearshore northern Gulf of Alaska (NGOA): the filter-feeding Pacific blue mussel (Mytilus trossulus), the mostly pelagic-feeding Black Rockfish (Sebastes melanops), and the benthic-feeding Kelp Greenling (Hexagrammos decagrammus). Mussels capture particles from the water column that they select based on size and quality (Bayne and Newell 1983). Mussels in the North Pacific are key trophic links as prey items for upper trophic levels, such as sea stars, sea otters (Enhydra lutris), sea ducks, and black oystercatchers (Haematopus bachmani; O’Clair and O’Clair 1998). Black Rockfish and Kelp Greenling are widely distributed across the North Pacific, from the Aleutian Islands to central California, but with locally small home and feeding ranges of ~ 1 km2 (Parker et al. 2007; Love 2011). Black Rockfish are generalist, pelagic feeders, consuming small fishes, zooplankton, and other prey found in coastal waters (< 55 m depth; Brodeur et al. 1987; Love 2011). Kelp Greenling are benthic feeders that prey on sea cucumbers, crabs, and other benthic species (Moulton 1977; Love 2011).
Here, we assess contributions of phytoplankton and macroalgae to the three focal consumers, assess whether primary producer pathways are associated with differences in their growth outcomes, and consider if environmental changes associated with the PMH shifted the contributions and growth outcomes in mussels. We employed a combination of stable isotope analysis to trace the two primary production sources (using carbon and nitrogen ratios; France 1995; Peterson 1999) and natural annual growth rings in mussel shells and fish otoliths (Millstein and O’Clair 2001; Black et al. 2005). Based on previous work showing strong reliance of many nearshore invertebrate and fish consumers in Alaska on macroalgal pathways (Duggins and Eckman 1997; von Biela et al. 2016; Siegert et al. 2022; Schloemer et al. 2023), we hypothesized that focal species would have higher growth rates with higher macroalgal contributions to their diets. We then used the PMH (2014–2016) as a natural experiment to test the hypothesis that a reduction in macroalgal abundance during the PMH (Weitzman et al. 2021) would lead to lower use of the macroalgal pathway in mussels, which, along with other abiotic stressors of the heatwave, would result in a decline in growth rates.
Methods
Sampling Region
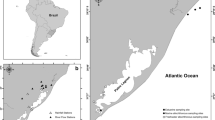
Sampling regions in the NGOA were based on current study locations of the Nearshore Component of the Gulf Watch Alaska Long-term Monitoring Program. Four study regions across the NGOA were included: Katmai National Park and Preserve (KATM), Kachemak Bay (KBAY), Kenai Fjords National Park (KEFJ), and Western Prince William Sound (WPWS). In each study region, mussels were collected at five or six rocky intertidal sites, for a total of 21 study sites (Fig. 1). Fish were collected in the subtidal environment of the four study regions.
Sample Collections
We used bulk stable isotope (carbon and nitrogen) analysis to trace phytoplankton and macroalgal pathways in the consumers. To isotopically characterize the macroalgal organic matter sources in the food web, samples of the dominant (% cover, see Iken and Konar 2022 and USGS Alaska Science Center and others 2022 for detailed data) species of red, green, and brown macroalgae were collected at each intertidal site in the summer (May to August) for most years from 2014 to 2021 (see Supplementary Material Table S1 for details). Most often, Odonthalia sp. or Neorhodomela sp. represented red algae, Ulva lactuca represented green algae, and Alaria marginata or Saccharina latissima, as well as Fucus distichus, represented brown algae. In addition, the ten most dominant macroalgal species from each site, based on percent cover (USGS Alaska Science Center and others 2022), were collected in the summer of 2021 (including representatives of red, green, and brown algae; Iken 2024a) to assess stable isotope variability across a larger range of macroalgal source species (see Supplementary Materials Table S1). Phytoplankton were sampled as POM from water samples > 100 m from shore at each sampling site in each year, and 500–1000 mL from each site was filtered onto Whatman GF/F filters and frozen until further analysis. POM water samples collected from the intertidal (2012–2018) were determined to be a mix between phytoplankton and macroalgal detritus based on preliminary analyses and, therefore, could not provide a useful isotopic value to distinguish primary producers.
For the consumers, ten M. trossulus (from here on called “mussels” when referring to this study) of an average size range (20–30 mm) were collected annually between 2014 and 2021 by hand at each site in each of the four study regions (total n = 1249). Black Rockfish and Kelp Greenling were collected on a regional scale rather than by site due to their mobility and use of larger foraging areas compared to mussels. Fish were caught in the nearshore by hook and line fishing, with a maximum of ten individuals per species collected per region and year (total n = 120 for Black Rockfish, total n = 108 for Kelp Greenling). Fish collections occurred in 2018, 2019, and 2021 in all regions (Iken 2024b). All mussel and fish samples were measured for total length (tip of snout to end of tail for fish, longest axis of the mussel) before further processing. The mussel adductor and fish epaxial muscle were sampled to be analyzed for bulk carbon and nitrogen stable isotopes.
Stable Isotope Analysis
Consumer muscle tissues, algae, and POM filter samples were dried at 60 °C until constant weight. Consumer tissue and algal samples were homogenized via manual crushing and 0.3–0.5 mg was weighed into aluminum tins. POM filters were acid-fumed for a minimum of 4 h, after which the surface layer was scraped into tin capsules. Samples in tin capsules were run through the continuous flow isotope ratio mass spectrometer (CF-IRMS) at the Alaska Stable Isotope Facility at the University of Alaska Fairbanks Water & Environmental Research Center to measure bulk stable carbon and nitrogen isotopes. The CF-IRMS uses a Thermo Scientific Flash 2000 elemental analyzer and Thermo Scientific Conflo IV interfaced with a Thermo Scientific DeltaVPlus Mass Spectrometer (Breman, Germany). Instrument precision was ± 0.11‰ for both isotopes, based on standard deviation from expected values of replicate standard measurements (n = 159). Results are reported relative to the international standards of Vienna Pee Dee Belemnite (V-PDB) for carbon and atmospheric N2 (air) for nitrogen. All results are reported in delta (δ) notation as parts per thousand (‰) difference between samples and the standard using the equation.
where X is the heavy isotope (13C or 15N) and R is the ratio of heavy to light isotope in the sample or standard. The ratio of carbon and nitrogen concentrations was calculated to ensure lipid correction was not necessary (C:N < 4; Hoffman et al. 2015).
Growth Rate Measurements
Growth rate measurements between shell ridges are widely interpreted and validated as annual growth rates (Millstein and O’Clair 2001; Black et al. 2005). To validate that major shell ridges represent annual growth rings in the mussels examined in this study, multiple mussels at each site were tagged with a piece of flat nylon filament (flattened fishing line) positioned at the edge of the mussel shell in the summer of 2021 (see Supplementary Materials Fig. S1a). These mussels were then recovered the following summer (same month as in 2021) and measured for the exact growth in one year (summer to summer) as the distance of the tag edge to the new shell edge. Measurements were taken on the mussel shell surface using electronic calipers (iGaging Absolute Origin, 6″ Origin Digital Caliper) with 0.01 mm accuracy (Iken 2024b). We compared this tag distance to the distance between the two most recent surface ridges on the same mussel shells to determine if it produced a comparable annual growth index, albeit for 1 year’s growth from winter to winter instead of summer to summer (Supplementary Materials Fig. S1a). Thus, tagged mussel growth and surface ridge-based growth represented slightly different time frames and overlapped for about 6 months, but this was unavoidable because field trips to the remote field sites occur only in summer. Because of the high correlation between tag and surface ridge measurement methods (R2 = 0.82, n = 51; see Supplementary Materials Fig. S2), surface ridges were then used to measure the annual growth of mussels.
