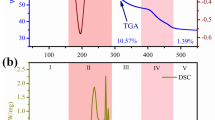
Abstract
The aim of the paper is to carry out a detailed investigation of the effect of graphene oxide-poly(amidoamine) (GO-PAMAM) incorporated in silica matrices on the corrosion behaviour of zinc. GO was modified with PAMAM dendrimer in order to improve its dispersion in the silica coatings prepared on zinc by dip-coating. Morpho-structural and physico-chemical characterization of the graphenes were made by FT-IR and Raman spectroscopy, TEM, and XRD. After incorporation, the effect of graphenes on the anti-corrosive performance of silica-coated zinc was investigated by electrochemical impedance spectroscopy. The results revealed that GO-PAMAM nanosheets dispersed in silica matrix significantly improved the corrosion resistance of the coatings. The polarization resistance Rp increased from 680 to 2489 kΩ cm2. The performances of SiO2-GO-PAMAM coatings were compared with those of SiO2 coatings incorporating GO, reduced graphene oxide (rGO), and 3-aminopropyl triethoxysilane (APTES)-modified GO. The influence of silica sol-ageing of the composite coatings was also investigated, as the polycondensation plays an important role in its anti-corrosive properties. The morphology of the composite coatings was examined by SEM, and their wettability by contact angle measurements. The protection offered by the SiO2-GO-PAMAM composite material is the leading. This can be associated with the GO-PAMAM’s high oxidation degree, low electrical conductivity, and by the fact that reduces the penetration of the electrolyte into the silica/zinc interface.















Similar content being viewed by others
Data availability
Authors can confirm that all relevant data are included in the article.
References
Ahmadi A, Ramezanzadeh B, Mahdavian M (2016) Hybrid silane coating reinforced with silanized graphene oxide nanosheets with improved corrosion protective performance. RSC Adv 59:54102–54112. https://doi.org/10.1039/C6RA04843A
Cotolan N, Varvara S, Albert E, Szabo G, Horvolgyi Z, Muresan L-M (2016) Evaluation of corrosion inhibition performance of silica sol–gel layers deposited on galvanised steel. Corros Eng Sci Technol 51:373. https://doi.org/10.1080/1478422X.2015.1120404
Li Y, Wu C, Xue M, Cai J, Huang Y, Yang H (2019) Preparation of sol-gel derived anticorrosive coating on Q235 carbon steel substrate with long-term corrosion prevention durability. Materials 12:1960. https://doi.org/10.3390/ma12121960
Xue B, Yu M, Liu J et al (2017) Corrosion protection of AA2024-T3 by sol-gel film modified with graphene oxide. J Alloys Compd 725:84–95. https://doi.org/10.1016/j.jallcom.2017.05.091
Albert E, Cotolan N, Nagy N, Gy S, Szabó G, Mureşan L, Hórvölgyi Z (2015) Mesoporous silica coatings with improved corrosion protection properties. Microporous Mesoporous Mater 206:102–113
Szabo G, Albert E, Both J, Kocs L, Gy S, Szoke A, Horvolgyi Z, Muresan LM (2019) Influence of embedded inhibitors on the corrosion resistance of zinc coated with mesoporous silica layers. Surf Interfaces 15:216–223. https://doi.org/10.1016/j.surfifin.2019.03.007
Pourhashem S, Vaezi MR, Rashidi A et al (2017) Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coating. Prog Org Coat 111:47–56. https://doi.org/10.1016/j.porgcoat.2017.05.008
Ramezanzadeh B, Niroumandrad S, Ahmadi A et al (2016) Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros Sci 103:283–304. https://doi.org/10.1016/j.corsci.2015.11.033
Ding R, Li W, Wang X et al (2018) A brief review of corrosion protective films and coatings on graphene and graphene oxide. J Alloys Compd 764:1039–1055. https://doi.org/10.1016/j.jallcom.2018.06.133
