Abstract
Objective
Macrophages show extreme heterogeneity and different subsets have been characterized by their activation route and their function. For instance, macrophage subsets are distinct by acting differently under pathophysiological conditions such as inflammation and cancer. Macrophages also contribute to angiogenesis, but the role of various specific subsets in angiogenesis has not been thoroughly investigated.
Methods and results
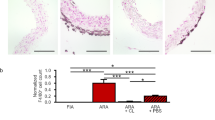
Matrigel supplemented with macrophage subsets [induced by IFNγ (M1), IL-4 (M2a) or IL-10 (M2c)] was injected subcutaneously in C57BL/6 J mice and analyzed by CD31 staining after 14 days. Increased numbers of endothelial cells and tubular structures were observed in M2-enriched plugs compared to control and other subsets. Additionally, more tubular structures formed in vitro in the presence of M2 macrophages or their conditioned medium. To identify a mechanism for the pro-angiogenic effect, gene expression of angiogenic growth factors was analyzed. Induced expression of basic fibroblast growth factor (Fgf2), insulin-like growth factor-1 (Igf1), chemokine (C–C motif) ligand 2 (Ccl2) and placental growth factor (Pgf) was observed in M2 macrophages. Using a blocking antibody of PlGF to inhibit M2c induced angiogenesis resulted in mildly reduced (40 %) tube formation whereas neutralization of FGF-2 (M2a) signaling by sFGFR1-IIIc affected tube formation by nearly 75 %.
Conclusions
These results indicate that macrophages polarized towards an M2 phenotype have a higher angiogenic potential compared to other subsets. Furthermore, we propose FGF signaling for M2a- and PlGF signaling for M2c-induced angiogenesis as possible working mechanisms, yet, further research should elucidate the exact mechanism for M2-induced angiogenesis.





Similar content being viewed by others
References
Virmani R et al (2005) Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 25(10):2054–2061
Khurana R et al (2005) Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 112(12):1813–1824
Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145(3):341–355
Sluimer JC et al (2008) Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol 51(13):1258–1265
Xue L, Greisler HP (2002) Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. Surgery 132(2):259–267
Xiong M et al (1998) Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol 153(2):587–598
Schulze-Osthoff K et al (1990) In situ detection of basic fibroblast growth factor by highly specific antibodies. Am J Pathol 137(1):85–92
Pakala R, Watanabe T, Benedict CR (2002) Induction of endothelial cell proliferation by angiogenic factors released by activated monocytes. Cardiovasc Radiat Med 3(2):95–101
Polverini PJ et al (1977) Activated macrophages induce vascular proliferation. Nature 269(5631):804–806
Sunderkotter C et al (1991) Macrophage-derived angiogenesis factors. Pharmacol Ther 51(2):195–216
Gratchev A et al (2006) Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology 211(6–8):473–486
Stout RD et al (2005) Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175(1):342–349
Wolfs IM, Donners MM, de Winther MP (2011) Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost 106(5):763–771
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3(1):23–35
Mantovani A et al (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686
Mantovani A et al (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Kodelja V et al (1997) Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology 197(5):478–493
Lin EY et al (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66(23):11238–11246
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4(1):71–78
Murdoch C et al (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8(8):618–631
Sica A et al (2008) Macrophage polarization in tumour progression. Semin Cancer Biol 18(5):349–355
De Palma M et al (2003) Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med 9(6):789–795
Kanters E et al (2004) Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood 103(3):934–940
Dirkx AE et al (2006) Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol 80(6):1183–1196
Zhang X et al (2006) Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 281(23):15694–15700
He H et al (2012) Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood 120(15):3152–3162
Murakami M et al (2011) FGF-dependent regulation of VEGF receptor 2 expression in mice. J Clin Invest 121(7):2668–2678
Jih YJ et al (2001) Distinct regulation of genes by bFGF and VEGF-A in endothelial cells. Angiogenesis 4(4):313–321
Taraboletti G et al (2002) Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 160(2):673–680
Presta M et al (2005) Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev 16(2):159–178
Anghelina M et al (2004) Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev 13(6):665–676
Fantin A et al (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116(5):829–840
Lamagna C, Aurrand-Lions M, Imhof BA (2006) Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol 80(4):705–713
Rolny C et al (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19(1):31–44
Lewis C, Murdoch C (2005) Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol 167(3):627–635
Leek RD et al (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56(20):4625–4629
Coffelt SB et al (2010) Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res 70(13):5270–5280
Porta C et al (2009) Cellular and molecular pathways linking inflammation and cancer. Immunobiology 214(9–10):761–777
De Palma M et al (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8(3):211–226
Ribatti D, Levi-Schaffer F, Kovanen PT (2008) Inflammatory angiogenesis in atherogenesis–a double-edged sword. Ann Med 40(8):606–621
Khallou-Laschet J et al (2010) Macrophage plasticity in experimental atherosclerosis. PLoS ONE 5(1):e8852
Stout RD, Suttles J (2004) Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol 76(3):509–513
Acknowledgments
This work was supported by the Netherlands Heart Foundation (Dr. E Dekker post-doctoral fellow Grant [Grant Numbers 2007T034, 2012T079] to Dr. Donners; Dr. E Dekker Established Investigator Grant [Grant Number 2007T067] and NWO-VIDI Grant [Grant Number 917.066.329] to Dr. de Winther). Dr Post is supported by Grants from BMM (PENT, iValve) and CTMM/Netherland Heart Foundation (EMINENCE): These research programs of the BioMedical Materials institute and the Center for Translational and Molecular Medicine are co-funded by the Dutch Ministry of economic affairs Agriculture and Innovation.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10456_2013_9381_MOESM1_ESM.tif
Supplemental figure 1. Gene expression profile of macrophage subsets after 24h polarization. After 8 days of culturing, macrophages are polarized with IFNγ, IL-4 or IL-10 (n=3). A, IFNγ stimulation results in increased expression of Nos2, Tnfa and Il10 (C). B, IL-4 stimulation results in upregulation of Chi3l3, Arg1 and Mrc1 (C) whereas IL-10 stimulates expression of Il10 and Mrc1(C). The statistical significance was determined by one-way ANOVA. **p<0.01. (TIFF 71 kb)
10456_2013_9381_MOESM2_ESM.tif
Supplemental figure 2. Fluorescently labeled macrophages (red) and endothelial cells (green) in a Matrigel plug. A, Co-localization of macrophages and endothelial cells is apparent in vivo. B, Microvessels are surrounded by macrophages. (TIFF 151 kb)
10456_2013_9381_MOESM3_ESM.tif
Supplemental figure 3. Inhibition of PlGF signaling using soluble Flt-1 in a tube formation assay. sFlt-1 reduced tube formation of endothelial cells alone (-M, 34.7%), endothelial cells co-cultured with M0 macrophages (27.4%) or M2c macrophages (44.5 %) compared to control. The data represent the mean ± SEM. The statistical significance was determined by one-way ANOVA. (TIFF 39 kb)
10456_2013_9381_MOESM4_ESM.tif
Supplemental figure 4. ELISA for PlGF. Concentration of PlGF protein was measured in concentrated conditioned medium of macrophage subsets. A trend could be observed towards more PlGF production in M2c macrophages. The data represent the mean ± SEM. (TIFF 35 kb)
Rights and permissions
About this article
Cite this article
Jetten, N., Verbruggen, S., Gijbels, M.J. et al. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17, 109–118 (2014). https://doi.org/10.1007/s10456-013-9381-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-013-9381-6