Estimating growth rates from fish otoliths followed methods described by von Biela et al. (2015). Otoliths were embedded in epoxy and thinly sectioned through the longitudinal center where all annual growth bands are present. A high-resolution digital image of each section was taken using a Leica M165 C dissecting microscope with a Leica DFC420 camera attachment and Leica Application Suite (Leica 2020). Annual growth was measured along the shorter dorsal growth axis (next to the sulcus) as the width between the outer edge of the two most recent growth rings for each individual from all regions (Supplementary Materials Fig. S1b). ImageJ (Schneider et al. 2012) measurements of otolith images were taken to 0.01 µm accuracy based on the calibration slide. Therefore, as individuals were collected in the summers of 2018, 2019, and 2021, the last complete increments represented growth of the fishes in 2017, 2018, and 2020, respectively.
To match the source contributions derived from stable isotope analysis with growth estimates for each species in a year, only the width of the last complete annual growth increment was measured from mussel shells and fish otoliths (Supplementary Materials Fig. S1b). The ongoing annual growth increment was not measured because it was incomplete at the time of summer sampling. Multiple measurements (3–5) of each last complete growth increment were taken per individual and averaged, along with standard deviation, for the final growth value used in statistical analyses. Standard deviations over a calculated limit were used to indicate that a measurement was uncertain and possibly inaccurate. This standard deviation limit was calculated for each species as the upper limit for outliers of standard deviation values (interquartile range × 1.5 + third quartile). The cutoff value was 0.53 mm for mussels, 0.20 µm for Black Rockfish, and 0.15 µm for Kelp Greenling. Measurements with larger standard deviations than those cutoffs were removed.
Temperature Measurements
To track environmental temperatures, HOBO (Onset Computers) temperature loggers were continuously deployed at the 0.5-m tidal elevation at each site to record the temperature (°C) every 30 min. Based on time and tide height, each temperature record was noted as being submerged, emerged, or transitioning. Submerged temperatures were then averaged for the annual summer season, defined as April through September, for each site and year (USGS Alaska Science Center and others 2016). Based on annual summer temperature records, select study years were categorized as being before (2012–2014), during (2015–2016), or after (2017–2018, 2020–2021) the PMH to be used in comparisons of macroalgal diet contributions and growth in mussels (see below). While 2019 was a warmer-than-average year, it was not part of the original PMH and was excluded from the analyses.
Data Analyses
Stable isotope mixing models that determine diet contributions from different primary production sources require the sources to be sufficiently isotopically different to allow partitioning of these sources. Therefore, macroalgal and POM carbon stable isotope values were compared using a t-test (α = 0.05) by site and by region. Sites without significantly different carbon isotope values between macroalgae and POM were excluded from further analyses as mixing models would not be able to distinguish the contributions of these sources to consumer diets. Organic matter contributions from POM and macroalgae to the diet for each species and site and/or region were then determined using a Bayesian mixing model, MixSIAR (Stock and Semmens 2016), in R software (R Core Team 2020). The model was run using what is considered a “long” run with three chains of length 300,000 with a burn-in of 200,000 and thinning of 100. Convergence was tested using Geweke (low z-score for each chain) and Gelman-Rubin (95% of variables < 1.05) diagnostics. Diet estimates were based on the average POM and macroalgal stable isotope data for carbon and nitrogen across all years for each region, along with the standard deviation and sample size. All consumer stable isotope data were run separately by species in MixSIAR, with site and region as nested random factors in the calculations for mussels and with region as a random factor for the two fish species. Fish samples were also analyzed with MixSIAR by sample ID to calculate the individual diet contributions to be compared to individual growth measurements. This was not done for mussels, as the individuals used for stable isotope analysis were often different from the individuals used for growth rate measurements; hence, mussel calculations and comparisons were done on the population level at each site and analyzed by region.
The MixSIAR model was also parameterized with a trophic discrimination factor (TDF) of 0.4 ± 1.3‰ for δ13C and 3.4 ± 1‰ for δ15N (Post 2002; França et al. 2011) per trophic level. Trophic level of each species was calculated as an input variable for the MixSIAR model following Markel and Shurin (2015, after Post 2002):
where TL is the trophic level, δ15Nc is the average stable nitrogen isotope value of the consumer, δ15NPOM is the average stable nitrogen isotope value of POM, δ15NMA is the average stable nitrogen isotope value of macroalgae, and Δn is the TDF of 3.4‰. The α variable is calculated as follows:
with δ13Cc being the average stable carbon isotope value of the consumer, δ13CMA is the average stable carbon isotope value of macroalgae, and δ13CPOM is the average stable carbon isotope value of POM. MixSIAR incorporates uncertainty in TDFs into relative source contribution calculations using Bayesian methods.
In accordance with our hypothesis, we chose to report the proportional contribution of macroalgae to the respective consumer diets, with the understanding that the POM contribution is the remaining proportion. To evaluate differences in macroalgal contributions by sites and/or regions, we calculated the probability that consumers at one site/region had greater macroalgal contributions than at another site/region using the package R2Jags (Su and Yajima 2015). The site/region with the larger average value was placed on the greater side of the test, so only one test was needed for each comparison and all probabilities were over 0.50. We considered probabilities over 0.95 as ecologically meaningful.
Linear regression analysis between individual body size and growth rate was used to produce standardized residuals and corrected growth rates for the individual size variations for mussels and both fish species. Stable isotope turnover time in muscle tissue of mussels and fish living in cold water is on the order of multiple months (McKinney et al. 2001; Perga and Gerdeaux 2005; Hill and McQuaid 2009; Weidel et al. 2011). This period of isotopic integration allowed us to align isotope values of consumers with the measured growth period. A linear mixed model then related growth rate residuals to macroalgal diet contribution for each species and region (α = 0.05). In addition, for mussels, diet and growth from three regions (KATM, KEFJ, WPWS, based on data availability) were categorized as being before (2014), during (2016), or after (2021) the PMH. These years were chosen because they had the largest datasets available. The PMH was first noticed in late 2014 after the majority of the summer growth had occurred, such that 2015 is considered the first PMH year, in accordance with other Gulf of Alaska biological studies (von Biela et al. 2019; Piatt et al. 2020; Danielson et al. 2022). Macroalgal diet contributions and growth rates were then compared among these time periods to determine differences in both metrics in relation to the PMH (one-way ANOVA, α = 0.05, with Tukey HSD). Linear relationships between macroalgal diet contribution and growth rate residuals were analyzed by time category in relation to the PMH (α = 0.05). All statistical analyses were performed using the R Stats Package in R (R Core Team 2020).
Results
Contributions of Phytoplankton- and Macroalgal-Based Pathways to Diet
The carbon stable isotope values of the extensive collections of macroalgal species from 2021 were similar within the same group (i.e., red, green, or brown algae) so that the reduced number of macroalgal species collected in other years was considered representative of the macroalgal sources in general (Supplementary Materials Table S1). At the site level, macroalgal δ13C values were significantly higher relative to POM at nearly all sites (t-test, all t ≥ 3.086, all p < 0.05), except for Bishop’s Beach (t-test, t = 2.335, p = 0.100) and Bluff Point (t-test, t = 2.827, p = 0.087) in the KBAY region. Therefore, sources could not be separated at these two sites, and they were removed from further analyses. Across all regions, macroalgal δ13C values ranged from − 32 to − 12‰, whereas POM δ13C values ranged more narrowly from − 25 to − 20‰, with significant differences between the two sources in all four regions (t-test, all t ≥ 9.434, all p < 0.001 for each region; Supplementary Materials Table S1). With macroalgal average δ13C values being higher than POM average δ13C in each region, we interpreted that consumers with higher δ13C values used more of the macroalgal-based pathway compared to consumers with lower δ13C values.
