Abstract
The consequences of warming-induced ‘shrubification’ on Arctic soil carbon storage are receiving increased attention, as the majority of ecosystem carbon in these systems is stored in soils. Soil carbon cycles in these ecosystems are usually tightly coupled with nitrogen availability. Soil microbial responses to ‘shrubification’ may depend on the traits of the shrub species that increase in response to warming. Increase in deciduous shrubs such as Betula nana likely promotes a loss of soil carbon, whereas the opposite may be true if evergreen shrubs such as Empetrum hermaphroditum increase. We analyzed soil organic matter stocks and 13C NMR fractions, microbial CO2 respiration, biomass, extracellular enzyme activities (EEAs), and their association with shrub density in northern Sweden after 20 years of experimental warming using open top chambers (OTCs). Our study sites were located in a tundra heath that stores high soil carbon quantities and where the OTCs had increased deciduous shrubs, and in a mountain birch forest that stores lower soil carbon quantities and where the OTCs had increased evergreen shrubs. We predicted that organic matter stocks should be lower and respiration and EEAs higher inside the OTCs than untreated plots in the tundra, whereas no effect should be detected in the forest. Soil organic matter stocks and 13C NMR fractions remained unaffected at both sites. When expressed as per gram microbial biomass, respiration and EEAs for carbohydrate and chitin degradation were higher inside the OTCs, and contrasting our prediction, this effect was stronger in the forest. Unexpectedly, the OTCs also led to a substantially lower microbial biomass carbon and nitrogen irrespective of habitat. The decline in the microbial biomass counteracted increased activities resulting in no effect of the OTCs on respiration and a lower phenol oxidase activity per gram soil. Microbial biomass nitrogen correlated negatively with evergreen shrub density at both sites, indicating that ‘shrubification’ may have intensified nutrient competition between plants and soil microorganisms. Nutrient limitation could also underlie increased respiration per gram microbial biomass through limiting C assimilation into biomass. We hypothesize that increasing nutrient immobilization into long-lived evergreen shrubs could over time induce microbial nutrient limitation that contributes to a stability of accumulated soil organic matter stocks under climate warming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Soil organic matter stocks and carbon quality were unchanged after 20 years of experimental warming with open-top chambers (OTCs) in a subarctic forest–tundra ecotone when shrubs had substantially increased
-
The OTCs increased respiration and EEAs per gram microbial biomass but simultaneously induced a decline in soil microbial biomass carbon and nitrogen
-
Soil microbial nitrogen correlated negatively with evergreen shrub density, indicating that ‘shrubification’ may have intensified nutrient competition between plants and soil microorganisms
Introduction
Because litter and soil organic matter decomposition in Arctic ecosystems has been slower than plant production for millennia, these ecosystems store substantial quantities of accumulated soil carbon (Jonasson and others 2001; Tarnocai and others 2009). If climate warming accelerates soil carbon decomposition faster than plant production, this could lead to a net release of CO2 into the atmosphere and induce a positive feedback to climate warming (Davidson and Janssens 2006; Hartley and others 2012; Bradford and others 2016). Experimental evidence from field studies offers support for a hypothesis that warming enhances microbial respiration and extracellular enzyme activities (EEAs) catalyzing organic matter depolymerization (Shaver and others 2006; Pendall and others 2008; Rinnan and others 2009; Henry 2012; Sistla and others 2013; Phillips and others 2018) leading to decreased soil organic layer carbon storage (Sistla and others 2013; Phillips and others 2018; Ylänne and others 2020). It is therefore urgent to better understand how contrasting Arctic vegetation communities sequester soil carbon and which mechanisms might trigger the destabilization of accumulated soil carbon (Parker and others 2021).
Although warmer temperatures affect soil processes directly both during (Wallenstein and Hall 2012) and outside (Schimel and others 2004; Buckeridge and Grogan 2008) the growing seasons, over the long term, warming-induced vegetation change starts to indirectly influence the soil environment (Weintraub and Schimel 2005a; Wookey and others 2009). Large parts of the circumpolar region are experiencing an increased distribution and biomass of dwarf and tall shrubs (Myers-Smith and others 2011; Kaarlejärvi and others 2012; Vowles and Björk 2018), which is commonly referred to as Arctic ‘greening’ or ‘shrubification’ (Sturm and others 2005; Myers-Smith and others 2011; Elmendorf and others 2012; Myers-Smith and others 2020). A taller and denser vegetation cover buffers the soil against warmer summer temperatures through increased shading, which cools the soil and reduces the direct temperature effect (Sturm and others 2005; Weintraub and Schimel 2005a). Moreover, ‘shrubification’ enhances soil microbial activities through increasing labile carbon flow via plant root exudation and fresh litter input, which fuels increasing degradation of the accumulated soil carbon (Weintraub and Schimel 2005a; Buckeridge and others 2010; Kardol and others 2010; Deslippe and others 2012; Sistla and Schimel 2013; Street and others 2020; Hicks and others 2021). This process where increasing labile carbon stimulates microbial degradation of accumulated carbon is termed as a ‘priming’ effect (Fontaine and others 2004), which is associated with increased ‘N mining’ to meet increased nitrogen requirements (Moorhead and Sinsabaugh 2006; Craine and others 2007). These phenomena may have a particularly important role in the Arctic (Hicks and others 2020; Street and others 2020; Parker and others 2021), and both correlative (Parker and others 2021; Clemmensen and others 2021; Kemppinen and others 2021; Parker and others 2021) and experimental (Nowinski and others 2008; Street and others 2020) evidence suggests that increased shrub abundance is associated with lower soil carbon stocks. Ultimately, warming in the Fennoscandian Arctic may advance the treeline, leading the treeless tundra to transform into forest cover that is dominated by mountain birch (Betula pubescens ssp. czerepanovii) with an ericaceous shrub understory. Although the consequences of this regime shift vary across regions (Devos and others 2022), mountain birch forest soils in the study area store substantially less soil carbon compared with tundra soils, and the advance of treeline would thus release vast quantities of carbon to the atmosphere (Sjögersten and others 2003; Hartley and others 2012; Clemmensen and others 2021).
However, soil microbial responses to ‘shrubification’ are not entirely straightforward due to the highly varying traits of the plant species that increase in response to climate warming. Increasing deciduous shrub abundance, such as that of Betula nana L. may lead to a lower soil carbon storage, because it enhances labile carbon flow to belowground (Street and others 2020) and its ectomycorrhizal symbionts exhibit a strong capacity for ‘N mining’ (Parker and others 2015; Parker and others 2021) that increase in activity in response to warming (Dunleavy and Mack 2021). Increasing evergreen shrubs, such as the ericoid mycorrhizal mountain crowberry (Empetrum nigrum ssp. hermaphroditum Hagerup), may not induce a similar effect, because they produce slowly decomposable litter with high concentrations of allelopathic compounds that generally decelerate soil nutrient and carbon cycles (van Wijk and others 2003; Wallstedt and others 2005; Bråthen and others 2010; Vowles and Björk 2018). Although ericoid mycorrhizae are highly efficient at acquiring nitrogen from recalcitrant soil organic matter (SOM) and producing oxidative enzymes that catalyze SOM degradation, the presence of highly melanized necromass inputs may also lead to more recalcitrant SOM (Read and Perez-Moreno 2003; Clemmensen and others 2015). The significant and ongoing increase in E. hermaphroditum detected across northern Fennoscandia and the high Arctic (Buizer and others 2012; Kaarlejärvi and others 2012; Vuorinen and others 2017) could drive tundra communities toward slower rather than faster process rates (Bråthen and others 2017; Vowles and Björk 2018). A climate-induced increase in evergreen shrubs has yet another consequence, as it increases the proportion of the total ecosystem C and N stock immobilized above- rather than belowground (Ylänne and others 2015), but the potential role of this phenomenon for soil processes has not been considered.
Here, we analyzed soil microbial activities, litter and soil organic matter stocks, and carbon quality after 20 years of simulated climate warming using open top chambers (OTCs) at a tundra site and a mountain birch forest site located in the forest–tundra ecotone in northernmost Sweden. The two sites were compared due to their varying susceptibilities to soil carbon loss under climate warming. The tundra site stores substantial amounts of soil carbon with a high proportion of chemically labile carbohydrates, which may easily be released into the atmosphere (Sjögersten and others 2003; Hartley and others 2012; Clemmensen and others 2021). In addition, at the tundra site, warming has increased the deciduous shrub B. nana (Kaarlejärvi and others 2012), that is, a species considered to promote increased decomposition of accumulated soil organic matter. By contrast, the mountain birch forest site stores both a lower amount and chemically more recalcitrant soil carbon (Sjögersten and others 2003). Here, the OTCs have mainly increased the evergreen dwarf shrub E. hermaphroditum (Kaarlejärvi and others 2012), whose impact on soil microbial activities should be divergent from that of B. nana (Bråthen and others 2017; Vowles and Björk 2018). We predicted that (1) the soil organic matter stock should be lower after 20 years of ‘shrubification’ in the tundra, but not in the forest, and (2) rates of microbial CO2 respiration and EEAs should be higher under ‘shrubification’ in the tundra, whereas no difference, or even lower rates, should be detected in the forest.
