Abstract
Bemcentinib is a newly developed AXL inhibitor that is currently under investigation in phase II trails for the treatment of acute myeloblastic leukemia (AML). Clinical and radiographic findings in this case were very similar to cases of MRONJ in patients receiving Sunitinib or other anti-angiogenetic substances, assuming that Bemcentinib may cause similar oral side effects. We present a male 81-year-old patient with a manifestation of alveolar bone necrosis at the central upper incisors following a 2-month regimen with the AXL-inhibitor Bemcentinib, administered for the treatment of secondary acute myeloblastic leukemia (sAML). Due to the duration of less than 8 weeks, the osteonecrosis was diagnosed as necrotizing periodontitis, but the intraoral clinical and radiographic findings were also compatible with the differential diagnosis of medication-related osteonecrosis of the jaw (MRONJ, stage II). Following to discontinuation of Bemcentinib, the affected bone was surgically revised including the removal of a demarcated bone sequester under preventive antibiotic treatment (metronidazole 400 mg t.i.d.). We hypothesize that Bemcentinib might increase the susceptibility for osteonecrosis of the jaw, probably related to its antiangiogenic effects and the resulting modulation of host immune response. Based on the current observations, it can be assumed that oro-dental health might be significant also prior and during treatment with Bemcentinib for the prevention of MRONJ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The idea of developing an extensive arsenal of active substances for targeted and individual cancer therapy has produced an array of anti-angiogenetic active substances, e.g., Sunitinib [1,2,3,4], that might induce medication-related osteonecrosis of the jaw as a side effect. Bemcentinib (BGB 324), currently under investigation in phase II trails (ClinicalTrials.gov/Identifier: NCT03965494, NCT03824080, NCT03654833, NCT03649321, NCT03184571, NCT03184558), is targeting AXL with additional antiangiogenic effects [5]. Herein, we report a case of medication-related osteonecrosis of the upper jaw after Bemcentinib regimen for the treatment of secondary acute myeloblastic leukemia. Clinical and radiographic findings were very similar to cases of MRONJ as previously described using Sunitinib [1,2,3] or other anti-angiogenetic substances [6, 7]. We show clinical findings and review the literature.
Case presentation
An 81-year-old male patient with a diagnosis of secondary acute myeloblastic leukemia (sAML) treated with the AXL inhibitor Bemcentinib for 2 months as part of a phase II trail was referred to the outpatient clinic of the Department of Conservative Dentistry and Periodontology, University Hospital Munich due to exposed alveolar bone of the upper anterior jaw persisting for approximately 4–5 weeks in June 2019. Despite discontinuation of AXL inhibitor therapy immediately upon first manifestation, the bone exposure remained unaffected. Besides general fatigue, the patient reported occasional pain in the area of exposure during mastication, whereas other signs of inflammation, such as fever or lymphadenopathy, were not observed. Secondary findings were atrial fibrillation, hypertension, aortic aneurysm, and cholelithiasis. Antihypertensive therapy was conducted with bisoprolol (1.25 mg o.d.), torasemid (10 mg o.d.), and candesartan (8 mg o.d.). In addition, the patient received apixaban (2.5 mg b.i.d.) for the prevention of ischemic stroke. According to anamnesis, the patient was currently not undergoing dental treatment and no oral examination had been performed prior to initiation of treatment with Bemcentinib. Preliminary clinical or radiological dental findings were not available. The patient did never receive radiation therapy of the head and neck region.
