Abstract
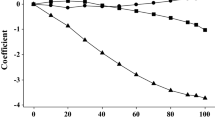
A few different theoretical methods for assigning the partial atomic charges were benchmarked for calculation of the hydrophilic/lipophilic index (HLI). The coefficients were selected to produce the best correlation of the HLI values with the experimental octanol-water partition. Different parameters were checked in calculations of partial charges to get the best performance of the HLI values obtained. Thus, four partitioning schemes (Coulson, Mulliken, Merz-Kollman, Ford-Wang) were benchmarked for calculations of atomic charges with six semiempirical methods (AM1, PM3, RM1, PM6, PM6-D3H4, PM7). Moreover, five distinct types of partial atomic charges (Mulliken, Hirshfeld, Löwdin, CHELPG, NPA), obtained at the Hartree–Fock and DFT levels of theory with three basis sets, were tested for their ability to produce the HLI values with the best correlation to experimental logP coefficients of 50 mono-charged organic anions. In the case of the semiempirical methods, the best correlation between the HLI and logP values (the correlation coefficient r = 0.9216) was obtained with the AM1 Ford–Wang parametric electrostatic potential charges. The Mulliken and Coulson charges calculated with the PM7 method can be used as an alternative to AM1, with the r values of 0.9107 and 0.8984, respectively. In the case of the DFT, the PBE/def2-TZVP natural population analysis charges produce the best correlation (r = 0.9220). Nevertheless, in spite of a marginally lower performance (r = 0.9159), the NPA charges computed at the PBE/def2-SVP level are more robust and can be regarded as the optimum choice for calculating the HLI values.

The hydrophilic/lipophilic index (HLI)



Similar content being viewed by others
References
Leo A, Hansch C, Elkins D (1971) Partition coefficients and their uses. Chem Rev 71:525–616
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2012) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 64:4–17
Hawrył AM, Popiołek ŁP, Hawrył MA, Świeboda RS, Niejedlia MA (2015) Chromatographic and calculation methods for analysis of the lipophilicity of newly synthesized thiosemicarbazides and their cyclic analogues 1,2,4-triazol-3-thiones. J Braz Chem Soc 26:1617–1624
Barzanti C, Evans R, Fouquet J, Gouzin L, Howarth NM, Kean G, Levet E, Wang D, Wayemberg E, Yeboah AA, Kraft A (2007) Potentiometric determination of octanol–water and liposome–water partition coefficients (logP) of ionizable organic compounds. Tetrahedron Lett 48:3337–3341
Remko M (2010) Molecular structure, pKa, lipophilicity, solubility and absorption of biologically active aromatic and heterocyclic sulfonamides. J Mol Struc THEOCHEM 944:34–42
Zou J-W, Zhao W-N, Shang Z-C, Huang M-L, Guo M, Yu Q-S (2002) A quantitative structure-property relationship analysis of logP for disubstituted benzenes. J Phys Chem A 106:11550–11557
Bannan CC, Calabró G, Kyu DY, Mobley DL (2016) Calculating partition coefficients of small molecules in octanol/water and cyclohexane/water. J Chem Theory Comput 12:4015–4024
Chen C-S, Lin S-T (2016) Prediction of pH effect on the octanol−water partition coefficient of ionizable pharmaceuticals. Ind Eng Chem Res 55:9284–9294
Jones MR, Brooks BR, Wilson AK (2016) Partition coefficients for the SAMPL5 challenge using transfer free energies. J Comput Aided Mol Des 30:1129–1138
Kah M, Brown CD (2008) LogD: lipophilicity for ionisable compounds. Chemosphere 72:1401–1408
Martel S, Begnaud F, Schuler W, Gillerat F, Oberhauser N, Nurisso A, Carrupt P-A (2016) Limits of rapid logP determination methods for highly lipophilic and flexible compounds. Anal Chim Acta 915:90–101
Nieto-Draghi C, Fayet G, Creton B, Rozanska X, Rotureau P, de Hemptinne J-C, Ungerer P, Rousseau B, Adamo C (2015) A general guidebook for the theoretical prediction of physicochemical properties of chemicals for regulatory purposes. Chem Rev 115:13093–13164
Mannhold R, Poda GI, Ostermann C, Tetko IV (2009) Calculation of molecular lipophilicity: state-of-the-art and comparison of logP methods on more than 96,000 compounds. J Pharm Sci 98:861–893
Dapson RW (2013) Alternative methods for estimating common descriptors for QSAR studies of dyes and fluorescent probes using molecular modeling software. 1. Concepts and procedures. Biotech Histochem 88:477–488