Nonahal M, Rastin H, Saeb MR et al (2018) Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings. Nonisothermal cure kinetics study. Prog Org Coat 114:233–243. https://doi.org/10.1016/j.porgcoat.2017.10.023
Kuila T, Bose S, Mishra AK et al (2012) Chemical functionalization of graphene and its applications. Prog Mater Sci 57:1061–1105. https://doi.org/10.1016/j.pmatsci.2012.03.002
Wang H, He Y, Fei G et al (2019) Functionalizing graphene with titanate coupling agents as reinforcement for one-component waterborne poly(urethane-acrylate) anticorrosion coatings. Chem Eng J 359:331–343. https://doi.org/10.1016/j.cej.2018.11.133
Othman NR, Yahya WZN, Ismail MC et al (2020) Highly dispersed graphene oxide–zinc oxide nanohybrids in epoxy coating with improved water barrier properties and corrosion resistance. J Coat Technol Res 17:101–114. https://doi.org/10.1007/s11998-019-00245-y
Yu Z, Di H, Ma Y et al (2015) Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings. Surf Coat Technol 276:471–478. https://doi.org/10.1016/j.surfcoat.2015.06.027
Yu Z, Lv L, Ma Y et al (2016) Covalent modification of graphene oxide by metronidazole for reinforced anti-corrosion properties of epoxy coatings. RSC Adv 6:18217–18226. https://doi.org/10.1039/C5RA23595B
Hosseinpour A, Abadchi MR, Mirzaee M et al (2021) Recent advances and future perspectives for carbon nanostructures reinforced organic coating for anti-corrosion application. Surf Interfaces 23:100994. https://doi.org/10.1016/j.surfin.2021.100994
Parhizkar N, Ramezanzadeh B, Shahrabi T (2018) Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl Surf Sci 439:45–59. https://doi.org/10.1016/j.apsusc.2017.12.240
Gholipour-Mahmoudalilou M, Roghani-Mamaqani H, Azimi R et al (2018) Preparation of hyperbranched poly (amidoamine)-grafted graphene nanolayers as a composite and curing agent for epoxy resin. Appl Surf Sci 428:1061–1069. https://doi.org/10.1016/j.apsusc.2017.09.237
Pogacean F, Socaci C, Pruneanu S et al (2015) Graphene based nanomaterials as chemical sensors for hydrogen peroxide—a comparison study of their intrinsic peroxidase catalytic behavior. Sens Actuators B Chem 213:474–483. https://doi.org/10.1016/j.snb.2015.02.124
Pruneanu S, Biris AR, Pogacean F et al (2015) The influence of uric and ascorbic acid on the electrochemical detection of dopamine using graphene-modified electrodes. Electrochim Acta 154:197–204. https://doi.org/10.1016/j.electacta.2014.12.046
Ovári TR, Katona G, Szabó G et al (2022) Electrochemical evaluation of the relationship between the thermal treatment and the protective properties of thin silica coatings on zinc substrates. Studia UBB Chemia 67:227–243. https://doi.org/10.24193/subbchem.2022.1.15
Ramos-Galicia L, Mendez LN, Martínez-Hernández AL et al (2013) Improved performance of an epoxy matrix as a result of combining graphene oxide and reduced graphene. Int J Polym Sci 2013:493147. https://doi.org/10.1155/2013/493147
Serodre T, Oliveira N, Miquita D et al (2019) Surface silanization of graphene oxide under mild reaction conditions. J Braz Chem Soc 30. https://doi.org/10.21577/0103-5053.20190167
Gu Y, Guo Y, Wang C et al (2017) A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery. Mater Sci Eng C 70:572–585. https://doi.org/10.1016/j.msec.2016.09.035
Ferrari AC, Basko DM (2013) Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat Nanotechnol 8:235–246. https://doi.org/10.1038/nnano.2013.46