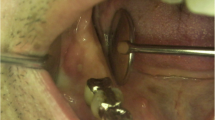
Clinical examination, including a standardized dental examination, as well as a localized periodontal examination, including the central maxillary incisors, was carried out. In order to avoid excessive bleeding resulting from AML-associated thrombopenia and drug-related anticoagulation, a periodontal examination of the remaining dentition was not conducted. However, a generalized accumulation of hard and soft dental plaque reflected the patient’s inadequate oral hygiene; thus, a pre-existing periodontal disease could not be excluded. Focusing on the maxillary anterior dentition, the interdental gingival tissue of the central upper incisors presented with significant ulceration causing extensive exposure of the crestal parts of the interdental bone. The margins of the soft tissue defect showed minor swelling and redness. The exposed bone appeared necrotic due to grey discoloration and was mobile upon gentle probing indicating partial demarcation and sequestration. Both incisors showed gingival recession defects > 6 mm at the mesial aspects of the tooth root (Fig. 1a, b). The probing pocket depth was 12 mm at the mesio-buccal and the mesio-palatal sites. Apical radiograph of the relevant area revealed a c-shaped radiolucent area of 5 mm in the middle third of the interdental osseous tissue between the central incisors compatible with a zone of demarcation (Fig. 1i). Based on the clinical and radiographic signs and the overall duration of only 4–5 weeks, a diagnosis of necrotizing periodontitis (NP) with the differential diagnosis of MRONJ (stage II) according to the AAOMS [4] and the indication for the surgical removal of the osseous sequester was made.
a, b Intraoral situation with exposed bone between incisors 11 and 21. c Surgically depicted sequestrum. d Situation after sequestrotomy. e, f Intraoral situation 7-day follow-up. g, h Intraoral situation 36-day follow-up. i X-ray of incisors 11, 21. j Surgically removed sequestrum, divided oro-vestibularly
Due to the aforementioned thrombopenia (platelet 50 × 103/μl), the patient received platelet concentrate 2 h preoperatively in order to improve hemostasis during the surgical procedure and to prevent postoperative secondary bleeding. Afterwards, the sequestrotomy was performed under local anesthesia (1.7 ml articain hydrochloride 4% with epinephrine 1:200,000) followed by subgingival scaling using curettes of the adjacent root surface (Fig. 1c, d) and suturing in order to approach wound margins and to ease coagulation. Immediately after completion of the surgical treatment, both central maxillary incisors presented with significantly increased mobility. To prevent further impairment, temporary splint using a resin-based composite was placed (Fig. 1e). One day preoperatively, the patient received metronidazole (400 mg t.i.d.) which was continued upon completion of wound closure after 7 days. After uneventful healing, except slight residual gingival swelling, there were no signs of inflammation detectable (Fig. 1f). At final clinical visit, 5-week postoperative gingival swelling had entirely ceased and the gingival recession increased 2 mm at both incisors (Fig. 1g, h). Histopathological examination of the surgically removed tissue depicted in Fig. 1j confirmed the clinical findings by revealing necrotic bone with inflammatory cell infiltrate and gram-positive, rod-shaped bacteria (Fig. 2a–d).
Discussion and conclusions
AXL receptor tyrosine kinase—effects and inhibition
Bemcentinib (BGB324) is an inhibitor of the AXL receptor whose therapeutic effect on sAML is currently under investigation in clinical phase II trials [8, 9]. The AXL receptor tyrosine kinase is a member of the Tyro-3, AXL, and Mer (TAM) subfamily that can be almost ubiquitously found within many different tissues [5, 10,11,12,13]. Upon Gas6 activation, AXL mediates cell proliferation and migration through Ras/Raf/MEK/ERK and the Src signaling pathway as well as cell survival by S6, AKT, or JNK [10, 13, 14]. Regarding the immune system, AXL mediates efferocytosis, a reduced TLR-dependent inflammatory response, and natural killer cell activity [12, 14]. In the tumor immune microenvironment, AXL is often overexpressed by dendritic cells, NK cells, or macrophages, promoting epithelial to mesenchymal transition, tumor angiogenesis, resistance to chemotherapeutic and targeted agents, and decreased antitumor immune response [5, 13, 15, 16]. AXL activation in dendritic cells inhibits TLR and TLR-induced cytokine-receptor cascades [12] and reduces dendritic cell activity as well as complement T cell checkpoint cascade [16]. Activation of the tumor-associated macrophage receptor AXL by Gas6 or Protein S has shown to shift macrophage polarization, inducing pro-tumor m2 polarization and inhibiting pro-inflammatory m1 polarization [11]. Several studies confirm a TAM-related downregulation of m1 polarization leading to decreased secretion of pro-inflammatory cytokines such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [17,18,19,20,21]. Moreover, another study demonstrated increased expression levels of these cytokines in Gas6 or Protein S-inhibition models [20]. On the other hand, AXL signaling increases the polarization of m2-like macrophages. Under physiological conditions, the m2 phenotype is involved in maintenance of tissue homeostasis, consolidation of inflammatory processes, and wound healing through efferocytosis, angiogenesis, and secretion of anti-inflammatory cytokines [11, 14, 22, 23]. In the tumor microenvironment, however, m2-like functions are overexpressed via TAM regulation, ultimately leading to tumor angiogenesis [22, 24], impaired anti-tumor immunity, tumor growth, and metastasis [23, 25].