Based on MixSIAR mixing model results, macroalgae were the main organic matter source contributor, always above 50%, to the diet of mussels, Black Rockfish, and Kelp Greenling across all regions when all sampling years were combined (Tables 1 and 2; Fig. 2). In KATM mussels, average macroalgal contribution by site ranged from 73 to 88% (Table 1). Macroalgal contributions to mussels in the KBAY region were similar among sites (73–78%). In the KEFJ region, macroalgal contributions by site ranged from 66 to 83% (Table 1). In WPWS, macroalgal contributions to mussels at two sites (78–80%) were lower than the three other sites (86–90%, Table 1). When macroalgal contributions to all consumer diets were compared among regions, contributions were similar (Table 3), although the KBAY region appeared to have consistently lower macroalgal contributions than other regions in all consumers (Fig. 2; Table 3). With all regions combined, Black Rockfish had an average macroalgal contribution of around 70%, while Kelp Greenling had a higher average macroalgal contribution of 87%.
Macroalgal contribution (%) to the diet of mussels, Black Rockfish, and Kelp Greenling in four regions in the northern Gulf of Alaska. Within boxplots, the box contains the 25th to 75th percentiles with the center line representing the 50th percentile (median). The asterisk marks the mean and whiskers reach out to the 2.5th and 97.5th percentiles. The same letters above boxplots indicate significant groupings (p ≤ 0.05) by region within each species. Sample sizes (n) are given for each region. The horizontal dotted line is for orientation at 50% macroalgal contribution. Katmai National Park and Preserve: KATM, Kachemak Bay: KBAY, Kenai Fjords National Park: KEFJ, Western Prince William Sound: WPWS
The Effect of Source Contributions on Growth Performance
Mussel residual growth was significantly positively correlated with macroalgal contribution to their diet when data were combined across all sites, years, and regions (linear model, n = 45, R2 = 0.27, p < 0.001; Table 4). When analyzed by individual regions, however, mussels in KATM were the only ones where macroalgal contribution was significantly positively correlated with growth (linear model, n = 14, R2 = 0.29, p = 0.048). Mussel growth was significantly different among regions (one-way ANOVA, n = 46, F(3, 42) = 3.897, p = 0.015; Table 5), with significantly higher growth in KBAY and WPWS than in KEFJ (Tukey HSD, p < 0.05; Table 5).
For Black Rockfish, there was no significant relationship between macroalgal contribution to the diet and growth when analyzed across all regions (linear model, n = 106, R2 = 0.0009, p = 0.755; Table 4). In individual regions, only Black Rockfish in KBAY grew significantly faster in years with higher macroalgal contributions to the diet (linear model, n = 27, R2 = 0.4, p < 0.001). Growth was significantly different among regions, independent of macroalgal diet contribution (one-way ANOVA, n = 106, F(3, 102) = 5.266, p = 0.002; Table 6), with higher growth in KATM than KEFJ or WPWS (Tukey HSD, p < 0.03 for both comparisons; Table 6). Kelp Greenling growth was not significantly related to macroalgal contribution when all regions were combined (linear model, n = 75, R2 = 0.028, p = 0.297) or in any of the regions individually (linear model, p > 0.05 for all regions; Table 4). Region alone, independent of macroalgal contribution, also had no significant relationship with Kelp Greenling growth (one-way ANOVA, n = 75, F(3, 71) = 1.546, p = 0.210; Table 6).
Mussel Diet and Growth in Relation to the Pacific Marine Heatwave
The average summer water temperature was warmest for each region in 2016 and 2019 and coolest in 2013, although these differences were not significant (one-way ANOVA, n = 40, F(9, 30) = 2.019, p = 0.072; Fig. 3). The average temperature during the peak of the heatwave in 2016 was about 1 °C warmer than the average temperatures across all years sampled for each of the regions (excluding 2019, which was not part of the original heatwave). In addition, there was a significant difference in average temperatures among regions (Table 7; one-way ANOVA, n = 40, F(3, 36) = 8.089, p = 0.0003), with WPWS having significantly warmer long-term average temperatures than KATM and KBAY (Tukey HSD, p = 0.022 for KATM, p = 0.0002 for KBAY).
Average summer (April to September) water temperatures (°C) by year (USGS Alaska Science Center and others 2016). Colors and shapes are for each region. Horizontal dashed lines are the average across all years for each region. Years considered affected by the Pacific Marine Heatwave (PMH) and the heat spike year 2019 (not included in analyses) are highlighted in gray. Katmai National Park and Preserve: KATM red squares, Kachemak Bay: KBAY purple circles, Kenai Fjords National Park: KEFJ blue triangles, Western Prince William Sound: WPWS green diamonds
During the PMH (2015–2016), macroalgal contribution to mussel diet combined for all regions was significantly lower than after the PMH (2017–2018, 2020–2021; Tukey HSD, p = 0.042; Fig. 4, Table 8) and was intermediate (but not significantly different) for the period before the PMH (2012–2014). Similarly, mussel growth was significantly different before, during, and after the PMH for all regions for which these long-term data were available (one-way ANOVA, n = 386, F(2, 383) = 95.36, p < 0.001; Fig. 4). Mussel growth was lowest during the PMH (2016) and highest after the PMH (2021; Table 8). Macroalgal contribution did not relate to growth before or after the PMH (linear model, n = 41, p = 0.581 before PMH, p = 0.277 after PMH) but was a significant predictor of growth during the PMH (linear model, p = 0.004; Table 4).
Macroalgal contribution to the diet of mussels and their growth increment residuals in relation to the Pacific Marine Heatwave (PMH). The box contains the 25th to 75th percentiles with the center line representing the 50th percentile (median). The asterisk marks the mean and whiskers reach out to the 2.5th and 97.5th percentiles. The same letters above the time periods indicate significant groupings (p ≤ 0.05). Sample sizes (n) for macroalgal contributions are the number of regions and years for each time period. Sample sizes (n) for growth increment residuals are the number of mussels measured. PMH time periods for macroalgal contributions are 2012–2014: Before, 2015–2016: During, and 2017–2018 and 2020–2021: After
Discussion
Consumers in the NGOA nearshore ecosystem sourced the majority of their organic matter from macroalgal-based trophic pathways, which challenges the common view that globally, phytoplankton is the main organic matter source for coastal consumers. This macroalgal importance observed in our study could even be spatially more extensive, as macroalgal detritus may reach coastal areas tens of kilometers away from algal stands because detritus is carried by winds and currents (Kaehler et al. 2006) and provide a diet subsidy to offshore or deeper regions (Britton-Simmons et al. 2009; Krumhansl and Scheibling 2012; Renaud et al. 2015). Growth outcomes suggested that a high proportional reliance on macroalgae either had no effect or was beneficial for consumers. This result implies that the quality of macroalgal matter may not be lower than phytoplankton (Dethier et al. 2014; Guo et al. 2016). The PMH was associated with both overall lower macroalgal contributions and lower growth rates of mussels, but the relationship of macroalgal contribution to growth in mussels was stronger during the PMH. This indicates that while climate events can result in changes to key lower trophic levels, consumers can be partially buffered from such climate effects by utilizing both macroalgal and phytoplankton organic matter.
Contributions of Phytoplankton- and Macroalgal-Based Pathways to Diet
The diet of mussels, Black Rockfish, and Kelp Greenling across all sites and regions in this study was overwhelmingly dominated by the macroalgal-based pathway. High reliance on the macroalgal pathway seems to be relatively common for northern high latitudes, with multiple studies finding many invertebrates (including mussels) and fishes deriving organic matter from macroalgae (Duggins and Eckman 1997; Fredriksen 2003; Renaud et al. 2015; von Biela et al. 2016; Siegert et al. 2022; Schloemer et al. 2023). High macroalgal reliance may be driven by availability at locations with high macroalgal biomass and diversity, such as in our study regions in the NGOA (Konar et al. 2010). It is also possible that the macroalgal-based pathways are prominently used in the NGOA because of the strong seasonality of phytoplankton, which is particularly pronounced in high-latitude systems because of winter light limitations and storms (Childers et al. 2005). Our results for mussels differ from those of Mytilus californianus in Oregon, which relied heavily on a phytoplankton-based pathway, responding to variations in phytoplankton availability and quality (Bracken et al. 2012). While macroalgal abundance and diversity along the Oregon coast are also high (e.g., Bracken and Nielsen 2004), the difference compared to our study may be related to the reduced seasonality, and thus more continuous availability of phytoplankton in the lower-latitude system (e.g., Du and Peterson 2014).
