Abstract
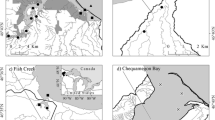
Efforts to conserve, restore, or otherwise manage large rivers and the services they provide are hindered by limited understanding of the functional dynamics of these systems. This shortcoming is especially evident with regard to trophic structure and energy flow. We used natural abundances of carbon and nitrogen isotopes to examine patterns of material flow in ten large-river food webs characterized by different landscape-scale hydrologic characteristics (low-gradient river, high-gradient river, river stretches downstream of reservoirs, and reservoirs), and tested predictions from three ecosystem concepts commonly applied to large-rivers: The River Continuum Concept, The Flood Pulse Concept and the Riverine Productivity Model. Carbon derived from aquatic C3 plants and phytoplankton were the dominant energy sources supporting secondary consumers across the ten large-river food webs examined, but relative contributions differed significantly among landscape types. For low-gradient river food webs, aquatic C3 plants were the principal carbon source, contributing as much as 80% of carbon assimilated by top consumers, with phytoplankton secondarily important. The estimated relative importance of phytoplankton was greatest for food webs of reservoirs and river stretches downriver from impoundments, although aquatic C3 plants contributed similar amounts in both landscape types. Highest 99th percentile source contribution estimates for C4 plants and filamentous algae (both approximately 40%) were observed for high-gradient river food webs. Our results for low-gradient rivers supported predictions of the Flood Pulse Concept, whereas results for the three other landscape types supported the Riverine Productivity Model to varying degrees. Incorporation of landscape-scale hydrologic or geomorphic characteristics, such as river slope or floodplain width, may promote integration of fluvial ecosystem concepts. Expanding these models to include hydrologically impacted landscapes should lead to a more holistic understanding of ecosystem processes in large-river systems.




Similar content being viewed by others
REFERENCES
Agostinho AA, Gomes LC, Suzuki HI, Júlio Jr HF. 2003. Migratory fishes of the upper Paraná River basin, Brazil. In: Carolsfeld J, Harvey J, Ross C, Baer A, Eds. Migratory fishes of South America: biology, fisheries and conservation status. Victoria (Canada): International Development Research Centre and The World Bank. pp 19–98
Agostinho AA, Gomes LC, Veríssimo S, Okada EK. 2004. Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Rev Fish Biol Fish 14:11–9
Agostinho AA, Júlio Jr HF, Gomes LC, Bini LM, Agostinho CS. 1997. Composição, abundância e distribuição espaço-temporal da ictiofauna. In: Vazzoler AEAM, Agostinho AA, Hahn NS, Eds. A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Maringá (Brazil): EDUEM. pp. 179–208
Agostinho AA, Thomaz SM, Minte-Vera CV, Winemiller KO. 2000. Biodiversity in the high Paraná River floodplain. In: Gopal B, Junk WJ, Davis JA, Eds. Biodiversity in wetlands: assessment, funciton and conservation. Leiden: Backhuys Publishers. pp 89–118
Agostinho AA, Vazzoler AEAM, Thomaz SM. 1995. The high Paraná River basin: Limnological and ichthyological aspects. In: Tundisi JG, Bicudo CEM, Matsumura-Tundisi T, Eds. Limnology in Brazil. Rio de Janeiro: Brazilian Academy of Science/Brazilian Limnological Society. pp 59–104
Allan JD, Flecker AS. 1993. Biodiversity conservation in running waters. Bioscience 43:32–43
Araujo-Lima CARM, Forsberg BR, Victoria RL, Martinelli LA. 1986. Energy sources for detritivorous fishes in the Amazon. Science 234:1256–8
Bayley PB. 1989. Aquatic environments in the Amazon basin, with an analysis of carbon sources, fish production, and yield. Dodge DP, Ed. In: Proceedings of the international large rivers symposium. Ottawa: Canadian Special Publication in Fisheries and Aquatic Sciences 106:399–408
Bonetto AA. 1986. The Paraná River system. In: Davies BR, Walker KF, Eds. The ecology of river systems. Dordrecht: Dr. W. Junk Publishers. pp 541–55
Boon PI, Bunn SE. 1994. Variations in the stable isotope composition of aquatic plants and their implications for food web analysis. Aquat Bot 48:99–108
Bunn SE, Arthington AH. 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manage 30:492–507
Bunn SE, Davies PM, Winning M. 2003. Sources of organic carbon supporting the food web of an arid zone floodplain river. Freshw Biol 48:619–35
Cummins KW. 1973. Trophic relations of aquatic insects. Annu Rev Entomol 18:183–206
Delong MD, Thorp JH. 2006. Significance of instream autotrophs in trophic dynamics of the Upper Mississippi River. Oecologia 147:76–85