Materials and Methods
Study Area, Experimental Design, and Sampling
The study was conducted in the forest–tundra ecotone in the oroarctic region (Virtanen and others 2016), a few kilometers south of the Abisko Scientific Research Station in northernmost Sweden. The study sites are located across an altitudinal gradient of between 520 and 600 m. The mean annual temperature is − 0.79°C, and the mean annual precipitation is 338 mm. Mountain birch (Betula pubescens Ehrh. subsp. tortuosa (Ledeb.) Nyman) forms the treeline and dominates the forests. The forest–tundra ecotone is a 3–4-km wide mosaic of mountain birch forest patches extending to tundra. In mountain birch forests, the understory is dominated by E. hermaphroditum, Vaccinium myrtillus, V. uliginosum and Deschampsia flexuosa, and the mosses Hylocomium splendens, Pleurozium schreberi, and Dicranum spp. and a few lichens (for example, Cladonia spp.; Kaarlejärvi and others 2012), and the soil is a micro-podzol (spodosol) with a shallow organic layer, underlain by a blue-gray (Munsell chart: 10YR 5/1) eluvial (albic) horizon and a light-orange-colored (Munsell chart: 10YR 4/3) illuvial (spodic) horizon above a coarse glacial till (Hartley and others 2012). The tundra area is dominated by E. hermaphroditum, V. vitis-idaea, and B. nana, V. uliginosum, Arctostaphylos uva-ursi and Festuca ovina, with Cladonia mitis, Cetraria cucullata, Nephroma arctica and Ptilidium ciliare at the bottom layer, and the soil is coarse glacial till with large clasts and only occasional pockets of fine-grained material (Hartley and others 2012).
The mountain birch forest and tundra sites used in this study (see photographs in Supplementary Figure 1) belong to a network of experiments established in mountain birch forests and tundra heath patches in 1998 (Sjögersten and others 2003; Kaarlejärvi and others 2012). We used seven control plots of 1 m2 and seven experimental warming plots randomly set out at the forest site, and eight control plots and warmed plots at the tundra site. The warming treatment was conducted using International Tundra Experiment (ITEX) hexagonal Open Top Chambers (hereafter OTCs) with a maximum basal diameter of 146 cm. On average, the OTCs increase air temperatures by 1.5–1.9°C and soil temperatures by 0.6–1.1°C, corresponding to predicted climate conditions in 2050 (Elmendorf and others 2012), while also decreasing wind speed and increasing air humidity (Bokhorst and others 2013). At the early phases of the experiment, OTCs surface air temperatures in July increased by 0.8 and 2.5°C in the forest and the tundra sites, respectively (Sjögersten and others 2003). We do not have air or soil surface temperature data from the time of the present investigation, but have no reason to believe that the OTCs would not warm the air temperatures also presently. The average soil temperature (measured every 2 h at 3 cm depth, 8th June–20th August 2018; EasyLog EL-USB-1, Lascar Electronics, Whiteparish) was 9.1 ± 0.4 and 9.4 ± 0.2 in control and OTC plots in the forest site, and 8.7 ± 0.3 and 8.1 ± 0.5 in control and OTC plots in the tundra site, respectively. Increased plant abundances insulated the ground to an extent that the OTCs experienced similar soil temperatures as the control plots in the forest, and even lower daily temperature maximums in the tundra site (Supplementary Figure 2), which aligns with earlier concepts that ‘shrubification’ exerts an important indirect impact on the soil microclimate (Weintraub and Schimel 2005a; Wookey and others 2009).
We took multiple soil samples (4–7 soil cores, diameter 2.5 cm) within each plot, which were then pooled to form one composite sample per plot during the early growing season (June 6), mid-growing season (July 11), and late growing season (August 17) in 2018. We separated out each core to the following layers: (1) litter layer (plant leaves and biomass fragments on the ground), (2) organic soil layer, and (3) mineral soil layer. We sampled the mineral soil layer until the corer hit a stone. The thicknesses of organic and mineral layers were recorded during sampling. At the tundra site with a thicker organic layer, the top 5 cm of each core (consequently referred to as “surface organic soil”) was further separated from the lower layer of the organic layer (consequently referred to as “bottom organic soil”). This separation was done to ensure that the detectable effect of warming would not be overshadowed by the more inert layer below most of the plant roots. Samples were homogenized (mesh size, 2 mm) during the day of sampling and stored at + 4°C before analyses that were finished within 3–4 days of sampling.
Soil Moisture, Organic Matter Content and Stock, pH, and C Quality
All samples were analyzed for soil moisture (12 h, 105°C; calculated as a percentage of soil fresh weight) and soil organic matter content (OMC%, loss on ignition, 475°C, 4 h; calculated as a percentage of soil dry weight). The sampling area and the total sample weight were used to calculate soil organic matter stocks individually for litter, organic, and mineral layer (g SOM m−2) and bulk density of the surface organic soil layer (g dry soil dm–3). Soil pH in the surface organic soil layer was measured in 3:5 v/v soil:water suspensions using distilled water, shaking and leaving samples stand overnight before measuring soil pH (Denver Instrument Model 220).
We determined the chemical structures of organic carbon in the July soil samples by cross-polarized magic angle spinning (CPMAS) NMR. The samples were first demineralized (see Supplementary Methods) to increase signal-to-noise ratio (Gélinas and others 2001). However, this had no influence on the spectra; therefore, we did not do this treatment subsequently but analyzed the samples directly. 13C CPMAS NMR spectra were measured with a Bruker Avance III 300 spectrometer, showing a 75.5 MHz resonance frequency for 13C. The magic angle spinning rate was 5 kHz. For all the spectra, 10,000 scans were accumulated using an acquisition time of 20 ms, a spectral width of 662 ppm, a ramped contact pulse of 1 ms, a relaxation delay of 2 s, and a Spinal64 proton decoupling during the signal acquisition. This meant an experiment duration of 5 h 38 min for each sample. Line broadening of 40 Hz was used to process the spectra. The spectra were referenced with respect to the glycine signal at 176 ppm. The resulting spectra were segmented into the following seven regions, with their integrals being proportional to the amount of corresponding functional classes of C (Väisänen and others 2015): aliphatic not-O-substituted: 0–50 ppm; methoxyls: 50–60 ppm; carbohydrates: 60–90 ppm; carbohydrates and aliphatic lignin: 90–110 ppm, aromatic lignin: 110–160 ppm; carboxyl/carbonyl: 160–210 ppm. The integrals of the regions are expressed as a percentage of the total integral, and therefore, they represent approximately the percentage of the functional class of the total C. The CPMAS integrals are not quantitative as, with the polarization transfer, we get relatively more intensity for the carbons that have more nearby protons. However, they reflect accurately the differences between the samples.
Soil and Microbial C and N Concentrations
A sub-sample of ~ 3 g fresh soil was extracted for 2 h with 50 mL of 0.5 M K2SO4. Dissolved organic carbon (DOC) concentrations in these extracts were analyzed with TOC-VCPH/N Total Organic Carbon Analyzer (Shimadzu Corporation, Kyoto, Japan). NH4-N and NO3-N concentrations were analyzed via flow injection analysis (Quickchem 8000 FIA Analyzer, A83200, Zellweger Analytics, USA). NO3-N concentrations were below detection limit and are therefore not presented in the results. Extractable organic N was calculated by subtracting inorganic N concentrations from the total. Microbial C and N were extracted from the samples using 0.5 M K2SO4 after chloroform fumigation for 18 h (Brookes and others 1985) and then analyzed as total extractable N and DOC. Microbial C and N were calculated from the difference between unfumigated and fumigated extracts. Chloroform fumigations, K2SO4-extractions and flow injection analyses for extractable organic N and DOC, were later repeated from stored frozen soil samples to check for potential problems in the first analyses. The re-analyses showed the same results for N but indicated that there had been a systematic error in the latter part of the original run for DOC resulting in overly high values. DOC values from the re-analyses were used in the final results. Because the error in the first run was systematic, we could calculate a correction coefficient derived from the average difference in values between the first and the second run for those samples that had run out of material (ca 40%). As the original error was systematic, the re-analyses did not change the detected difference between habitats and treatments.