For consumers to be responsive to variable energy input from multiple trophic pathways, they have to be able to exhibit trophic flexibility (Bridier et al. 2021; Timmerman et al. 2021). Flexibility to use multiple pathways seems especially common in coastal systems (Kopp et al. 2015; Siegert et al. 2022). Flexible feeding on various primary producer sources is particularly important for lower trophic level consumers that feed directly on these resources, rather than upper trophic levels that integrate trophic pathways from various basal resources through their intermediate prey. In the NGOA, intertidal mussels are an example of such trophic flexibility, being able to use both phytoplankton- and macroalgal-derived pathways, depending on environmental conditions and resource availability (Siegert et al. 2022). This could allow mussels to respond to changes in the availability of these resources due to seasonal or climatic variation, such as low macroalgal abundance during marine heatwaves (Román et al. 2020; Weitzman et al. 2021) or from combined pressures, such as warming and acidification (Gao et al. 2021). Similar results have been seen in both Black Rockfish and Kelp Greenling, with those individuals at locations with limited phytoplankton availability relying more on the kelp-derived carbon pathway (von Biela et al. 2016). However, with these fish species being more generalist feeders and consuming prey at multiple trophic levels, rather than directly feeding on primary producers, their pathway use depends largely on the resource use of their various prey species. The prominence of the macroalgal pathway in both fish species in our study suggests that the dominant use of this pathway is widespread across lower trophic level consumers in the system and is not limited to primary consumers like mussels studied here specifically. This is especially true for Kelp Greenling, which consume mostly prey that are part of benthic food webs that are more prone to very high macroalgal organic matter contributions, either through direct grazing or detrital material (Fredriksen 2003; Renaud et al. 2015). The prominence of macroalgal organic matter in the more pelagic-feeding Black Rockfish was less expected, although previous work in the region suggested similar patterns (von Biela et al. 2016). The high percentage of macroalgal contribution to Black Rockfish could suggest that macroalgal organic matter efficiently enters pelagic food webs through “reversed” coupling (the uptake of macroalgal detritus by zooplankton to pass into pelagic food webs; McManus et al. 2004; Feehan et al. 2018).
While macroalgal contributions to consumer diets were high in all focal species and regions, there were some site-specific differences in contributions to mussels, which may be related to environmental factors. One of these factors could be freshwater influence from river or glacial discharge, which has also been determined as a major driver of mussel demographics (LaBarre et al. 2023). For example, mussels at Kukak (KATM) and Whale Bay (WPWS) had significantly lower macroalgal contributions than at the rest of the sites in their respective regions. Kukak is glacially influenced with five glacial rivers feeding into the bay (Hildreth et al. 1999). The Whale Bay site is near glacial input both from the Whale Bay watershed and from glacial water from nearby Icy Bay (Gay and Vaughan 2001). Glacial runoff can carry high amounts of terrestrially-derived nutrients, as well as highly labile terrestrial DOM (Fellman et al. 2010). We did not include terrestrial matter in our mixing model, so that potential terrestrial matter uptake based on stable isotope data would have been attributed to a higher proportion of phytoplankton in our study. However, a recent study of organic matter sources in glacially-influenced estuaries for lower trophic level consumers in the KBAY study region indicated that terrestrial matter was not a significant food source for mussels in this system (Schloemer et al. 2023).
The Effect of Source Contributions on Growth Performance
We hypothesized that higher reliance on the macroalgal-based pathway would promote higher growth in the three focal species due to macroalgae being a more consistent food source. In contrast, we observed only a moderate increase in growth with higher macroalgal-contributions to the diet in mussels across all sites and regions and no effect across all regions in either fish species. It should be noted, however, that proportional contributions are not a measure of total food availability, and that the overall ranges (57–99% for mussels) of macroalgal contributions to the focal species were relatively narrow. The narrow range and overall high level of macroalgal contributions to the diet of all species may limit our ability to detect stronger effects on growth. A positive relationship of macroalgal contributions across a range from 45 to 85% across all sites and growth is similar to patterns observed in M. edulis in the Aleutian Islands, another high-latitude system, where higher contributions of kelp-derived carbon also led to higher growth rates (Duggins et al. 1989). Conversely, M. californianus in Oregon had higher growth with more POM, responding to variations in phytoplankton availability and quality (Bracken et al. 2012). While these differences may relate to latitude or oceanographic current systems, they also suggest that there is likely not a single consistently better source of primary production to support growth across a wide range of environmental conditions. Another aspect to consider is that there can be high variability in individual growth performance in some mussel species (e.g., M. galloprovincialis), where individual growth differs even if specimens are fed a consistent diet (Fuentes-Santos et al. 2018). Strong performance differences between “slow and fast growers” in mussels could obscure diet quality effects.
Similar to mussels, the range of macroalgal contribution for the two fish species was narrow and had no relationship to growth. In a different example with a wide range of macroalgal contributions (0–60%), the growth of juvenile Black Rockfish from Vancouver Island, Canada, was positively associated with more kelp-derived carbon (Markel and Shurin 2015). The growth variation examined by Markel and Shurin (2015) was likely wider because juvenile fish allocate most of their energy into growth, while adult fish examined in this study allocate most of their energy into reproduction and maintenance (Rombough 1994). Therefore, we cannot rule out the possibility that macroalgae contributions do affect fish growth, at least in some life stages, in the NGOA.
Mussel Diet and Growth in Relation to the Pacific Marine Heatwave
While the PMH was a major perturbation in the NGOA, it may not have had as drastic effects in the nearshore environment (Robinson et al. 2023). The higher intertidal temperatures in 2016 were noticeable but had enough cooler periods in each year that the summer average water temperatures were not significantly higher than in previous years across all regions. The intertidal macroalgal die-off reported by other studies was a significant effect of these warmer periods (Weitzman et al. 2021) and was likely due to the faster response times and higher sensitivity of macroalgae compared to the growth of nearshore consumers.
Macroalgal contributions to mussel diet were significantly lower during the PMH (2015–2016) compared to after the PMH (2017–2018, 2020–2021). On a population level, many macroalgal species significantly decline in growth and standing biomass under warmer temperatures or during heatwaves (Gouvêa et al. 2017; Román et al. 2020; Weitzman et al. 2021). For example, during the PMH, Fucus distichus and other fleshy macroalgae in the NGOA declined, with a shift to more invertebrate-dominated intertidal systems (Weitzman et al. 2021). Under warmer conditions, macroalgal respiration increases while photosynthesis decreases, leading to overall less growth (Davison 1991), a pattern that is especially prominent in cold-water macroalgae that have low photosynthetic temperature optima (Ji and Gao 2021). Reduced macroalgal productivity could explain our observed lower macroalgal contributions to mussel diets during the heatwave. Macroalgal contributions, however, increased significantly after the PMH, possibly due to an increase of macroalgal biomass in the detrital pool deriving from the macroalgal die-off during the warm phases (Gao et al. 2021; Weitzman et al. 2021; Ji and Gao 2021; McPherson et al. 2021). Macroalgal detrital lability for lower trophic level invertebrate consumers increases over the weeks to months after initial detrital production (e.g., Kristensen et al. 1992; Dethier et al. 2014), which may explain a delay in observing the increase of macroalgal contribution, considering the PMH lasted 2 years. It also should be noted that there is a lag in detectable changes in food sources in consumer tissues based on stable isotope turnover time (several months for Mytilus spp.; Hawkins 1985) and our annual sampling frequency. With the intertidal macroalgal die-off starting in 2015 and likely peaking in 2016 at the height of the heatwave (Weitzman et al. 2021), an increased amount of macroalgal detritus would be expected to enter the food web a few months later, as it increases in lability. It would then require several months for that increase in macroalgal consumption to be reflected in the isotope composition of the consumers, likely in late 2016. Based on our annual sampling scheme, we were then able to detect this signal in 2017.