Depetris PJ, Kempe S. 1993. Carbon dynamics and sources in the Paraná River. Limnol Oceanogr 38:382–95
Dudgeon D. 2000. Large-scale hydrological changes in tropical Asia: prospects for riverine biodiversity. Bioscience 50:793–806
Dynesius M, Nilsson C. 1994. Fragmentation and flow regulation of river systems in the Northern Third of the World. Science 266:753–62
Elmoor-Loureiro LMA. 1997. Manual de Identificação de Cladóceras Límnicos do Brasil. Brasília: Universa
England LE, Rosemond AD. 2004. Small reductions in forest cover weaken terrestrial-aquatic linkages in headwater streams. Freshw Biol 49:721–34
Finlay JC. 2001. Stable-carbon-isotope ratios of river biota: implications for energy flow in lotic food webs. Ecology 82:1052–64
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK. 2005. Global consequences of land use. Science 309:570–4
Forsberg BR, Araujo-Lima CARM, Martinelli LA, Victoria RL, Bonassi JA. 1993. Autotrophic carbon sources for fish of the central Amazon. Ecology 74:643–52
Gomes LC, Miranda LE. 2001. Hydrologic and climatic regimes limit phytoplankton biomass in reservoirs of the Upper Paraná River Basin, Brazil. Hydrobiologia 457:205–14
Gore JA, Shields FD. 1995. Can large rivers be restored? Bioscience 45:142–52
Grey J, Jones RI, Sleep D. 2000. Stable isotope analysis of the origins of zooplankton carbon in lakes of differing trophic state. Oecologia 123:232–40
Hamilton SK, Lewis WM, Sippel SJ. 1992. Energy sources for aquatic animals in the Orinoco River floodplain: evidence from stable isotopes. Oecologia 89:324–30
Herwig BR, Soluk DA, Dettmers JM, Wahl DH. 2004. Trophic structure and energy flow in backwater lakes of two large floodplain rivers assessed using stable isotopes. Can J Fish Aquat Sci 61:12–22
Humphreys WF. 1979. Production and respiration in animal populations. J Anim Ecol 48:427–53
Huryn AD, Riley RH, Young RG, Arbuckle CJ, Peacock K. 2002. Natural-abundance stable C and N isotopes indicate weak upstream-downstream linkage of food webs in a grassland river. Archiv Fur Hydrobiol 153:177–96
Huryn AD, Riley RH, Young RG, Arbuckle CJ, Peacock K, Lyon G. 2001. Temporal shift in contribution of terrestrial organic matter to consumer production in a grassland river. Freshw Biol 46:213–26
Jackson RB, Carpenter SR, Dahm CN, McKnight DM, Naiman RJ, Postel SL, Running SW. 2001. Water in a changing world. Ecol Appl 11:1027–45
Jepsen DB, Winemiller KO. 2002. Structure of tropical river food webs revealed by stable isotope ratios. Oikos 96:46–55
Jepsen DB, Winemiller KO. 2007. Basin geochemistry and isotopic ratios of fishes and basal production sources in four neotropical rivers. Ecol Freshw Fish (in press)
Johnson BL, Richardson WB, Naimo TJ. 1995. Past, present, and future concepts in large river ecology. Bioscience 45:134–41
Junk WJ, Bayley PB, Sparks RE. 1989. The flood pulse concept in river-floodplain systems. Dodge DP, Eds. In: Proceedings of the international large rivers symposium. Ottawa: Canadian Special Publication in Fisheries and Aquatic Sciences 106:110–127
Lewis WM, Hamilton SK, Rodriguez MA, Saunders JF, Lasi MA. 2001. Foodweb analysis of the Orinoco floodplain based on production estimates and stable isotope data. Journal of the North American Benthological Society 20:241–254
Lowe-McConnell RH. 1987. Ecological studies in tropical fish communities. Cambridge: Cambridge University Press
MacLeod NA, Barton DR. 1998. Effects of light intensity, water velocity, and species composition on carbon and nitrogen stable isotope ratios in periphyton. Can J Fish Aquat Sci 55:1919–25
McCutchan Jr JH, Lewis Jr WM, Kendall C, McGrath CC. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–90
Mihuc TB. 1997. The functional trophic role of lotic primary consumers: generalist versus specialist strategies. Freshw Biol 37:455–62
Minagawa M, Wada E. 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between d15N and animal age. Geochim Cosmochim Acta 48:1135–40
Nilsson C, Reidy CA, Dynesius M, Revenga C. 2005. Fragmentation and flow regulation of the world’s large river systems. Science 308:405–8
Pease AA, Davis JJ, Edwards MS, Turner TF. 2006. Habitat and resource use by larval and juvenile fishes in an arid-land river (Rio Grande, New Mexico). Freshw Biol 51:475–86
Peterson BJ, Fry B. 1987. Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Phillips DL, Gregg JW. 2003. Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–9
Phillips DL, Newsome SD, Gregg JW. 2005. Combining sources in stable isotope mixing models: alternative methods. Oecologia 144:520–7
Post DM. 2002a. The long and short of food-chain length. Trends Ecol Evol 17:269–77
Post DM. 2002b. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–18
Postel SL, Daily GC, Erhlich PR. 1996. Human appropriation of renewable fresh water. Science 271:785–8