Microbial Respiration and Extracellular Enzyme Activities
We analyzed microbial respiration as a proxy for organic matter decomposition, which includes various sources such as respiration for growth, maintenance, overflow respiration, and synthesizing enzymes (Schimel and Weintraub 2003). Soil samples (3 g fresh weight) were pre-incubated for 2 days in the laboratory in 120-mL glass vials and incubated for 14 days at a range of incubation temperatures (4°C, 9°C, 14°C) in field moisture. Gas samples (250 µL) were taken from the headspace every 48 h of incubation. The CO2 concentration of the gas sample was analyzed with an Agilent 6890N GC equipped with a ShinCarbon ST 100/120 mesh 2 m × 1 mm ID micropacked column (Restek) and thermal conductivity detector. Respiration rates at different temperatures were used to calculate the Q10 value (Wallenstein and others 2009).
As the soil microbial potential for organic compound depolymerization, a major driver of soil carbon decomposition (Schimel and Weintraub 2003; Allison 2006; Schmidt and others 2011), we analyzed potential EEAs on fresh soils in optimal substrate conditions to provide a metric for detecting differences in enzyme pool size between treatments and time points (Wallenstein and Weintraub 2008). Enzyme activities depict the capacity of soil microorganisms to depolymerize organic substrates, an important determinant for soil carbon decomposition (Schimel and Weintraub 2003). As climate warming may also impact the temperature adaptation of the soil microbial community (Wallenstein and others 2011), we used three different temperatures (4°C, 9°C, 14°C) to calculate Q10 values (Wallenstein and others 2009). We used a microplate method with the following chromogenic substrates: β-glucosidase (BG, paranitrophenyl(pNP)-β-glucopyranoside, 5 mM), cellobiase hydrolase (CBH; 2 mM pNP-cellobioside), β-N-acetylglucosaminidase (NAG, pNP-β-N-acetylglucosaminide, 2 mM), leucine aminopeptidase (LAP, leucine p-nitroanilide, 5 mM), acid-phosphatase (AP, pNP-phosphate, 5 mM), and phenol oxidase (PO, pyrogallol, 50 mM) (Martz and others 2016). BG, CBH, NAG, LAP, and AP catalyze reactions that hydrolyze the terminal linkages of oligomers released from polymers. BG and CBH release glucose and cellobiose from cellulose, NAG hydrolyzes N-acetyl glucosamide residues from chitin-derived oligomers, LAP catalyzes the hydrolytic release of leucine and other amino acids from peptides, AP catalyzes the release of phosphate by hydrolyzing the phosphoric ester bonds of phosphate groups in organic molecules, and PO catalyzes oxidative reactions in the decomposition of phenols (Sinsabaugh and others 2008).
The stoichiometric requirement of carbon and nutrients for soil microorganisms is generally an important determinant for the effects of global changes (Sistla and others 2014). EEAs were categorized based on whether they catalyze the degradation of organic compounds that contain only carbon (for example, BG, CBH) or compounds that contain both carbon and nitrogen (for example, NAG, LAP), after which the enzymatic C:N stoichiometric ratio was calculated (Sinsabaugh and others 2008). This deviation is not absolute, because enzymes degrading chitin and proteins may be synthesized either for microbial N- or C-acquisition (Henry 2012; Koyama and others 2013) and, along with their resource requirement, are promoted by the availability of chitin and proteins in the soil (Hernandez and Hobbie 2010; Kielak and others 2013; Zeglin and others 2013). We also calculated BG:PO ratio, used as an index for microbial potential to degrade carbohydrates versus phenols (Sinsabaugh and Follstad Shah 2011). PO may be associated with ‘N mining’ (Sinsabaugh 2010; Chen and others 2017), but also catalyzes decomposition of easily decomposable phenolics (Fierer and others 2001; Sinsabaugh 2010).
A soil homogenate (2 g fresh soil, 30 mL of MilliQ-water) was prepared, and 100-μL aliquots were used for assay samples and homogenate controls. For substrate controls, 100-µL aliquots of a sodium acetate buffer (50 mM, pH 5.0) were added. For assay samples and substrate controls, 200 µL of each substrate (BG, CBH, NAG, AP, LAP, PO) was added, and 200 µL of buffer was added for homogenate controls. The samples were incubated and centrifuged, and the supernatants analyzed as the oxidation of chromogenic substrates (405 nm, Multiscan FC microplate reader, Thermo Scientific). Five microliters of 1.0 M NaOH was added to samples with paranitrophenyl-based substrates (AP, BG and NAG) prior to measurement to halt the reaction and cause color development. The absorbances of homogenate and substrate controls were subtracted from the assay absorbance. Extinction coefficients for calculating final EEAs were obtained based on standard curves for paranitrophenol (BG, NAG, AP), paranitroaniline (LAP), and the oxidation of pyrogallol by mushroom tyrosinase (PO).
Vegetation Analyses
The point intercept method was used with a total of 87 vertical pins systematically distributed along the three diagonals of OTC and control plots (29 per diagonal) (Kaarlejärvi and others 2012). The total number of the hits per species per pin was recorded individually for each of the pins. Here, we present the total plant density, that is the sum of all hits, E. hermaphroditum and B. nana, to analyze the relationships among vegetation, soil microbial activities, and organic matter stocks. The results were normalized as touches per 100 pins.
Statistical Analyses
The effects of the OTCs, habitat, month, and their interactions on soil N and C concentrations, microbial respiration, and EEAs were tested using the linear mixed model with treatment, habitat, and month as fixed factors and plot as a random factor. A month was assigned as a repeated factor with the plot as the subject. For repeated factors, we used either a diagonal covariance structure or first-order autoregressive structure (AR1) with homogenous variances depending on which model gave the best fit based on Akaike’s criterion. The effects of the OTCs and habitat, and their interactions, on SOM stocks and 13C-NMR fractions were tested using the linear mixed model with treatment and habitat as fixed factors. Logarithmic transformations were used as necessary to meet the assumptions of ANOVA or the linear mixed model. To test for associations between plant densities and microbial biomass and respiration, we used linear mixed effect models using forward selection (that is, explanatory variables added one at a time until the best model, based on AIC, was found) separately for habitat. Pearson’s correlation was used to check possible autocorrelation among explanatory variables. Statistical analyses were conducted using SPSS 26 Statistical Software.
Results
Litter and Soil Organic Matter Stocks and Soil Carbon Fractions
The total organic matter stock was over two times larger in the tundra site than in the forest site (Table 1, Figure 1). Of different layers, the litter organic matter stock was two times higher and the organic layer stock was 3.4 times higher in the tundra site when compared to the forest site (Table 1, Figure 1). The OTCs saw an increase in organic matter stock in the litter layer by 57% at the tundra site while there was no perceived effect in the forest site (OTC × Habitat interaction; Table 1, Figure 1). There was no effect of the OTCs on the soil organic and mineral layer organic matter stocks, or the total stock (Table 1, Figure 1). 13C NMR analyses showed that the proportion of carbohydrate-C in the surface organic layer was 14% higher in the tundra than in the forest, whereas the opposite was true for aliphatic-C (Table 1, Figure 1). Yet, OTCs had no effect on the organic fractions (Table 1, Figure 1).
Organic matter stocks in the litter layer, the organic soil layer and the mineral soil layer, and carbon fractions in the surface organic soil layer (top 5 cm) in untreated plots (control) and after 20 years of experimental warming (OTC) in a mountain birch forest and a tundra heath in Abisko, northern Sweden. Values are mean, N = 7 in the forest site, N = 8 in the tundra site.
Soil organic layer thickness and OMC% in the surface organic layer showed a similar pattern as the organic matter stock (Tables 1, 2). There were no effects of habitat or the OTCs on the bulk density, and the soil pH was higher in the tundra site than in the forest site (Tables 1, 2). Soil moisture was at its lowest during the early growing season and at its highest during late growing season (main effect of Month; Tables 2, 3). On average, soils were about 20% drier in the forest site than in the tundra site and 7% drier inside the OTCs than in the control plots (main effects of Habitat and OTC; Tables 2, 3).