Cançado LG, Takai K, Enoki T et al (2006) General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl Phys Lett 88:1–4. https://doi.org/10.1063/1.2196057
Stefan-van Staden RI, Gheorghe DC, Ilie-Mihai RM et al (2021) Stochastic biosensors based on N- and S-doped graphene for the enantioanalysis of aspartic acid in biological samples. RSC Adv 11:23301–23309. https://doi.org/10.1039/D1RA02066H
Ngo HT, Nguyen MTT, Do DP et al (2020) Low operating voltage resistive random access memory based on graphene oxide–polyvinyl alcohol nanocomposite thin films. J Sci: Adv Mater Devices 5:199–206. https://doi.org/10.1016/j.jsamd.2020.04.008
Pokhrel J, Bhoria N, Anastasiou S et al (2018) CO2 adsorption behavior of amine-functionalized ZIF-8, graphene oxide, and ZIF-8/graphene oxide composites under dry and wet conditions. Microporous Mesoporous Mater 267:53–67. https://doi.org/10.1016/j.micromeso.2018.03.012
Balzar D (1993) X-ray diffraction line broadening: modeling and applications to high-Tc superconductors. J Res Natl Inst Stand Technol 98:321. https://doi.org/10.6028/jres.098.026
Jun SC (2015) Fundamental of Graphene. In: Rashid bin Mohd Yusoff, A. (ed) Graphene-based energy devices, pp. 1–48. Hoboken, NJ, John Wiley & Sons. https://doi.org/10.1002/9783527690312.ch1
Li J, Cui J, Yang J et al (2016) Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection. Prog Org Coat 99:443–451. https://doi.org/10.1016/j.porgcoat.2016.07.008
Khaled KF, Atta AA, Abdel-Shafi NS (2014) A structure/function study of polyamidoamine dendrimer as a steel corrosion inhibitor. J Mater Environ Sci 5:831–840
Shuanger S, Zhiming Z, Liangmin Y (2017) Hydrophobic polyaniline/modified SiO2 coatings for anticorrosion protection. Synth Met 233:94–100. https://doi.org/10.1016/j.synthmet.2017.10.002
McDonagh C, Sheridan F, Butler T, MacCraith BD (1996) Characterisation of sol-gel-derived silica films. J Non-Cryst Solids 194:72–77. https://doi.org/10.1016/0022-3093(95)00488-2
Jiang Z, Jiang Z, Tian X et al (2014) Amine-functionalized holey graphene as a highly active metal-free catalyst for the oxygen reduction reaction. J Mater Chem A 2:441–450. https://doi.org/10.1039/C3TA13832A
Ramezanzadeh M, Ramezanzadeh B, Sari MG et al (2020) Corrosion resistance of epoxy coating on mild steel through polyamidoamine dendrimer-covalently functionalized graphene oxide nanosheets. J Ind Eng Chem 82:290–302. https://doi.org/10.1016/j.jiec.2019.10.025
Both J, Szabó G, Katona G et al (2022) Tannic acid reinforced sol-gel silica coatings for protection of zinc substrates. Mat Chem Phys 282:1–11. https://doi.org/10.1016/j.matchemphys.2022.125912
Funding
The present work has received financial support through the project: Entrepreneurship for innovation through doctoral and postdoctoral research, POCU/380/6/13/123886, co-financed by the European Social Fund, through the Operational Program for Human Capital 2014- 2020.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Tamara Ovari, Gabriella Szabo, G. Katona, and Maria Coros. The first draft of the manuscript was written by Tamara Ovari, and all authors commented on previous versions of the manuscript. The work was carried out under the supervision of Liana Maria Muresan, who elaborated the final version of the paper. All authors red and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ovari, TR., Katona, G., Coros, M. et al. Corrosion behaviour of zinc coated with composite silica layers incorporating poly(amidoamine)-modified graphene oxide. J Solid State Electrochem 27, 1795–1811 (2023). https://doi.org/10.1007/s10008-022-05358-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05358-w