Pringle CM, Freeman MC, Freeman BJ. 2000. Regional effects of hydrologic alterations on riverine macrobiota in the New World: tropical—temperate comparisons. Bioscience 50:807–23
Rosenberg DM, McCully P, Pringle CM. 2000. Global-scale environmental effects of hydrological alterations: Introduction. Bioscience 50:746–51
Rosi-Marshall EJ, Wallace JB. 2002. Invertebrate food webs along a stream resource gradient. Freshw Biol 47:129–41
Santos-Silva EN. 2000. Revisão das espécies do “complexo nordestinus” (Wright, 1935) de Notodiaptomus Kiefer, 1936 (Copepoda: Calanoida: Diaptomidae). PhD Dissertation. Universidade de São Paulo, São Paulo
Santos-Silva EN, Robertson BA, Reid JW, Hardy ER. 1989. Atlas de copépodos planctônicos, Calanoida e Cyclopoida (Crustacea), da Amazônia Brasileira. I. Reprêsa de Curuá-Unu, Pará. Revista Brasiliera Zoologica 6:725–58
Sedell JR, Richey JE, Swanson FJ. 1989. The river continuum concept: a basis for the expected ecosystem behavior of very large rivers? Dodge DP, Ed. In: Proceedings of the international large rivers symposium. Ottawa: Canadian Special Publication in Fisheries and Aquatic Sciences 106:49–55
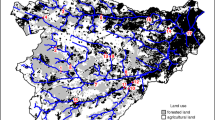
Souza MC, Romagnolo MB, Kita KK. 2004. Riparian vegetation: ecotones and plant communities. In: Thomaz SM, Agostinho AA, Hahn NS, editors. The Upper Paraná River and its floodplain: physical aspects, ecology and conservation. Leiden: Backhuys Publishers. pp 353–67
Tan FC, Edmond JM. 1993. Carbon isotope geochemistry of the Orinoco basin. Estuarine Coastal Shelf Sci 36:541–7
Thomaz SM, Agostinho AA, Hahn NS, Eds. 2004a. The Upper Paraná River and its Floodplain: Physical Aspects, Ecology and Conservation. Leiden: Backhuys Publishers
Thomaz SM, Bini LM, Pagioro TA, Murphy KJ, Santos AM, Souza DC. 2004b. Aquatic macrophytes: diversity, biomass and decomposition. In: Thomaz SM, Agostinho AA, Hahn NS, Eds. The Upper Paraná River and its floodplain: physical aspects, ecology and conservation. Leiden: Backhuys Publishers. pp 331–52
Thorp JH, Delong MD. 1994. The riverine productivity model: an heuristic view of carbon sources and organic processing in large river ecosystems. Oikos 70:305–8
Thorp JH, Delong MD. 2002. Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos 96:543–50
Thorp JH, Delong MD, Greenwood KS, Casper AF. 1998. Isotopic analysis of three food web theories in constricted and floodplain regions of a large river. Oecologia 117:551–63
Thorp JH, Thoms MC, Delong MD. 2006. The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Res Appl 22:123–47
Vanderklift MA, Ponsard S. 2003. Sources of variation in consumer-diet d15N enrichment: a meta-analysis. Oecologia 136:169–82
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. 1980. The river continuum concept. Can J Fish Aquat Sci 37:130–7
Ward JV, Stanford JA. 1983. The serial discontinuity concept of lotic ecosystems. In: Fontaine TD, Bartell SM, editors. Dynamics of lotic ecosystems. Ann Arbor: Ann Arbor Science. pp 29–42
Ward JV, Stanford JA. 1995. The serial discontinuity concept—extending the model to floodplain rivers. Regul Rivers Res Manage 10:159–68
Winemiller KO. 1990. Spatial and temporal variation in tropical fish trophic networks. Ecol Monogr 60:331–67
Winemiller KO. 1991. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr 61:343–65
ACKNOWLEDGEMENTS
Colleagues at the Research Nucleus in Limnology, Ichthyology and Aquaculture (Nupelia) at the State University of Maringá (UEM), Brazil, aided in field collections and identification of species, and allowed access to laboratory equipment, especially Evanilde Benedito-Cecilio, Claudia Bonecker, Luiz Gomes, Horácio Júlio Jr., Elaine Kashiwaqui, João Latini, Edson Okada, Fernando Pelicice, Pitágoras Piana and Sidinei Thomaz. Helpful comments and suggestions on earlier versions of this manuscript were provided by Steve Davis, Walter Dodds, Michelle Evans-White, Alexandre Garcia, Keith Gido, Luiz Gomes, Craig Layman, Dan Roelke, Deb Walks and Bob Wharton. Jaime Luiz helped prepare Figure 1. This study was supported in part by grants from the Society of Wetland Scientists (DJH) and Texas A&M University Office of Graduate Studies (DJH), a Mills Scholarship from the Texas Water Resources Institute (DJH), and NSF DEB #0089834 (KOW). The Upper Paraná River Floodplain is site 6 of the Brazilian Long Term Ecological Research Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoeinghaus, D.J., Winemiller, K.O. & Agostinho, A.A. Landscape-Scale Hydrologic Characteristics Differentiate Patterns of Carbon Flow in Large-River Food Webs. Ecosystems 10, 1019–1033 (2007). https://doi.org/10.1007/s10021-007-9075-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-007-9075-2