Soil and Microbial C and N Concentrations
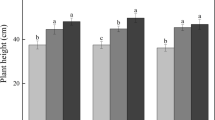
Microbial biomass C concentration, analyzed in surface organic soil layer, was lower inside the OTCs, and in the tundra site this difference was particularly strong during the mid-growing season (main effect of OTC, OTC × Month and Habitat × Month interactions; Table 3, Figure 2). The concentration of DOC was 70% higher in the forest site than in the tundra site, and it was not affected by the OTCs (Tables 2, 3). Soil extractable organic N and microbial N concentrations followed same seasonal patterns as DOC and microbial C concentrations and were 61% and 54% larger in the forest site than in the tundra site, respectively (main effect of Habitat; Table 2, Figure 2), and—except for NH4–N concentration—varied seasonally (main effect of Month; Table 2). The OTCs decreased microbial biomass N irrespective of habitat (main effects of OTC, no OTC × Habitat interaction), but this pattern was especially strong during the early growing season in the forest site, whereas it was stronger during the mid- and late growing season in the tundra site (OTC × Habitat × Month interaction; Table 2, Fig, 2). The OTCs also increased the microbial C:N ratio in the tundra site (Habitat × OTC interaction; Tables 2, 3). The effect of treatment on NH4-N concentrations varied, depending on the month and the habitat (OTC × Habitat- and OTC × Habitat × Month interactions; Table 2). During the late growing season, NH4-N concentrations were higher inside the OTCs in the forest site, but lower inside the OTCs in the tundra site (Figure 2). The same pattern was seen in extractable organic N, but only in the forest site (OTC × Habitat × Month interaction; Tables 2, 3).
Soil microbial biomass C and N and NH4–N concentrations in the surface organic soil layer (top 5 cm) in untreated plots (control) and after 20 years of experimental warming (OTC) in a mountain birch forest and a tundra heath in June (early growing season), July (mid-growing season) and August (late growing season) in Abisko, northern Sweden. Values are mean + S.E., N = 7 in the forest site, N = 8 in the tundra site.
Microbial Respiration and EEAs
Microbial respiration and EEAs per gram soil organic matter were generally higher in the forest site than in the tundra site (main effect of Habitat; Table 4, Figure 3A–F, Supplementary Table 1). Many activities varied among the three sampling months depending on the habitat (main effect of Month, Month × Habitat interactions; Table 4). As seasonal variation per se is not the primary focus of our study, we present microbial activities pooled for the whole growing season. Overall, activities were particularly high during the early growing season in the forest site with weaker seasonal variations detected in the tundra site (Supplementary Table 1).
Soil microbial respiration (A) and extracellular enzyme activities (B-G) in the surface organic soil layer (top 5 cm) in untreated plots (control) and after 20 years of experimental warming (OTC) in a mountain birch forest and a tundra heath in Abisko, northern Sweden. Values are mean + S.E. pooled from three sampling months (June, July and August), N = 7 in the forest site, N = 8 in the tundra site.
When expressed as per gram microbial biomass, the OTCs increased microbial respiration (main effect of OTC; Table 4; Figure 3A, left). The OTCs enhanced BG, CBH, and NAG activities to a greater extent in the forest than in the tundra site (main effect of OTC, OTC × Habitat interaction; Table 4; Figure 3B, C, E, left), while there was no effect on PO activity (Figure 3D). The OTCs decreased LAP activity to a stronger extent in the tundra than in the forest site (main effect of OTC, OTC × Habitat interaction; Table 4; Figure 3F). The OTCs increased AP activity in the forest site but decreased it in the tundra site (OTC × Habitat interaction; Table 4; Figure 3G, left).
The overall treatment effect was highly different when activities were expressed as per gram of soil organic matter. There were no effects of the OTCs on microbial respiration, BG, CBH and NAG activities, and both PO and LAP activities were lower inside the OTCs irrespective of habitat (Table 4, Figure 3A–E, right). AP activity was lower inside the OTCs in the tundra site during the mid-growing season (Table 4; Supplementary Table 1; Figure 2F, right). There were no effects of habitat or treatment on enzymatic C:N stoichiometry (Table 4, Supplementary Table 2). The BG:PO ratio was 67% higher in the tundra site than in the forest site and increased in response to treatment by 40% (Table 4, Supplementary Table 2). The Q10 of microbial respiration was at its highest during the early growing season, whereas the Q10 of enzyme activities were at their highest during mid- and late growing season. The OTCs lowered the Q10 of microbial respiration by 9%, but did not influence the Q10 of enzyme activities (Table 4; Supplementary Table 2).
Shrub Densities and Their Relationships with Microbial Biomass and Respiration
The total vascular plant and evergreen shrub densities were approximately 50% higher and deciduous shrub density two times higher in the tundra than the forest site (Table 1; Figure 4). Of different evergreen shrubs, E. hermaphroditum constituted 78.3% and 87.4% of the evergreen shrubs in the forest and the tundra sites, respectively. In the forest site, B. nana was absent and V. myrtillus was the dominant deciduous shrub, whereas in the tundra site, B. nana constituted 65.6% of the total deciduous shrubs. The total vascular plant and evergreen shrub densities were significantly higher inside the OTCs irrespective of habitat (main effect of OTC; Table 1, Figure 4), whereas deciduous shrubs increased inside the OTCs only in the tundra site (Habitat × OTC interaction; Table 1, Figure 4).
A Densities of deciduous shrubs, evergreen shrubs, and other vascular plants analyzed with point frequency and expressed as hits/100 pins in untreated plots (control) and after 20 years of experimental warming (OTC) in a mountain birch forest and a tundra heath in Abisko, northern Sweden. B Correlation between evergreen shrubs density (hits/100 pins) and the concentration of microbial N (mg kg−1 SOM) in a mountain birch forest and a tundra heath in Abisko, northern Sweden. C Correlation between microbial C and microbial N (mg kg−1 SOM) in the same mountain birch forest and tundra heath.
The best predictor for microbial biomass N was evergreen shrub density in both the forest and the tundra site (Supplementary Tables 3 and 4), accounting for 69.7% and 38.5% of the variation, respectively, in a negative correlation (Figure 4b). In both sites, microbial C concentration was best explained by microbial N, accounting for 44.6% and 26.0% of the variation in the forest and the tundra site, respectively (Supplementary Tables 3 and 4, Figure 4c). In turn, the best predictor for respiration per gram microbial biomass was microbial biomass C, which accounted for 52.8% and 38.2% of the variation in the forest and tundra site, respectively, in a negative correlation (Supplementary Tables 3 and 4).
Discussion
Because increasing deciduous shrub abundance has been found to be associated with lower soil carbon stocks (Parker and others 2015, 2021; Kemppinen and others 2021), we predicted that organic matter stocks would be lower after 20 years of ‘shrubification’ in the tundra site, where previous studies had demonstrated an increasing deciduous shrub abundance (Kaarlejärvi and others 2012). We did not predict a similar effect for the forest site, where only evergreen shrubs had increased (Kaarlejärvi and others 2012). However, soil organic matter stocks and quality remained unaffected in both the tundra and forest sites. As increasing deciduous shrub abundances are known to be associated with enhanced microbial activities (Deslippe and others 2012; Sistla and Schimel 2013; Street and others 2020; Hicks and others 2021), we also predicted that microbial respiration rate and EEAs should be higher inside the OTCs in the tundra site, whereas no such effect should be detected in the forest site. When expressed as per gram microbial biomass, the respiration rates and EEAs for carbohydrate and chitin degradation were higher inside the OTCs; however, this effect was much more pronounced in the forest site. Additionally, the OTCs induced a substantial decline in the microbial biomass in both habitats leading to no change in microbial respiration rate and EEAs per gram soil, an outcome that was not considered in our hypotheses.
‘Shrubification’ Induced a Shift in Carbohydrate Versus Phenol Degradation and Increased Respiration per Gram Microbial Biomass
In contrast with predictions, the only treatment effect on organic matter stocks was a higher litter stock inside the OTCs in the tundra site, which likely results from the combination of increased litterfall and the fact that the chambers prevent fallen leaves from being blown away by the wind. 13C NMR analyses showed that the proportion of carbohydrate-C was significantly lower in the forest site than the tundra site, whereas the opposite was true for aliphatic-C, which aligns with earlier results from the same sites (Sjögersten and others 2003) and elsewhere (Dai and others 2002; Väisänen and others 2015) that tundra soil organic matter contains a high proportion of labile carbon compounds.
Owing to the combined effects of the shrub growth form (that is, evergreen or deciduous shrub) and the initial soil carbon quality (that is, the proportion of labile and ‘recalcitrant’ carbon compounds), we predicted that the OTCs would experience increased microbial respiration and EEAs in the tundra site, but not in the forest site. The differences between habitats were in fact the opposite of our predictions, as the responses of BG, CBH, and NAG activities were stronger in the forest site. Soil and microbial C and N concentrations and respiration were also greater overall in the forest site, which confirms previous studies in the same study area (Hartley and others 2012; Clemmensen and others 2021). We suggest that the stronger effect of the OTCs on microbial activities in the forest sites reflects this higher overall microbial activity. The higher activities in the forest coincide with a shift in the microbial community composition into taxa with a stronger capacity for ‘N mining’ through organic matter degradation (Clemmensen and others 2021).