During the PMH period of lower macroalgal contributions, we found that mussel growth rates were also lower, but we found a strong relationship between trophic pathway sourcing and growth during this time. This finding suggests that macroalgae can provide an important energy pathway to maintain mussel growth under stressful environmental conditions, even if the relationship during other times was low. Conversely, mussel growth rates during the PMH could also be driven by changes in phytoplankton availability (Batten et al. 2022; Wyatt et al. 2022), a pathway that could then become proportionally more pronounced during heatwaves. However, while phytoplankton production may increase during marine heatwaves, phytoplankton species composition during warmer temperatures typically shifts to smaller-celled taxa (Batten et al. 2022; Wyatt et al. 2022). If indeed a specific source of primary production does not consistently enhance growth, as discussed above, mussels may be able to withstand some of these fluctuations by switching main primary producer pathways.
Growth performance of nearshore species can also be affected by environmental habitat conditions, independent of food supply (e.g., Ruckelshaus et al. 1993). Typically, mussels are more likely to grow faster during warmer temperatures if food abundance is high (Lesser et al. 2010), i.e., higher growth and metabolic demand can be fueled by increased food intake (Lesser et al. 1994), although this ability depends on the thermal physiology of a species (Schneider 2008). M. trossulus, the species investigated here, has low tolerance towards high temperatures (Evans and Somero 2010; Tomanek and Zuzow 2010), indicating that compensation of increased energy demands for growth during warm periods may be limited. In particular, stressful conditions, such as during heatwaves, could cause energy allocation priorities in Mytilus to switch from growth to maintenance or heat shock protection (Petes et al. 2008; Lesser et al. 2010; Tomanek and Zuzow 2010). It should also be noted that the temperature mussels experience internally is driven by complex interactions of microclimate resulting from the position on a rock, surrounding organisms, size of the mussel, wind and water spray, etc. (Helmuth 1998). Therefore, mussels in our study could have experienced much higher or lower body temperatures than the proxy of environmental data loggers can convey. While our PMH results for mussels showed both lower macroalgal contribution and lower growth rates, these could both be related to temperature instead of to each other, although there is evidence that food availability is a more important driver of Mytilus growth than temperature (Page and Hubbard 1987).
Implications for the Nearshore Ecosystem
The climate and state of the ocean are changing at a faster rate than seen before (Jones and Driscoll 2022). It is reasonable to expect more frequent marine heatwaves (Frölicher et al. 2018) will lead to lower macroalgal abundance (Weitzman et al. 2021) with effects on the food web, as seen in this study. If performance of foundational species, such as mussels, became compromised, they could become less competitive under changed ocean conditions, which could increasingly open the door for range extensions of southern species (Therriault et al. 2018). For example, the currently more southerly-distributed species, M. galloprovincialis, is better able to tolerate warmer conditions than the NGOA-native M. trossulus, including maintaining growth performance at higher temperatures (Schneider and Helmuth 2007; Shinen and Morgan 2009). Given the importance of macroalgae to nearshore consumers demonstrated here and elsewhere (e.g., Duggins et al. 1989), understanding and anticipating nearshore responses to bottom-up climate forcing can benefit from the inclusion of monitoring of macroalgal production. Management actions aimed at conserving and expanding macroalgae are likely to benefit nearshore food webs under all climate scenarios and especially during marine heatwaves.
Data Availability
All data used in this work are publicly available. Stable isotope data are available in Iken (2024a). Growth data are available in Iken (2024b). Temperature data are at the USGS Alaska Science Center, National Park Service Southwest Alaska Inventory and Monitoring Network, University of Alaska Fairbanks College of Fisheries and Ocean Sciences (2016).
References
Amsler, C., K. Iken, J. McClintock, M. Amsler, K. Peters, J. Hubbard, F. Furrow, and B. Baker. 2005. Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Marine Ecology Progress Series 294: 141–159.
Arimitsu, M.L., J.F. Piatt, S. Hatch, R.M. Suryan, S. Batten, M.A. Bishop, R.W. Campbell, H. Coletti, D. Cushing, K. Gorman, R.R. Hopcroft, K.J. Kuletz, C. Marsteller, C. McKinstry, D. McGowan, J. Moran, S. Pegau, A. Schaefer, S. Schoen, J. Straley, and V.R. von Biela. 2021. Heatwave-induced synchrony within forage fish portfolio disrupts energy flow to top pelagic predators. Global Change Biology 27 (9): 1859–1878.
Arteaga, L.A., and C.S. Rousseaux. 2023. Impact of Pacific Ocean heatwaves on phytoplankton community composition. Communications Biology 6 (1): 1–13.
Batten, S.D., S. Moffitt, W.S. Pegau, and R. Campbell. 2016. Plankton indices explain interannual variability in Prince William Sound herring first year growth. Fisheries Oceanography 25 (4): 420–432.
Batten, S.D., C. Ostle, P. Hélaouët, and A.W. Walne. 2022. Responses of Gulf of Alaska plankton communities to a marine heat wave. Deep Sea Research Part II: Topical Studies in Oceanography 195: 105002.
Bayne, B.L., and C. Newell. 1983. In Physiological energetics of marine molluscs, ed. A.S.M. Saleuddin and K.M. Wilbur, 407–515. Academic Press: The mollusca.
Black, B.A., G.W. Boehlert, and M.M. Yoklavich. 2005. Using tree-ring crossdating techniques to validate annual growth increments in long-lived fishes. Canadian Journal of Fisheries and Aquatic Sciences 62 (10): 2277–2284.
Bracken, M.E.S., and K.J. Nielsen. 2004. Diversity of intertidal macroalgae increases with nitrogen loading by invertebrates. Ecology 85 (10): 2828–2836.
Bracken, M.E., B.A. Menge, M.M. Foley, C.J. Sorte, J. Lubchenco, and D.R. Schiel. 2012. Mussel selectivity for high-quality food drives carbon inputs into open-coast intertidal ecosystems. Marine Ecology Progress Series 459: 53–62.
Bridier, G., F. Olivier, L. Chauvaud, M.K. Sejr, and J. Grall. 2021. Food source diversity, trophic plasticity, and omnivory enhance the stability of a shallow benthic food web from a high-Arctic fjord exposed to freshwater inputs. Limnology and Oceanography 66: S259–S272.
Britton-Simmons, K.H., G. Foley, and D. Okamoto. 2009. Spatial subsidy in the subtidal zone: Utilization of drift algae by a deep subtidal sea urchin. Aquatic Biology 5 (3): 233–243.
Brodeur, R. D., H. V. Lorz, and W. G. Pearcy. 1987. Food habits and dietary variability of pelagic nekton off Oregon and Washington, 1979–1984. Technical Report NMFS 57, NOAA.
Chen, C.-T.A., and A.V. Borges. 2009. Reconciling opposing views on carbon cycling in the coastal ocean: Continental shelves as sinks and near-shore ecosystems as sources of atmospheric CO2. Deep Sea Research Part II: Topical Studies in Oceanography 56 (8): 578–590.
Childers, A.R., T.E. Whitledge, and D.A. Stockwell. 2005. Seasonal and interannual variability in the distribution of nutrients and chlorophyll a across the Gulf of Alaska shelf: 1998–2000. Deep Sea Research Part II: Topical Studies in Oceanography 52 (1–2): 193–216.
Danielson, S.L., T.D. Hennon, D.H. Monson, R.M. Suryan, R.W. Campbell, S.J. Baird, K. Holderied, and T.J. Weingartner. 2022. Temperature variations in the northern Gulf of Alaska across synoptic to century-long time scales. Deep Sea Research Part II: Topical Studies in Oceanography 203: 105155.
Davison, I.R. 1991. Environmental effects on algal photosynthesis: Temperature. Journal of Phycology 27 (1): 2–8.