AXL inhibitors such as Bemcentinib counteract these numerous effects. By inhibiting the formerly altered macrophage polarization mentioned above, angiogenesis is impaired and the previously inhibited secretion of pro-inflammatory cytokines is increased consequently. These two effects might present a possible link to the complex pathogenesis of periodontal disease as occurred in this case. Actually, the aforementioned cytokines IL-1β and TNF-α are two key marker molecules in periodontitis that are known to play a crucial role in the development and maintenance of inflammatory tissue degradation and alveolar bone resorption [26, 27]. Interacting synergistically, they stimulate the expression of the receptor activator of nuclear factor-κB ligand (RANKL) on the surface of osteoblasts and stroma cells, thus promoting osteoclast formation and corresponding bone loss [28]. Additionally, the Bemcentinib-induced inhibition of angiogenesis in conjunction with the increased inflammatory component could favor a rapid necrotic course of periodontitis.
Diagnosis—necrotizing periodontitis or medication-related osteonecrosis of the jaw
Necrotizing periodontal conditions as observed in the present case are of infectious etiology and strongly associated with impairment of the host immune system [29]. According to the current classification for periodontal and peri-implant diseases and conditions [29], necrotizing periodontitis (NP) is an acute inflammatory process of the periodontium characterized by necrosis/ulcer of the interdental papilla, gingival bleeding, pain, and rapid bone loss [30, 31]. As the clinical findings of this case were consistent with all these characteristics, the working diagnosis necrotizing periodontitis was adjusted accordingly.
However, considering the numerous aforementioned effects of Bemcentinib on signaling cascades and the associated anti-angiogenic and ultimately pro-inflammatory effects, the possible influence of the AXL inhibitor on the periodontal condition herein must be taken into account when determining a suspect diagnosis. Hence, the differential diagnosis of a medication-related osteonecrosis of the jaw was considered.
According to the 2014 AAOMS position paper, MRONJ is defined as “exposed bone or bone that can be probed through an intraoral or extraoral fistula(e) in the maxillofacial region that has persisted for more than eight weeks” in a patient that has currently or previously been treated with antiresorptive or antiangiogenic agents and shows no history of radiation therapy or obvious metastasis of the jaws [4].
Herein, there has been recorded no history of radiation therapy or obvious metastasis of the jaws and the AXL inhibitor Bemcentinib clearly has antiangiogenic effects but the exposure of jawbone did not persist for more than 8 weeks at the time of diagnosis. Currently, there exists considerably controversy on the AAOMS criteria and the definition as published by the International Task Force on ONJ in 2015 [32] specifically regarding the diagnosis of early stages and non-exposed cases of MRONJ that might precede clinical and radiographic evident sequestration and/or exposure of the jawbone [33]. Accordingly, one might argue that the necrosis of the alveolar bone as seen in the present case was already existent unperceived well before being clinically noticeable for the first time.
Moreover, histological analysis of the surgically removed bone clearly revealed colonization with gram-positive, rod-shaped bacteria. Regarding BRONJ, the colonization of the affected parts of the alveolar bone specifically with gram-positive, rod-shaped Actinomyces species is highly prevalent and was thus proposed to play a central etiologic role [34]. Apart from the microscopic analysis, bacteria have not been, however, further characterized in the present case.