Besides the difference in magnitude, the responses of EEAs were relatively uniform between the habitats. NAG activity, which has a major role in tundra C and N cycles (Schimel and Mikan 2005; Koyama and others 2013) and an assumed link with increasing ‘N mining’ (Deslippe and others 2012; Ylänne and others 2020) was higher under ‘shrubification’ at both habitats. Yet, the OTCs simultaneously decreased LAP, which is also synthesized for N acquisition (Sinsabaugh and others 2008), which together with the higher BG activity led to no change in enzymatic C:N stoichiometry, thus differing from some other experiments (Sistla and others 2014). However, the BG:PO ratio was higher under ‘shrubification,’ indicating that soil microorganisms were increasingly synthesizing extracellular enzymes for carbohydrate rather than phenol degradation (Sinsabaugh and Follstad Shah 2011) thereby differing from many other studies witnessing increases in PO activity in response to warming in tundra (Sistla and Schimel 2013; Seo and others 2015) and globally (Chen and others 2017). The OTCs increased AP activity in the forest site but not in the tundra site, indicating that forest soil microorganisms maintained the same N:P stoichiometry under ‘shrubification. By contrast, in the tundra site, phosphorus acquisition in relation to nitrogen acquisition was downregulated; a phenomenon found also previously in tundra (Phillips and others 2018; Stark and others 2018; Ylänne and others 2020) and elsewhere (Sardans and others 2021) and suggested to reflect increasing nitrogen in relation to phosphorus limitation for soil microorganisms (Stark and others 2018; Sardans and others 2021).
A Decline in Microbial Biomass Carbon and Nitrogen in a Strong Inverse Relationship Between Evergreen Shrub Density
We found a decline in microbial biomass C and N concentrations inside the OTCs, which was not predicted in our hypotheses. The lower amount of microbial biomass diminished the effect of increasing activities, and per gram soil, there were no effects on microbial respiration and BG and NAG activities, and a decrease in PO activity. These findings align with the generalization that the amount of microbial biomass is a strong determinant of the potential for soil carbon decomposition (Schmidt and others 2011). Interestingly, we found a tight inverse correlation between microbial N and evergreen shrub density in both sites. This could be interpreted as that ‘shrubification’—when continued for two decades—intensified nutrient competition between plants and soil microorganisms to such an extent that it may have limited the amount of microbial biomass. This hypothesis is not contradictory with the observation of higher microbial respiration per gram microbial biomass, as this could also result from a nutrient-limitation of microorganisms. According to a general framework, microbial CO2 respiration may increase either in response to enhanced labile C availability, or the nutrient limitation of soil microbial growth; in the latter case, enhanced respiration depicts an overflow metabolism resulting from a nutrient limitation of C assimilation into the microbial biomass (Schimel and Weintraub 2003). ‘Shrubification’ could therefore enhance microbial respiration per biomass through an overflow metabolism via nutrient limitation of microbial growth, and not only via enhanced labile C availability.
It has previously been hypothesized that as tundra soils are generally nitrogen-poor (Jonasson and others 2001; Schimel and Bennett 2004), and nitrogen constitutes an important component of both microbial biomass and extracellular enzymes, the N-limitation of soil microorganisms for growth and synthesizing extracellular enzymes is a major determinant for overall soil carbon decomposition rate (Schimel and Weintraub 2003; Schimel and Bennett 2004; Wallenstein and others 2009; Weedon and others 2011; Koyama and others 2013; Sistla and Schimel 2013; Sistla and others 2014; Stark and others 2014). More recently, it has also been suggested that phosphorus may under some conditions limit soil microbial growth and promote organic matter accumulation in the Arctic (Pold and others 2022). Studies conducted near our study site in Abisko indicated that nutrients did limit the depolymerization of soil organic compounds (Hicks and others 2020) and that soil microbial growth was limited by soil nutrients (Jonasson and others 2006; Demoling and others 2007; Rinnan and others 2007). When considering whether N limitation induces increasing ‘N mining,’ it is worth noting that nutrient limitation may induce opposite outcomes for microbial processes depending on the severity of this limitation, and shifting between conditions under which resource deficiency induces a higher microbial activity and where resource availability limits the microbial activity (Weedon and others 2011). Tundra soil microorganisms may become increasingly N-limited during the mid-growing season, which increases their enzyme production (Weintraub and Schimel 2005b, c; Buckeridge and Grogan 2008), but N limitation during peak growing season may be so severe that it actually limits extracellular enzyme synthesis (Wallenstein and others 2009; Sistla and others 2012). Similarly, ‘shrubification’ in nutrient-limited Arctic ecosystems could over time reach a threshold where it turns a demand-driven ‘N mining’ into a supply-driven limitation of microbial biomass to the extent that declining microbial biomass counteracts the impact of its increasing activity. This mechanism could constitute one of the main reasons why increased shrub abundance has not translated into changes in soil organic matter stocks in our study system. Tundra plants may outcompete soil microorganisms for soil nutrients, particularly because they store nutrients in their biomass for an extended time period (Jonasson and others 1999; Hodge and others 2000; Stark and Kytöviita 2005). Importantly, other warming experiments have found no effect on microbial biomass (Sistla and others 2013; Phillips and others 2018; Stark and others 2018), or a decline in it (Rinnan and others 2009), which shows that increased carbon flow to belowground through plant litter and root exudates does not enable soil microbial biomass to increase.
We predicted that the OTCs increasing deciduous or evergreen shrubs would have a decisive role for the consequences on the SOM stocks and microbial activities, but—in terms of the lack of effect on the SOM stock—the outcome was the same in both habitats. Interestingly, the responses of soil microbial C and N to ‘shrubification’ were also independent of the habitat. We suggest that the potential for intensified microbial nutrient limitation under ‘shrubification’ may still be dependent on the traits of the plant species’ that become dominant. E. hermaphroditum constituted a common species at both sites, although B. nana was also abundant at the tundra site. We did not take plant biomass samples to avoid damaging our long-term experiment, but other warming experiments have demonstrated increased ecosystem C and N stocks in the plant biomass in response to long-term warming, especially when E. hermaphroditum increased (Ylänne and others 2015). E. hermaphroditum may enhance its own performance through producing acidic, low-quality organic matter that decreases soil pH (Cornelissen and others 2006) and decelerates soil nutrient cycling (Bråthen and others 2017), as this species also possesses several traits favoring adaptation to nutrient-poor conditions (Eskelinen and others 2009). The potential of E. hermaphroditum to bind significant proportions of the ecosystem nutrient pool into a long-living biomass could constitute a major component in its ecological ‘niche construction’ into conditions of lower soil nutrient availability and ever more increasing abundance (sensu Bråthen and others 2017). The slope of the correlation between evergreen shrubs and microbial N was particularly steep in the forest with a largely evergreen-dominated ground vegetation. In the tundra, B. nana may exert more ambiguous impact on soil process rates through producing leaf litter that may accelerate soil nutrient cycling (for example, Buckgeridge and others 2010) and while simultaneously also binding carbon and nutrients for a long time in woody stems that form slow decomposing litter (Hobbie 1996; Weintraub and Schimel 2005a; Wookey and others 2009). Soil microorganisms are also able to modify their internal N concentrations depending on the nutrient availability, manifested as changes in the microbial C:N ratio (Jonasson and others 1999). Microbial C:N ratio increased inside the OTCs in the tundra but not in the forest, indicating that this flexibility may be higher in the tundra.
Our experiment represents a case where warming increases the abundance of shrubs, which may differ from situations where shrubs are expanding in distribution. However, studies from tundra sites that have compared graminoid and Betula nana-dominated vegetation found a higher carbon-use efficiency under shrubs, indicating that shrub expansion could promote stabilization of organic matter into the soil (Rousk and others 2016; Lynch and others 2018). Indeed, the ability to adjust carbon use efficiency is a strong determinant of the balance between microbial C assimilation and respiration under warming (Billings and Ballantyne 2013; Wieder and others 2013; Allison 2014). In our experiment, the OTCs no longer increased soil temperatures due to the buffering effect of a thicker vegetation cover. On the one hand, increased shrubs cool the soils due to an increased canopy shading which binds nutrients into recalcitrant woody biomass, but on the other, they also promote winter snow accumulation, which leads into higher microbial activities and a positive feedback on soil nutrient availability during the summer (Sturm and others 2005; Wookey and others 2009; Buckeridge and others 2010). This ‘dual’ effect of shrubs means that it is the balance between positive and negative factors that eventually decide the effects on carbon cycling and nutrient mineralization. Indeed, responses in N mineralization are mixed, showing increased (DeMarco and others 2011), decreased (Sturm and others 2005), no effects (Jonasson and others 1999; DeMarco and others 2014), or a variance depending on the time scale so that N mineralization may be enhanced in the short term but not in the long term (Hartley and others 1999).