Dethier, M.N., A.S. Brown, S. Burgess, M.E. Eisenlord, A.W.E. Galloway, J. Kimber, A.T. Lowe, C.M. O’Neil, W.W. Raymond, and E.A. Sosik. 2014. Degrading detritus: Changes in food quality of aging kelp tissue varies with species. Journal of Experimental Marine Biology and Ecology 460: 72–79.
Du, X., and W.T. Peterson. 2014. Seasonal cycle of phytoplankton community composition in the coastal upwelling system off central Oregon in 2009. Estuaries and Coasts 37: 299–311.
Duggins, D.O., and J.E. Eckman. 1997. Is kelp detritus a good food for suspension feeders? Effects of kelp species, age and secondary metabolites. Marine Biology 128 (3): 489–495.
Duggins, D.O., C.A. Simenstad, and J.A. Estes. 1989. Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245: 170–173.
Estes, J.A., and P.D. Steinberg. 1988. Predation, herbivory, and kelp evolution. Paleobiology 14 (1): 19–36.
Evans, W., and J.T. Mathis. 2013. The Gulf of Alaska coastal ocean as an atmospheric CO2 sink. Continental Shelf Research 65: 52–63.
Evans, T.G., and G.N. Somero. 2010. Phosphorylation events catalyzed by major cell signaling proteins differ in response to thermal and osmotic stress among native (Mytilus californianus and Mytilus trossulus) and invasive (Mytilus galloprovincialis) species of mussels. Physiological and Biochemical Zoology 83 (6): 984–996.
Feehan, C.J., B.C. Grauman-Boss, R.R. Strathmann, M.N. Dethier, and D.O. Duggins. 2018. Kelp detritus provides high-quality food for sea urchin larvae. Limnology and Oceanography 63 (S1): S299–S306.
Fellman, J.B., R.G.M. Spencer, P.J. Hernes, R.T. Edwards, D.V. D’Amore, and E. Hood. 2010. The impact of glacier runoff on the biodegradability and biochemical composition of terrigenous dissolved organic matter in near-shore marine ecosystems. Marine Chemistry 121 (1): 112–122.
França, S., R.P. Vasconcelos, S. Tanner, C. Máguas, M.J. Costa, and H.N. Cabral. 2011. Assessing food web dynamics and relative importance of organic matter sources for fish species in two Portuguese estuaries: A stable isotope approach. Marine Environmental Research 72 (4): 204–215.
France, R. 1995. Carbon-13 enrichment in benthic compared to planktonic algae: Foodweb implications. Marine Ecology Progress Series 124: 307–312.
Fredriksen, S. 2003. Food web studies in a Norwegian kelp forest based on stable isotope (δ13C and δ15N) analysis. Marine Ecology Progress Series 260: 71–81.
Frölicher, T.L., E.M. Fischer, and N. Gruber. 2018. Marine heatwaves under global warming. Nature 560: 360–364.
Fuentes-Santos, I., U. Labarta, and M.J. Fernández-Reiriz. 2018. Characterizing individual variability in mussel (Mytilus galloprovincialis) growth and testing its physiological drivers using Functional Data Analysis. PLoS ONE 13 (10): e0205981.
Gao, G., X. Zhao, M. Jiang, and L. Gao. 2021. Impacts of marine heatwaves on algal structure and carbon sequestration in conjunction with ocean warming and acidification. Frontiers in Marine Science 8: 758651.
Gay, Shelton, and M., and S. L. Vaughan. 2001. Seasonal hydrography and tidal currents of bays and fjords in Prince William Sound, Alaska. Fisheries Oceanography 10: 159–193.
Gouvêa, L.P., N. Schubert, C.D.L. Martins, M. Sissini, F. Ramlov, and E. R. de O. Rodrigues, E. O. Bastos, V. C. Freire, M. Maraschin, J. Carlos Simonassi, D. A. Varela, D. Franco, V. Cassano, A. L. Fonseca, B. Barufi J, and P. A. Horta. 2017. Interactive effects of marine heatwaves and eutrophication on the ecophysiology of a widespread and ecologically important macroalga. Limnology and Oceanography 62 (5): 2056–2075.
Grant, J., and C. Bacher. 1998. Comparative models of mussel bioenergetics and their validation at field culture sites. Journal of Experimental Marine Biology and Ecology 219 (1–2): 21–44.
Guo, F., M.J. Kainz, F. Sheldon, and S.E. Bunn. 2016. The importance of high-quality algal food sources in stream food webs – Current status and future perspectives. Freshwater Biology 61 (6): 815–831.
Hawkins, A.J.S. 1985. Relationships between the synthesis and breakdown of protein, dietary absorption and turnovers of nitrogen and carbon in the blue mussel. Mytilus Edulis l. Oecologia 66 (1): 42–49.
Helmuth, B.S.T. 1998. Intertidal mussel microclimates: Predicting the body temperature of a sessile invertebrate. Ecological Monographs 68 (1): 51–74.
Hildreth, W., J. Fierstein, M.A. Lanphere, and D.F. Siems. 1999. Snowy Mountain: A pair of small andesite-dacite stratovolcanoes in Katmai National Park. Geologic Studies in Alaska by the US Geological Survey 1633: 13–34.
Hill, J., and C. McQuaid. 2009. Effects of food quality on tissue-specific isotope ratios in the mussel Perna perna. Hydrobiologia 635: 81–94.
Hoegh-Guldberg, O.V.E., and J.S. Pearse. 1995. Temperature, food availability, and the development of marine invertebrate larvae. American Zoologist 35 (4): 415–425.
Hoffman, J.C., M.E. Sierszen, and A.M. Cotter. 2015. Fish tissue lipid - C: N relationships for correcting δ13C values and estimating lipid content in aquatic food-web studies. Rapid Communications in Mass Spectrometry 29 (21): 2069–2077.
Iken, K., and B. Konar. 2022. Long-term monitoring of ecological communities in Kachemak Bay, 2012–2022, Gulf Watch Alaska Nearshore Component. Research Workspace. https://doi.org/10.24431/rw1k1o.
Iken, K. 2024a. Stable carbon and nitrogen isotope data of nearshore producers and consumers in four regions of the Gulf of Alaska, 2014–2023. Research Workspace. https://doi.org/10.24431/rw1k8e2.
Iken, K. 2024b. Growth measurements of mussels and fish in the Gulf of Alaska, 2014–2021. Research Workspace. https://doi.org/10.24431/rw1k8e3.
Ji, Y., and K. Gao. 2021. Effects of climate change factors on marine macroalgae: A review. Advances in Marine Biology 88: 91–136.
Johnson, S.W., J.F. Thedinga, A.D. Neff, P.M. Harris, M.R. Lindeberg, J.M. Maselko, and S.D. Rice. 2010. Fish assemblages in nearshore habitats of Prince William Sound. Alaska. Northwest Science 84 (3): 266–280.
Jones, J.A., and C.T. Driscoll. 2022. Long-term ecological research on ecosystem responses to climate change. BioScience 72 (9): 814–826.
Kaehler, S., E. Pakhomov, R. Kalin, and S. Davis. 2006. Trophic importance of kelp-derived suspended particulate matter in a through-flow sub-Antarctic system. Marine Ecology Progress Series 316: 17–22.
Konar, B., K. Iken, J.J. Cruz-Motta, L. Benedetti-Cecchi, A. Knowlton, G. Pohle, P. Miloslavich, M. Edwards, T. Trott, E. Kimani, R. Riosmena-Rodriguez, M. Wong, S. Jenkins, A. Silva, I.S. Pinto, and Y. Shirayama. 2010. Current patterns of macroalgal diversity and biomass in northern hemisphere rocky shores. PLoS ONE 5 (10): e13195.
Kopp, D., S. Lefebvre, M. Cachera, M.C. Villanueva, and B. Ernande. 2015. Reorganization of a marine trophic network along an inshore–offshore gradient due to stronger pelagic–benthic coupling in coastal areas. Progress in Oceanography 130: 157–171.