Considerations and conclusion
Regarding the pathogenesis of bone necrosis associated with MRONJ, different etiologic models have been intensively discussed considering mainly the effects of bisphosphonates [1, 4]. Currently, an over-suppression of bone turnover [4, 35,36,37] as well as the necrosis as final result of infection in combination with attenuation of the proliferation of various cell types which are centrally involved into the immune response are discussed [4, 38]. Moreover, MRONJ has been considered to result from ischemia triggered by medication-related antiangiogenic effects [4, 36], a general toxicity of the medication [4, 39,40,41], or medication-induced shifts in pH [42]. Oversuppression of bone turnover, comparable to osteoclast suppression by bisphosphonates or inhibition of RANK ligand mediated by Denosumab [1, 38, 43], is not yet known for the AXL inhibitor Bemcentinib. On the other hand, the above mentioned hypothesis of necrosis as the result of an underlying infection with an additional drug-related alteration of immune response from which a MRONJ may develop [34] seems just as conceivable as an impaired angiogenesis. In particular with respect to the previously mentioned shift of macrophage polarization by AXL and the assumed opposite shift by inhibition of AXL receptor, an excessive immune reaction induced by increased secretion of pro-inflammatory cytokines (m1-like phenotype induced) and simultaneously inhibited angiogenesis and efferocytosis (m2-like phenotype induced) is a possible mechanism of osteonecrosis of the jaw.
Furthermore, cellular toxicity caused by previously administered cytostatics might also play a role through modulation of the immune defense mechanisms, facilitating the necrosis. Nevertheless, it has to be emphasized that the necrosis developed within a comparably short period after the onset of the Bemcentinib regimen. Therefore, the AXL inhibitor might be at least the ultimate promoting factor. Finally, a reduction of the pH value might have been mediated through excessive inflammation and represents another possible mechanism of necrosis [42].
Taken together, it can be concluded that an irreversible necrosis and exposure of parts of the alveolar bone have developed under a short regimen of Bemcentinib in the present case consistent with the commonly accepted pathogenesis of MRONJ.
Oro-dental infections, especially periodontal disease, have been shown to significantly increase the risk for the development of MRONJ. Based on the present clinical case, we strongly recommend meticulous preventive dental measures also prior and during the administration of AXL inhibitors such as Bemcentinib.
Data Availability
Not applicable.
Abbreviations
- μl:
-
microliter
- AAOMS:
-
American Association of Oral and Maxillofacial Surgeons
- AML:
-
Acute myeloblastic leukemia
- b.i.d.:
-
Bis in die
- BGB 324:
-
Bemcentinib
- BRONJ:
-
Bisphosphonate-related osteonecrosis of the jaw
- Fig.:
-
Figure
- h:
-
Hour(s)
- H&E:
-
Haematoxylin and eosin stain
- IL-1β:
-
Interleukin-1 beta
- IL-6:
-
Interleukin-6
- LMU:
-
Ludwig-Maximilians-Universität
- mg:
-
Milligram
- ml:
-
Milliliter
- mm:
-
Millimeter
- MRONJ:
-
Medication-related osteonecrosis of the jaw
- NK cells:
-
Natural killer cells
- NP:
-
Necrotizing periodontitis
- o.d.:
-
Omni die
- RANKL:
-
Receptor activator of nuclear factor-κB ligand
- sAML:
-
Secondary acute myeloblastic leukemia
- t.i.d:
-
Ter in die
- TAM:
-
Tyro-3, Axl and Mer receptor tyrosine kinases
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-α
References
Agrillo A, Nastro Siniscalchi E, Facchini A, Filiaci F, Ungari C (2012) Osteonecrosis of the jaws in patients assuming bisphosphonates and sunitinib: two case reports. Eur Rev Med Pharmacol Sci 16(7):952–957