Conclusions and Implications
Based on our findings, we hypothesize that in nutrient-limited subarctic habitats where warming increases long-lived evergreen shrubs, ‘shrubification’ may intensify nutrient competition between plants and soil microorganisms and even induce a decline in the amount of microbial biomass. This hypothesis ties together the concepts that nutrients limit soil microbial biomass and function in the tundra (Fierer and Schimel 2003; Weintraub and Schimel 2003; Sistla and others 2012, 2014; Hicks and others 2020) and that evergreen shrub expansion leads to divergent effects on soil carbon sequestration when compared to deciduous shrub expansion (Vowles and Björk 2018; Kemppinen and others 2021). Yet, it highlights the important role of nutrient binding into long-lived aboveground plant biomass as the main plant trait mediating these impacts. Consequently, a warmer climate may not induce an accelerated release of CO2 to the atmosphere, but induce a neutral or even slightly positive effect on ecosystem C storage as a result of increasing plants. Substantial losses from the soil to the atmosphere in this area could likely only take place once the forest tree line advances, inducing a regime shift from tundra to forest (Hartley and others 2012; Clemmensen and others 2021).
Our findings add to previous studies showing limited or mixed impacts of warming on soil nutrient dynamics, microbial stoichiometry, and soil C and N stocks in the tundra (Rousk and others 2016; Lynch and others 2018; Salazar and others 2020; McLaren and Buckeridge 2021; Pold and others 2021). A recent meta-analysis demonstrated that Arctic ecosystems respond to warming in a habitat-specific manner, with plant community shifts and microbial responses ultimately altering nutrient competition between plants and microorganisms variably across tundra habitats (Pold and others 2021). The outcome of warming and associated vegetation change may depend on an intricate balance between demand-driven ‘N mining’ into a supply-driven limitation of microbial biomass (sensu Weedon and others 2011) together with species interactions (Urcelay and others 2003). Similarly, some Arctic sites show a strong nutrient-limitation of microbial function (Sistla and others 2012), while other sites demonstrate an indifferent relationship with soil nutrient availability (Stark and Väisänen 2014; Melle and others 2015). Our results suggest a potential key role of nutrient competition in determining soil C and N dynamics under ‘shrubification,’ a fuller understanding of which should be recognized as a critical research target.
References
Allison SD. 2006. Brown ground: A soil carbon analogue for the Green World Hypothesis? Am Nat 167:619–627.
Allison SD. 2014. Modeling adaptation of carbon use efficiency in microbial communities. Front Microbiol 5:Article 571.
Billings SA, Ballantyne FI. 2013. How interactions between microbial resource demands, soil organic matter stoichiometry, and substrate reactivity determine the direction and magnitude of soil respiratory responses to warming. Glob Change Biol 19:90–102.
Bokhorst SF, Huiskes A, Aerts R, Convey P, Cooper EJ, Dalen L, Erschbamer B, Gudmundsson J, Hofgaard A, Hollister RD, Johnstone J, Jónsdóttir IS, Lebouvier M, van de Vijver B, Wahren CH, Dorrepaal E. 2013. Variable temperature effects of Open Top Chambers at polar and alpine sites explained by irradiance and snow depth. Glob Change Biol 19:64–74.
Bradford MA, Wieder WR, Bonan GB, Fierer N, Raymond PA, Crowther TW. 2016. Managing uncertainty in soil carbon feedbacks to climate change. Nat Clim Change 6:751–757.
Brookes PC, Kragt JF, Powlson DS, Jenkinson DS. 1985. Chloroform fumigation and the release of soil nitrogen: the effects of fumigation time and temperature. Soil Biol Biochem 17:831–835.
Bråthen KA, Fodstad CH, Gallet C. 2010. Ecosystem disturbance reduces the allelopathic effects of Empetrum hermaphroditum humus on tundra plants. J Veg Sci 21:786–795.
Bråthen KA, Gonzales VT, Yoccoz NG. 2017. Gatekeepers to the effects of climate warming? Niche construction restricts plant community changes along a temperature gradient. Perspect Plant Ecol Evol Syst 30:71–81.
Buckeridge KM, Grogan P. 2008. Deepened snow alters soil microbial nutrient limitations in arctic birch hummock tundra. Appl Soil Ecol 39:210–222.
Buckeridge KM, Zufelt E, Chu H, Grogan P. 2010. Soil nitrogen cycling rates in low arctic shrub tundra are enhanced by litter feedbacks. Plant Soil 330:407–421.
Buizer B, Weijers S, Bodegom PV, Alsos IG, Eidesen PB, Breda JV, Korte M, Rijckevorsel JV, Rozema J. 2012. Range shifts and global warming: ecological responses of Empetrum nigrum L. to experimental warming at its northern (high Arctic) and southern (Atlantic) geographical range margin. Environ Res Lett 7:1–9.
Chen J, Luo Y, García-Palacios P, Cao J, Dacal M, Zhou X, Li J, Xia J, Niu S, Yang H, Shelton S, Guo W, Groenigen KJV. 2017. Differential responses of carbon-degrading enzyme activities to warming: implications for soil respiration. Glob Change Biol 24:4816–4826.
Clemmensen KE, Durling MB, Michelsen A, Hallin S, Finlay RD, Lindahl BD. 2021. A tipping point in carbon storage when forest expands into tundra is related to mycorrhizal recycling of nitrogen. Ecol Lett.
Clemmensen KE, Finlay R, Dahlberg A, Stenlid J, Wardle DA, Lindahl B. 2015. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytologist 205:1525–1536.
Cornelissen JHC, Quested HM, van Logtestijn RSP, Perez-Harguindeguy N, Gwynn-Jones D, Diaz S, Callaghan TV, Press MC, Aerts R. 2006. Foliar pH as a new plant trait: Can in explain variation in foliar chemistry and carbon cycling processes among subarctic plant species and types? Oecologia 147:315–326.
Craine JM, Morrow C, Fierer N. 2007. Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113.
Dai XY, White D, Ping C-L. 2002. Comparing bioavailability in five Arctic soils by pyrolysis-gas chromatography/mass spectrometry. J Anal Appl Pyrol 62:249–258.
Davidson EA, Janssens IA. 2006. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173.
Demoling F, Figueroa D, Bååth E. 2007. Comparison of factors limiting bacterial growth in different soils. Soil Biol Biochem 39:2485–2495.
Deslippe J, Hartmann M, Simard SW, Mohn WW. 2012. Long-term warming alters the composition of Arctic soil microbial communities. FEMS Microbiol Ecol 82:303–315.
Devos CC, Ohlson M, Nasset E, Bollandsås OM. 2022. Soil carbon stocks in forest-tundra ecotones along a 500 km latitudinal gradient in northern Norway. Sci Rep 12:13358.
DeMarco J, Mack MC, Bret-Harte MS. 2011. The effects of snow, soil microenvironment, and soil organic matter quality on N availability in three Alaskan arctic plant communities. Ecosyst 14:804–817.
DeMarco J, Mack MC, Bret-Harte MS. 2014. Effects of arctic shrub expansion on biophysical vs. biogeochemical drivers of litter decomposition. Ecol 95:1861–1875.
Dunleavy HR, Mack MC. 2021. Long-term experimental warming and fertilization have opposing effects on ectomycorrhizal root enzyme activity and fungal community composition in Arctic tundra. Soil Biol Biochem 154:108151.
Elmendorf S, Henry GHR, Hollister RD, al. e. 2012. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat Clim Change 2:453–457.
Eskelinen A, Stark S, Männistö MK. 2009. Links between plant community composition, soil organic matter quality and microbial communities in contrasting tundra habitats. Oecologia 161:113–123.
Fierer N, Schimel JP. 2003. A proposed mechanism for the pulse in the carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci Soc Am J 67:798–805.
Fierer N, Schimel JP, Cates RG, Zou J. 2001. Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol Biochem 33:1827–1839.
Fontaine S, Bardoux G, Abbadie L, Mariotti A. 2004. Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320.