Kristensen, E., F.Ø. Andersen, and T.H. Blackburn. 1992. Effects of benthic macrofauna and temperature on degradation of macroalgal detritus: The fate of organic carbon. Limnology and Oceanography 37 (7): 1404–1419.
Krumhansl, K.A., and R.E. Scheibling. 2012. Production and fate of kelp detritus. Marine Ecology Progress Series 467: 281–302.
LaBarre, A., B. Konar, and K. Iken. 2023. Influence of environmental conditions on Mytilus trossulus size frequency distributions in two glacially influenced estuaries. Estuaries and Coasts 46 (5): 1253–1268.
Leica. 2020. Leica Application Suite software. Wetzkarm, Germany: Leica Microsystems. Version 4.12.0. https://www.leica-microsystems.com/products/microscope-software/p/leica-application-suite/.
Lesser, M.P., J.D. Witman, and K.P. Sebnens. 1994. Effects of flow and seston availability on scope for growth of benthic suspension-feeding invertebrates from the Gulf of Maine. The Biological Bulletin 187 (3): 319–335.
Lesser, M.P., M.A. Bailey, D.G. Merselis, and J.R. Morrison. 2010. Physiological response of the blue mussel Mytilus edulis to differences in food and temperature in the Gulf of Maine. Comparative Biochemistry and Physiology Part a: Molecular & Integrative Physiology 156 (4): 541–551.
Love, M. 2011. Certainly more than you want to know about the fishes of the Pacific coast. First edition. Really Big Press.
Marin, M., M. Feng, H.E. Phillips, and N.L. Bindoff. 2021. A global, multiproduct analysis of coastal marine heatwaves Distribution characteristics and long‐term trends. Journal of Geophysical Research Oceans 126 (2): e2020JC016708.
Markel, R.W., and J.B. Shurin. 2018. Indirect effects of sea otters on rockfish (Sebastes spp.) in giant kelp forests. Ecology 96 (11): 2877–2890.
McKinney, R.A., W.G. Nelson, M.A. Charpentier, and C. Wigand. 2001. Ribbed mussel nitrogen isotope signatures reflect nitrogen sources in coastal salt marshes. Ecological Applications 11 (1): 203–214.
McManus, G.B., H. Zhang, and S. Lin. 2004. Marine planktonic ciliates that prey on macroalgae and enslave their chloroplasts. Limnology and Oceanography 49 (1): 308–313.
McPherson, M.L., D.J.I. Finger, H.F. Houskeeper, T.W. Bell, M.H. Carr, L. Rogers-Bennett, and R.M. Kudela. 2021. Large-scale shift in the structure of a kelp forest ecosystem co-occurs with an epizootic and marine heatwave. Communications Biology 4 (1): 1–9.
Millstein, J., and C.E. O’Clair. 2001. Comparison of age-length and growth-increment general growth models of the Schnute type in the Pacific Blue Mussel, Mytilus trossulus Gould. Journal of Experimental Marine Biology and Ecology 262 (2): 155–176.
Moulton, L. L. 1977. An ecological analysis of fishes inhabiting the rocky nearshore regions of northern Puget Sound, Washington. PhD Thesis, University of Washington.
O’Clair, R.M., and C.E. O’Clair. 1998. Southeast Alaska’s rocky shores: Animals. Auke Bay, Alaska: Plant Press.
Oliver, E.C., M.T. Burrows, M.G. Donat, A. Sen Gupta, L.V. Alexander, S.E. Perkins-Kirkpatrick, J.A. Benthuysen, A.J. Hobday, N.J. Holbrook, and P.J. Moore. 2019. Projected marine heatwaves in the 21st century and the potential for ecological impact. Frontiers in Marine Science 6: 734.
Page, H.M., and D.M. Hubbard. 1987. Temporal and spatial patterns of growth in mussels Mytilus edulis on an offshore platform: Relationships to water temperature and food availability. Journal of Experimental Marine Biology and Ecology 111 (2): 159–179.
Parker, S.J., P.S. Rankin, J.M. Olson, and R.W. Hannah. 2007. Movement patterns of black rockfish (Sebastes melanops) in Oregon coastal waters. In Biology, assessment, and management of North Pacific rockfishes, 39–57. Alaska Sea Grant: University of Alaska Fairbanks. Alaska Sea Grant publication AK-SG-07-01.
Perga, M.E., and D. Gerdeaux. 2005. ‘Are fish what they eat’ all year round? Oecologia 144 (4): 598–606.
Peterson, B.J. 1999. Stable isotopes as tracers of organic matter input and transfer in benthic food webs: A review. Acta Oecologica 20 (4): 479–487.
Petes, L.E., B.A. Menge, and A.L. Harris. 2008. Intertidal mussels exhibit energetic trade-offs between reproduction and stress resistance. Ecological Monographs 78 (3): 387–402.
Piatt, J.F., J.K. Parrish, H.M. Renner, S.K. Schoen, T.T. Jones, M.L. Arimitsu, K.J. Kuletz, B. Bodenstein, M. García-Reyes, and R.S. Duerr. 2020. Extreme mortality and reproductive failure of common murres resulting from the northeast Pacific marine heatwave of 2014–2016. PLoS ONE 15 (1): e0226087.
Post, D.M. 2002. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83 (3): 703–718.
R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/.
Renaud, P.E., T.S. Løkken, L.L. Jørgensen, J. Berge, and B.J. Johnson. 2015. Macroalgal detritus and food-web subsidies along an Arctic fjord depth-gradient. Frontiers in Marine Science 2: 1–15.
Robinson, B., H. A. Coletti, B. Ballachey, J. L. Bodkin, K. Kloecker, S. B. Traiger, and D. Esler. 2023. Lack of strong responses to the Pacific marine heatwave by benthivorous marine birds indicates importance of trophic drivers. Marine Ecology Progress Series.
Rogers-Bennett, L., and C.A. Catton. 2019. Marine heat wave and multiple stressors tip bull kelp forest to sea urchin barrens. Scientific Reports 9 (1): 15050.
Román, M., S. Román, E. Vázquez, J. Troncoso, and C. Olabarria. 2020. Heatwaves during low tide are critical for the physiological performance of intertidal macroalgae under global warming scenarios. Scientific Reports 10 (1): 1–14.
Rombough, P.J. 1994. Energy partitioning during fish development: Additive or compensatory allocation of energy to support growth? Functional Ecology 8 (2): 178–186.
Ruckelshaus, M.H., R.C. Wissmar, and C.A. Simenstad. 1993. The importance of autotroph distribution to mussel growth in a well-mixed, temperate estuary. Estuaries 16 (4): 898–912.
Santelices, B., and J. Correa. 1985. Differential survival of macroalgae to digestion by intertidal herbivore molluscs. Journal of Experimental Marine Biology and Ecology 88 (2): 183–191.
Schloemer, J. W., L. A. Munk, and K. Iken. 2023. Marine and not terrestrial resources support nearshore food webs across a gradient of glacial watersheds in the Northern Gulf of Alaska. Estuaries and Coasts In press.
Schneider, K.R. 2008. Heat stress in the intertidal: Comparing survival and growth of an invasive and native mussel under a variety of thermal conditions. The Biological Bulletin 215 (3): 253–264.
Schneider, K.R., and B. Helmuth. 2007. Spatial variability in habitat temperature may drive patterns of selection between an invasive and native mussel species. Marine Ecology Progress Series 339: 157–167.
Schneider, C.A., W.S. Rasband, and K.W. Eliceiri. 2012. NIH image to ImageJ: 25 years of image analysis. Nature Methods 9 (7): 671–675.
Shelley, C.E., and D.W. Johnson. 2022. Larval fish in a warming ocean: A bioenergetic study of temperature-dependent growth and assimilation efficiency. Marine Ecology Progress Series 691: 97–114.
Shinen, J.S., and S.G. Morgan. 2009. Mechanisms of invasion resistance: Competition among intertidal mussels promotes establishment of invasive species and displacement of native species. Marine Ecology Progress Series 383: 187–197.