Nicolatou-Galitis O, Migkou M, Psyrri A, Bamias A, Pectasides D, Economopoulos T, Raber-Durlacher JE, Dimitriadis G, Dimopoulos MA (2012) Gingival bleeding and jaw bone necrosis in patients with metastatic renal cell carcinoma receiving sunitinib: report of 2 cases with clinical implications. Oral Surg Oral Med Oral Pathol Oral Radiol 113(2):234–238
Fleissig Y, Regev E, Lehman H (2012) Sunitinib related osteonecrosis of jaw: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol 113(3):e1–e3
Medication-related osteonecrosis of the Jaw 2014 Update [https://www.aaoms.org/docs/govt_affairs/advocacy_white_papers/mronj_position_paper.pdf]
Tanaka M, Siemann DW (2019) Axl signaling is an important mediator of tumor angiogenesis. Oncotarget 10(30):2887–2898
Antonuzzo L, Lunghi A, Petreni P, Brugia M, Laffi A, Giommoni E, Mela MM, Mazzoni F, Balestri V, Costanzo FD (2017) Osteonecrosis of the jaw and angiogenesis inhibitors: a revival of a rare but serous side effect. Curr Med Chem 24(28):3068–3076
Nicolatou-Galitis O, Kouri M, Papadopoulou E, Vardas E, Galiti D, Epstein JB, Elad S, Campisi G, Tsoukalas N, Bektas-Kayhan K et al (2019) Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review. Supportive Care in cancer : official Journal of the Multinational Association of Supportive Care in Cancer 27(2):383–394
Ben-Batalla I, Schultze A, Wroblewski M, Erdmann R, Heuser M, Waizenegger JS, Riecken K, Binder M, Schewe D, Sawall S et al (2013) Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood 122(14):2443–2452
Janning M, Ben-Batalla I, Loges S (2015) Axl inhibition: a potential road to a novel acute myeloid leukemia therapy? Expert Rev Hematol 8(2):135–138
Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G (2014) Diversification of TAM receptor tyrosine kinase function. Nat Immunol 15(10):920–928
Myers KV, Amend SR, Pienta KJ (2019) Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer 18(1):94
Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G (2007) TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131(6):1124–1136
Antony J, Huang RY (2017) AXL-driven EMT state as a targetable conduit in cancer. Cancer Res 77(14):3725–3732
Zhu C, Wei Y, Wei X (2019) AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer 18(1):153
Gay CM, Balaji K, Byers LA (2017) Giving AXL the axe: targeting AXL in human malignancy. Br J Cancer 116(4):415–423
Akalu YT, Rothlin CV, Ghosh S (2017) TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev 276(1):165–177
Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC (2010) TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol 87(5):869–875
Shibata T, Habiel DM, Coelho AL, Hogaboam CM (2014) Axl receptor blockade protects from invasive pulmonary aspergillosis in mice. Journal of immunology (Baltimore, Md : 1950) 193(7):3559–3565
Deng T, Zhang Y, Chen Q, Yan K, Han D (2012) Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology 135(1):40–50
Fukazawa T, Hiraiwa N, Umemura T, Mise-Omata S, Obata Y, Doi T (2015) Egress of mature murine regulatory T cells from the thymus requires RelA. Journal of immunology (Baltimore, Md : 1950) 194(7):3020–3028
Shen Y, Cui X, Rong Y, Zhang Z, Xiao L, Zhou T, Chen W (2016) Exogenous Gas6 attenuates silica-induced inflammation on differentiated THP-1 macrophages. Environ Toxicol Pharmacol 45:222–226
Kodelja V, Muller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S (1997) Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology 197(5):478–493
Werfel TA, Cook RS (2018) Efferocytosis in the tumor microenvironment. Semin Immunopathol 40(6):545–554
Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM (2014) Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17(1):109–118
Vaught DB, Stanford JC, Cook RS: Efferocytosis creates a tumor microenvironment supportive of tumor survival and metastasis. Cancer cell & microenvironment 2015, 2(1).