Gélinas Y, Baldock JA, Hedges JI. 2001. Demineralization of marine and freshwater sediments for CP/MAS 13C NMR analysis. Org Geochem 32:677–693.
Hartley AE, Neill C, Melillo JM, Crabtree R, Bowles FP. 1999. Plant performance and soil nitrogen mineralization in response to simulated climate change in subarctic dwarf shrub heath. Oikos 86:331–343.
Hartley IP, Garnett MH, Sommerkorn M, Hopkins DW, Fletcher BJ, Sloan VL, Phoenix GK, Wookey PA. 2012. A potential loss of carbon associated with greater plant growth in the European Arctic. Nat Clim Change. https://doi.org/10.1038/NCLIMATE1575.
Henry HAL. 2012. Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47:53–59.
Hernandez DL, Hobbie SE. 2010. The effects of substrate composition, quantity, and diversity on microbial activity. Plant Soil 335:397–411.
Hicks LC, Leizeaga A, Rousk K, Michelsen A, Rousk J. 2020. Simulated rhizosphere deposits induce microbial N-mining that may accelerate shrubification in the subarctic. Ecology 101:e03094.
Hicks LC, Yan M, Brangari A, Rousk K, Rousk J. 2021. Increased above- and belowground plant input can both trigger microbial nitrogen mining in subarctic tundra soils. Ecosystems in press.
Hobbie SE. 1996. Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522.
Hodge A, Robinson D, Fitter A. 2000. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308.
Jonasson S, Castro J, Michelsen A. 2006. Interactions between plants, litter and microbes in cycling of nitrogen and phosphorus in the arctic. Soil Biol Biochem 38:526–532.
Jonasson S, Chapin FSIII, Shaver GR. 2001. Biogeochemistry in the Arctic: patterns, processes and controls. Global biogeochemical cycles in the climate system. San Diego: Academic Press. pp 139–150.
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV. 1999. Responses of microbes and plants to changed temperature, nutrient, and light regimes in the arctic. Ecology 80:1828–1843.
Kaarlejärvi E, Baxter R, Hofgaard A, Hytteborn H, Khitun O, Molau U, Sjögersten S, Wookey PA, Olofsson J. 2012. Effects of warming on shrub abundance and chemistry drive ecosystem-level changes in a forest-tundra ecotone. Ecosystems 15:1219–1233.
Kardol P, Cregger MA, Campany CE, Classen AT. 2010. Soil ecosystem functioning under climate change: plant species and community effects. Ecology 91:767–781.
Kemppinen J, Niittynen P, Virkkala A-M, Happonen K, Riihimäki H, Aalto J, Luoto M. 2021. Dwarf shrubs impact tundra soils: drier, colder, and less organic carbon. Ecosystems in press.
Kielak AM, Cretoiu MA, Semenov AA, Sorensen SJ, van Elsas JD. 2013. Bacterial chitinolytic communities respond to chitin and pH alteration in soil. Appl Environ Microbiol 79:263–272.
Koyama A, Wallenstein MD, Simpson RT, Moore JC. 2013. Carbon-degrading enzyme activities stimulated by increased nutrient availability in Arctic tundra soils. PLoS One 8(e77212):77211–77210.
Lynch LM, Machmuller MB, Cotrufo MF, Paul EA, Wallenstein MD. 2018. Tracking the fate of fresh carbon in the Arctic tundra: Will shrub expansion alter responses of soil organic matter to warming? Soil Biol Biochem 120:134–144.
Martz F, Vuosku J, Ovaskainen A, Stark S, Rautio P. 2016. The snow must go on: ground ice encasement, snow compaction and absence of snow differently cause soil hypoxia, CO2 accumulation and tree seedling damate in boreal forest. PLoS One 11:e0156620.
McLaren JR, Buckeridge KM. 2021. Enhanced plant leaf P and unchanged P stocks after a quarter century of warming in the arctic tundra. Ecosphere 12:e03838.
Melle C, Wallenstein M, Darrouzet-Nardi A, Weintraub MN. 2015. Microbial activity is not always limited by nitrogen in Alaskan tundra soils. Soil Biol Biochem 90:52–61.
Moorhead DL, Sinsabaugh RL. 2006. A theoretical model of litter decay and microbial interactions. Ecol Monogr 76:151–174.
Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T, Blok D, Tape KD, Macias Fauria M, Sass-Klaassen U, Lévesque E, Boudreau S, Ropars P, Hermanutz L, Trant A, Collier LS, Weijers S, Rozema J, Rayback SA, Schmidt NM, Schaepman-Strub G, Wipf S, Rixen C, Ménard CB, Venn S, Goetz S, Andreu-Hayles L, Elmendorf S, Ravolainen V, Welker JM, Grogan P, Epstein HE, Hik DS. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6:045509.
Myers-Smith IH, Kerby JT, Phoenix GK, Bjerke JW, Epstein HE, Assmann JJ, John C, Andreu-Hayles L, Angers-Blondin S, Beck PSA, Berner LT, Bhatt US, Bjorkman AD, Blok D, Bryn A, Christiansen CT, Cornelissen JHC, Cunliffe AM, Elmendorf SC, Forbes BC, Goetz SJ, Hollister RD, de Jong R, Loranty MM, Macias-Fauria M, Maseyk K, Normand S, Olofsson J, Parker TC, Parmentier F-JW, Post E, Schaepman-Strub G, Stordal F, Sullivan PF, Thomas HJD, Tømmervik H, Treharne R, Tweedie CE, Walker DA, Wilmking M, Wipf S. 2020. Complexity revealed in the greening of the Arctic. Nat Clim Change 10:106–117.
Nowinski NS, Trumbore SE, Schuur EAG, Mack MC, Shaver GR. 2008. Nutrient addition prompts rapid detabilization of organic matter in an arctic tundra ecosystem. Ecosystems 11:16–25.
Parker TC, Subke J-A, Wookey PA. 2015. Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob Change Biol 21:2070–2081.
Parker TC, Thurston AM, Raundrup K, Subke J-A, Wookey PA, Hartley IP. 2021. Shrub expansion in the Arctic may induce large-scale carbon losses due to changes in plant-soil interactions. Plant and Soil in press.
Pendall E, Rustad L, Schimel J. 2008. Towards a predictive understanding of belowground process responses to climate change: have we moved any closer? Funct Ecol 22:937–940.
Phillips CA, Elberling B, Michelsen A. 2018. Soil carbon and nitrogen stocks and turnover following 16 years of warming and litter addition. Ecosystems. https://doi.org/10.1007/s10021-018-0256-y.
Pold G, Baillergeon N, Lepe A, Rastetter EB, Sistla SA. 2021. Warming effect on arctic tundra biogeochemistry are limited by habitat-dependent: a meta-analysis. Ecosphere 12:e03777.
Pold G, Kwiatkowski BL, Rastetter EB, Sistla SA. 2022. Sporadic P limitation constrains microbial growth and facilitates SOM accumulation in the stoichiometrically coupled, acclimating microbe–plant–soil model. Soil Biol Biochem 165:108489.
Read DJ, Perez-Moreno J. 2003. Mycorrhizas andn nutrient cycling in ecosystems—A journey towards relevance? New Phytologist 157:475–492.
Rinnan R, Michelsen A, Bååth E, Jonasson S. 2007. Mineralization and carbon turnover in subarctic heath soil as affected by warming and additional litter. Soil Biol Biochem 39:3014–3023.
Rinnan R, Stark S, Tolvanen A. 2009. Responses of vegetation and soil microbial communities to warming and simulated herbivory in a subarctic heath. J Ecol 97:788–800.
Rousk K, Michelsen A, Rousk J. 2016. Microbial control of soil organic matter mineralisation responses to labile carbon in subarctic climate change treatments. Glob Change Biol 22:4150–4161.
Salazar A, Rousk K, Jonsdottir IS, Bellenger J-P, Adresson OS. 2020. Faster nitrogen cycling and more fungal and root biomass in cold ecosystems under experimental warming: a meta-analysis. Ecology 101:e029938.
Sardans J, Janssens IA, Ciais P, Obersteiner M, Penuelas J. 2021. Recent advances and future research in ecological stoichiometry. Perspect Plant Ecol Evol Syst 50:125611.
Schimel JP, Bennett J. 2004. Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602.
Schimel JP, Bilbrough C, Welker JM. 2004. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biol Biochem 36:217–227.
Schimel JP, Mikan CJ. 2005. Changing microbial substrate use in Arctic tundra soils through a freeze-thaw cycle. Soil Biol Biochem 37:1411–1418.
Schimel JP, Weintraub MN. 2003. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563.
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE. 2011. Persistence of soil organic matter as an ecosystem property. Nature 478:49–56.