Siegert, D., B. Konar, M.R. Lindeberg, S. Saupe, and K. Iken. 2022. Trophic structure of key taxa in rocky intertidal communities in two contrasting high-latitude environments. Deep Sea Research Part II: Topical Studies in Oceanography 198: 105050.
Simenstad, C.A., and R.C. Wissmar. 1985. δ13C evidence of the origins and fates of organic carbon in estuarine and nearshore food webs. Marine Ecology Progress Series 22: 141–152.
Smaal, A.C., and M.R. van Stralen. 1990. Average annual growth and condition of mussels as a function of food source. In North Sea-Estuaries Interactions, ed. D.S. McLusky, V.N. de Jonge, and J. Pomfret, 179–188. Springer.
Steeves, L.E., R. Filgueira, T. Guyondet, J. Chassé, and L. Comeau. 2018. Past, present, and future: Performance of two bivalve species under changing environmental conditions. Frontiers in Marine Science 5: 1–14.
Steinberg, P.D. 1988. Effects of quantitative and qualitative variation in phenolic compounds on feeding in three species of marine invertebrate herbivores. Journal of Experimental Marine Biology and Ecology 120 (3): 221–237.
Stock, B.C. and B.X. Semmens. 2016. MixSIAR GUI user manual. Version 3.1. R. https://github.com/brianstock/MixSIAR.0.5281/zenodo.1209993.
Su, Y.S., and M. Yajima. 2015. R2jags: Using R to run ‘JAGS’. R Package Version 0.7–1.
Tallis, H. 2009. Kelp and rivers subsidize rocky intertidal communities in the Pacific Northwest (USA). Marine Ecology Progress Series 389: 85–96.
Therriault, T.W., J.C. Nelson, J.T. Carlton, L. Liggan, M. Otani, H. Kawai, D. Scriven, G.M. Ruiz, and C.C. Murray. 2018. The invasion risk of species associated with Japanese tsunami marine debris in Pacific North America and Hawaii. Marine Pollution Bulletin 132: 82–89.
Timmerman, C.-A., C. Giraldo, P. Cresson, B. Ernande, M. Travers-Trolet, M. Rouquette, M. Denamiel, and S. Lefebvre. 2021. Plasticity of trophic interactions in fish assemblages results in temporal stability of benthic-pelagic couplings. Marine Environmental Research 170: 105412.
Tomanek, L., and M.J. Zuzow. 2010. The proteomic response of the mussel congeners Mytilus galloprovincialis and M trossulus to acute heat stress Implications for thermal tolerance limits and metabolic costs of thermal stress. Journal of Experimental Biology 213 (20): 3559–3574.
Traiger, S.B., J.L. Bodkin, H.A. Coletti, B. Ballachey, T. Dean, D. Esler, K. Iken, B. Konar, M.R. Lindeberg, D. Monson, B. Robinson, R.M. Suryan, and B.P. Weitzman. 2022. Evidence of increased mussel abundance related to the Pacific marine heatwave and sea star wasting. Marine Ecology 43 (4): e12715.
USGS Alaska Science Center, National Park Service Southwest Alaska Inventory and Monitoring Network. 2022. Rocky intertidal data from Prince William Sound, Katmai National Park and Preserve, and Kenai Fjords National Park (ver. 2.0, October 2023): U.S. Geological Survey data release, https://doi.org/10.5066/F7513WCB.
USGS Alaska Science Center, National Park Service Southwest Alaska Inventory and Monitoring Network, and University of Alaska Fairbanks College of Fisheries and Ocean Sciences. 2016. Intertidal Temperature Data from Kachemak Bay, Prince William Sound, Katmai National Park and Preserve, and Kenai Fjords National Park (ver. 4.0, September 2023). U.S. Geological Survey data release, https://doi.org/10.5066/F7WH2N3T.
Valiela, I., J. McClelland, J. Hauxwell, P.J. Behr, D. Hersh, and K. Foreman. 1997. Macroalgal blooms in shallow estuaries Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography 42 (5part2): 1105–1118.
von Biela, V.R., C.E. Zimmerman, B.R. Cohn, and J.M. Welker. 2013. Terrestrial and marine trophic pathways support young-of-year growth in a nearshore Arctic fish. Polar Biology 36 (1): 137–146.
von Biela, V.R., G.H. Kruse, F.J. Mueter, B.A. Black, D.C. Douglas, T.E. Helser, and C.E. Zimmerman. 2015. Evidence of bottom-up limitations in nearshore marine systems based on otolith proxies of fish growth. Marine Biology 162 (5): 1019–1031.
von Biela, V.R., S.D. Newsome, J.L. Bodkin, G.H. Kruse, and C.E. Zimmerman. 2016. Widespread kelp-derived carbon in pelagic and benthic nearshore fishes suggested by stable isotope analysis. Estuarine, Coastal and Shelf Science 181: 364–374.
von Biela, V.R., M.L. Arimitsu, J.F. Piatt, B. Heflin, S.K. Schoen, J.L. Trowbridge, and C.M. Clawson. 2019. Extreme reduction in nutritional value of a key forage fish during the Pacific marine heatwave of 2014–2016. Marine Ecology Progress Series 613: 171–182.
Weidel, B.C., S.R. Carpenter, J.F. Kitchell, and M.J. Vander Zanden. 2011. Rates and components of carbon turnover in fish muscle: Insights from bioenergetics models and a whole-lake 13C addition. Canadian Journal of Fisheries and Aquatic Sciences 68 (3): 387–399.
Weitzman, B., B. Konar, K. Iken, H. Coletti, D. Monson, R. Suryan, T. Dean, D. Hondolero, and M. Lindeberg. 2021. Changes in rocky intertidal community structure during a marine heatwave in the northern Gulf of Alaska. Frontiers in Marine Science 8: 556820.
Wernberg, T., D.A. Smale, T.L. Frölicher, and A.J.P. Smith. 2021. Science brief review: climate change increases marine heatwaves harming marine ecosystems. In Critical issues in climate change science, ed. P. Liss, C. Le Quéré, and P. Forster. https://doi.org/10.5281/zenodo.5596820.
Wyatt, A.M., L. Resplandy, and A. Marchetti. 2022. Ecosystem impacts of marine heat waves in the northeast Pacific. Biogeosciences 19 (24): 5689–5705.
Acknowledgements
We thank the scientific team of the Gulf Watch Alaska long-term monitoring program for their expertise and resources in support of this publication. We thank our numerous colleagues at the U.S. Geological Survey (USGS) and the University of Alaska Fairbanks (UAF) for assistance in the field and fruitful discussions, especially Brenda Konar and Sarah Mincks (UAF), Dan Monson and Brian Robinson (USGS), Mandy Lindeberg (NOAA), and many other colleagues in the field. We thank two anonymous reviewers for their constructive comments.
Funding
The research described in this publication was supported by the Exxon Valdez Oil Spill Trustee Council through the Gulf Watch Alaska Program Restoration Project 21120114-H. Additional funding was provided by the National Park Service, the University of Alaska Fairbanks (UAF) Dr. Donald Hood Memorial Scholarship and Robert and Kathleen Byrd Award, Alaska EPSCoR NSF award #OIA-1757348, and the State of Alaska. This research was approved by the UAF Institutional Animal Care and Use Committee under project number 1725779. Samples were collected under the Alaska Department of Fish and Game Aquatic Resource Permit CF-21–044.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The findings and conclusions presented by the authors are their own and do not necessarily reflect the views or position of the Trustee Council. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Additional information
Communicated by Ken Dunton
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corliss, K., von Biela, V., Coletti, H. et al. Relative Importance of Macroalgae and Phytoplankton to Nearshore Consumers and Growth Across Climatic Conditions in the Northern Gulf of Alaska. Estuaries and Coasts (2024). https://doi.org/10.1007/s12237-024-01371-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12237-024-01371-6