Afacan B, Ozturk VO, Pasali C, Bozkurt E, Kose T, Emingil G (2019) Gingival crevicular fluid and salivary HIF-1alpha, VEGF, and TNF-alpha levels in periodontal health and disease. J Periodontol 90(7):788–797
Aral K, Milward MR, Kapila Y, Berdeli A, Cooper PR (2020) Inflammasomes and their regulation in periodontal disease: a review. J Periodontal Res
Hienz SA, Paliwal S, Ivanovski S (2015) Mechanisms of bone resorption in periodontitis. J Immunol Res 2015:615486
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F et al (2018) Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 89(Suppl 1):S173–s182
Holmstrup P, Plemons J, Meyle J (2018) Non-plaque-induced gingival diseases. J Clin Periodontol 45(Suppl 20):S28–s43
Herrera D, Retamal-Valdes B, Alonso B, Feres M (2018) Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Clin Periodontol 45(Suppl 20):S78–s94
Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O'Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S et al (2015) Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. Journal of Bone and Mineral Research : the official journal of the American Society for Bone and Mineral Research 30(1):3–23
Otto S, Pautke C, Van den Wyngaert T, Niepel D, Schiødt M (2018) Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev 69:177–187
De Ceulaer J, Tacconelli E, Vandecasteele SJ (2014) Actinomyces osteomyelitis in bisphosphonate-related osteonecrosis of the jaw (BRONJ): the missing link? European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology 33(11):1873–1880
Orriss IR, Key ML, Colston KW, Arnett TR: Inhibition of osteoblast function in vitro by aminobisphosphonates. Journal of cellular biochemistry 2009, 106(1):109-118 %@ 0730-2312.
Walter C, Klein MO, Pabst A, Al-Nawas B, Duschner H, Ziebart T (2010) Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin Oral Investig 14(1):35–41
Ristow O, Gerngross C, Schwaiger M, Hohlweg-Majert B, Kehl V, Jansen H, Hahnefeld L, Otto S, Pautke C (2014) Is bone turnover of jawbone and its possible over suppression by bisphosphonates of etiologic importance in pathogenesis of bisphosphonate-related osteonecrosis? Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 72(5):903–910
Katsarelis H, Shah NP, Dhariwal DK, Pazianas M (2015) Infection and medication-related osteonecrosis of the jaw. J Dent Res 94(4):534–539
Ayllon J, Launay-Vacher V, Medioni J, Cros C, Spano JP, Oudard S (2009) Osteonecrosis of the jaw under bisphosphonate and antiangiogenic therapies: cumulative toxicity profile? Annals of oncology : official journal of the European Society for Medical Oncology 20(3):600–601
Christodoulou C, Pervena A, Klouvas G, Galani E, Falagas ME, Tsakalos G, Visvikis A, Nikolakopoulou A, Acholos V, Karapanagiotidis G et al (2009) Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 76(3):209–211
Landesberg R, Cozin M, Cremers S, Woo V, Kousteni S, Sinha S, Garrett-Sinha L, Raghavan S (2008) Inhibition of oral mucosal cell wound healing by bisphosphonates. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 66(5):839–847
Otto S, Hafner S, Mast G, Tischer T, Volkmer E, Schieker M, Sturzenbaum SR, von Tresckow E, Kolk A, Ehrenfeld M et al (2010) Bisphosphonate-related osteonecrosis of the jaw: is pH the missing part in the pathogenesis puzzle? Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 68(5):1158–1161
Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K et al (2000) T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-$$. Nature 408(6812):600
Acknowledgments
Open Access funding provided by Projekt DEAL. We would like to thank the Institute of Pathology of the LMU Munich for a precise histopathological examination of the osseous specimen and the corresponding high-qualitiy photomicrographs.
Author information
Authors and Affiliations
Contributions
CVB, MF, UCW designed and wrote the paper, CVB performed the examination and surgery. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bumm, C.V., Folwaczny, M. & Wölfle, U.C. Necrotizing periodontitis or medication-related osteonecrosis of the jaw (MRONJ) in a patient receiving Bemcentinib—a case report. Oral Maxillofac Surg 24, 353–358 (2020). https://doi.org/10.1007/s10006-020-00851-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-020-00851-w