Seo J, Jang I, Young Jung J, Kyung Lee Y, Kang H. 2015. Warming and increased precipitation enhance phenol oxidase activity in soil while warming induces drought stress in vegetation of an Arctic ecosystem. Geoderma 259–260:347–353.
Shaver GR, Giblin AE, Nadelhoffer KJ, Thieler KK, Downs MR, Laundre JA, Rastetter EB. 2006. Carbon turnover in Alaskan tundra soils: effects of organic matter quality, temperature, moisture and fertilizer. J Ecol 94:740–753.
Sinsabaugh RL. 2010. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404.
Sinsabaugh RL, Follstad Shah JJ. 2011. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry 102:31–43.
Sinsabaugh RL, Lauber CL, Weintraub MN, Bony A, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH. 2008. Stoiciometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264.
Sistla SA, Asao S, Schimel JS. 2012. Detecting microbial N-limitation in tussock tundra soil: implications for Arctic soil organic carbon cycling. Soil Biol Biochem 55:78–84.
Sistla SA, Moore JC, Simpson RT, Gough L, Shaver GR, Schimel JP. 2013. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature 497:615.
Sistla SA, Rastetter EB, Schimel JP. 2014. Responses of a tundra system to warming using SCAMPS: a stoihiometrically coupled, acclimating microbe-plant-soil model. Ecol Monogr 84:151–170.
Sistla SA, Schimel JP. 2013. Seasonal patterns of microbial extracellular enzyme activities in an arctic tundra soil: Identifying direct and indirect effects of long-term summer warming. Soil Biol Biochem 66:119–129.
Sjögersten S, Turner BL, Mahieu N, Condron LM, Wookey PA. 2003. Soil organic matter biochemistry and potential susceptibility to climatic change across the forest-tundra ecotone in the Fennoscandian mountains. Glob Change Biol 9:759–772.
Stark S, Kytöviita M-M. 2005. Simulated grazer effects on microbial respiration in a subarctic meadow: implications for nutrient competition between plants and soil microorganisms. Applied Soil Ecology in press.
Stark S, Männistö MK, Eskelinen A. 2014. Soil nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 383:373–385.
Stark S, Väisänen M. 2014. Insensitivity of soil microbial activity to temporal variation in soil N in subarctic tundra—evidence from responses to large migratory grazers. Ecosystems 17:906–917.
Stark S, Ylänne H, Tolvanen A. 2018. Long-term warming alters soil N: P stoichiometry in nutrient-poor subarctic tundra. Soil Biol Biochem 124:184–188.
Street LE, Garnett MH, Subke J-A, Baxter R, Dean JF, Wookey PA. 2020. Plant carbon allocation drives turnover of old soil organic matter in permafrost tundra soils. Glob Change Biol 26:4559–4571.
Sturm M, Schimel JP, Michaelson G, Welker JM, Oberbauer SF, Liston GE, Fahnestock J, Romanovsky VE. 2005. Winter biological processes could help convert Arctic tundra to shrubland. BioScience 55:17–26.
Tarnocai C, Canadell JG, Schuur EAG, Kuhry P, Mazhitova G, Zimov S. 2009. Soil organic carbon pools in the northern circumpolar permafrost region. Glob Biogeochem Cycles. https://doi.org/10.1029/2008GB003327.
Urcelay C, Bret-Harte MS, Diaz S, Chapin FS III. 2003. Mycorrhizal colonization mediated by species interactions in arctic tundra. Oecologia 137:399–404.
van Wijk AJ, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FS III, Cornelissen JHC, Gough L, Hobbie SE, Jonasson S, Lee JA, Michelsen A, Press MC, Richardson SJ, Rueth H. 2003. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Glob Change Biol 10:105–123.
Virtanen R, Oksanen L, Oksanen T, Cohen J, Forbes BC, Johansen B, Käyhkö J, Olofsson J, Pulliainen J, Tommervik H. 2016. Where do the treeless tundra areas of northern highlands fit in the global biome system: toward an ecologically natural subvision of the tundra biome. Ecol Evol 6:143–158.
Vowles T, Björk RG. 2018. Implications of evergreen shrub expansion in the Arctic. J Ecol 107:650–655.
Vuorinen KEM, Oksanen L, Oksanen T, Pyykönen A, Olofsson J, Virtanen R. 2017. Open tundra persist, but arctic features decline—vegetation changes in the warming Fennoscandian tundra. Glob Change Biol 23:3794–3807.
Väisänen M, Sjögersten S, Large D, Drage T, Stark S. 2015. Long-term reindeer grazing limits warming-induced increase in C release: potential role of soil C quality. Environ Res Lett 10:094020.
Wallenstein MD, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh RL. 2011. Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A, Eds. Soil Enzymology, . Berlin: Springer. pp 245–258.
Wallenstein MD, Hall EK. 2012. A trait-based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochemistry 109:35–47.
Wallenstein MD, McMahon SK, Schimel JP. 2009. Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Change Biol 15:1631–1639.
Wallenstein MD, Weintraub MN. 2008. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol Biochem 40:2098–2106.
Wallstedt A, Gallet C, Nilsson M-C. 2005. Behaviour and recovery of the secondary metabolite batatasin-III from boreal forest humus: influence of temperature, humus type and microbial community. Biochem Syst Ecol 33:385–407.
Weedon JT, Aerts R, Kowalchuk GA, Bodegom PMv. 2011. Enzymology under global change: organic nitrogen turnover in alpine and sub-Arctic soils. Biochem Soc Trans 39:309–314.
Weintraub MN, Schimel JP. 2003. Interactions between carbon and nitrogen mineralization and soil organic matter chemistry in arctic tundra soils. Ecosystems 6:129–143.
Weintraub MN, Schimel JP. 2005a. Nitrogen cycling and the spread of shrubs control changes in the carbon balance of arctic tundra ecosystems. BioScience 55:408–415.
Weintraub MN, Schimel JP. 2005b. The seasonal dynamics of amino acids and other nutrients in Alaskan Arctic tundra soils. Biogeochemistry 73:359–380.
Weintraub MN, Schimel JP. 2005c. Seasonal protein dynamics in Alaskan arctic tundra soils. Soil Biol Biochem 37:1469–1475.
Wieder WR, Bonan GB, Allison SD. 2013. Global soil carbon projections are improved by modelling microbial processes. Nat Clim Change 3:909–912.
Wookey PA, Aerts R, Bardgett RD, Baptist F, Bråthen KA, Cornelissen JHC, Gough L, Hartley IP, Hopkins DW, Lavorel S, Shaver GR. 2009. Ecosystem feedbacks and cascade processes: understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Glob Change Biol 15:1153–1172.
Ylänne H, Kaarlejärvi E, Väisänen M, Ahonen SH, Männistö MK, Olofsson J, Stark S. 2020. Removal of grazers alters the response of tundra soil carbon to warming and enhanced nitrogen availability. Ecol Monogr 90:e01396.
Ylänne H, Stark S, Tolvanen A. 2015. Vegetation shift from deciduous to evergreen dwarf shrubs in response to selective herbivory offsets carbon losses: evidence from 19 years of warming and simulated herbivory in the subarctic tundra. Glob Change Biol 21:3696–3711.
Zeglin LH, Kluber LA, Myrold DD. 2013. The importance of amino sugar turnover to C and N cycling in organic horizons of old-growth Douglas-fir forest soils colonized by ectomycorrhizal mats. Biogeochemistry 112:679–693.
Acknowledgements
We thank Sirkka-Liisa Aakkonen for helping with laboratory analyses, Jonas Gustafsson for helping with vegetation analyses, Juho Haveri-Heikkilä for helping with soil sampling, and Philip Burgess for checking the language. This project was funded by the Academy of Finland (Decision Number 310776 to Minna K. Männistö).
Funding
Open Access funding provided by University of Lapland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
JO and SSj established and maintained the field experiment; MKM and SS designed this study. MK analyzed microbial respiration, EM analyzed vegetation, and JV, AMK, and VVT analyzed carbon fractions. SS analyzed enzyme activities, organic matter stocks, and nutrient concentrations, conducted statistical analyses, and led the writing process to which all other authors contributed with discussion and text.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stark, S., Kumar, M., Myrsky, E. et al. Decreased Soil Microbial Nitrogen Under Vegetation ‘Shrubification’ in the Subarctic Forest–Tundra Ecotone: The Potential Role of Increasing Nutrient Competition Between Plants and Soil Microorganisms. Ecosystems 26, 1504–1523 (2023). https://doi.org/10.1007/s10021-023-00847-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-023-00847-z