Dapson RW, Horobin RW (2013) Alternative methods for estimating common descriptors for QSAR studies of dyes and fl uorescent probes using molecular modeling software. 2. Correlations between logP and the hydrophilic/lipophilic index, and new methods for estimating degrees of amphiphilicity. Biotech Histochem 88:489–497
Mulliken RS (1955) Electronic population analysis on LCAOMO molecular wave functions. I. J Chem Phys 23:1833–1840
Mulliken RS (1962) Criteria for the construction of good self consistent field molecular orbital wave functions, and the significance of LCAOMO population analysis. J Chem Phys 36:3428–3439
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. WIREs Comput Mol Sci 2:1–42
Wang B, Ford GP (1994) Atomic charges derived from a fast and accurate method for electrostatic potentials based on modified AM1 calculations. J Comput Chem 15:200–207
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J Comput Chem 11:361–373
Besler BH, Merz Jr KM, Kollman PA (1990) Atomic charges derived from semiempirical methods. J Comput Chem 11:431–439
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138
Löwdin P-O (1970) On the nonorthogonality problem. Adv Quantum Chem 5:185–199
Cusachs LC, Politzer P (1968) On the problem of defining the charge on an atom in a molecule. Chem Phys Lett 1:529–531
Mayer I (2004) Lowdin population analysis is not rotationally invariant. Chem Phys Lett 393:209–212
Bruhn G, Davidson ER, Mayer I, Clark AE (2006) Lowdin population analysis with and without rotational invariance. Int J Quantum Chem 106:2065–2072
Löwdin P-O (1955) Quantum theory of many-particle systems. I. Physical interpretations by means of density matrices, natural spin-orbitals, and convergence problems in the method of configurational interaction. Phys Rev 97:1474–1489
Coulson CA, Longuet-Higgins HC (1947) The electronic structure of conjugated systems. II. Unsaturated hydrocarbons and their hetero-derivatives. Proc Roy Soc A 192:16–32
Chirgwin BH, Coulson CA (1950) The electronic structure of conjugated systems. VI. Proc Roy Soc A 201:196–209
Leahy DE (1986) Intrinsic molecular volume as a measure of the cavity term in linear solvation energy relationships: octanol-water partition coefficients and aqueous solubilities. J Pharm Sci 75:629–636
Leahy DE, Taylor PJ, Wait AR (1989) Model solvent systems for QSAR. Part I. Propylene glycol dipelargonate (PGDP). A new standard solved for use in partition coefficient determination. Quant Struct-Act Relat 8:17–31
Leahy DE, Morris JJ, Taylor PJ, Wait AR (1992) Model solvent systems for QSAR. Part 3. An LSER analysis of the ‘critical quartet.’ New light on hydrogen bond strength and directionality. J Chem Soc Perkin Trans 2:705–722
Leo AJ (1993) Calculating log Poct from structures. Chem Rev 93:1281–1306
Leo A (1983) The octanol-water partition coefficient of aromatic solutes: the effect of electronic interactions, alkyl chains, hydrogen bonds, and ortho-substitution. J Chem Soc Perkin Trans 2:825–838
Leo AJ (1991) Calculating the hydrophobicity of chlorinated hydrocarbon solutes. Sci Total Environ 109–110:121–130
Paley O (2014) Cetylpyridinium chloride. Synlett 25:599–600
Sigma-Aldrich Chemie GmbH (2018) Sodium benzoate, safety data sheet, version 5.1. Revision date 19.09.2014. https://www.sigmaaldrich.com
Balon K, Riebesehl BU, Müller BW (1999) Drug liposome partitioning as a tool for the prediction of human passive intestinal absorption. Pharm Res 16:882–888
Sigma-Aldrich Chemie GmbH, Sodium xylenesulfonate, Safety Data Sheet, Version 5.1 Revision Date 20.05.2013
Schwarrenbach RP, Stierli R, Folsom BR, Zeyer J (1988) Compound properties relevant for assessing the environmental partitioning of nitrophenols. Environ Sci Technol 22:83–92
Takacs-Novak K, Jozan M, Szasz G (1995) Lipophilicity of amphoteric molecules expressed by the true partition coefficient. Int J Pharm 113:47–55
Terada H, Inagi T (1977) Partition of methyl orange between H2O and n-octanol. Membranes 2:63–68
Sigma-Aldrich Chemie GmbH (2018) Sodium p-toluenesulfonate, safety data sheet, version 5.1. Revision date 06.02.2013. https://www.sigmaaldrich.com
Sigma-Aldrich Chemie GmbH (2018) 2-Mercaptopyridine N-oxide sodium salt, safety data sheet, version 5.0. Revision date 03.08.2012. https://www.sigmaaldrich.com
DHI, Milieu Ltd., Protection Through Knowledge Ltd. (2018) Review of annex IV of the regulation No. 1907/2006 (REACH). Evaluation of existing entries, p 113. http://ec.europa.eu/environment/chemicals/reach/pdf/6b_appendix_2.pdf
Lewis KA, Tzilivakis J, Warner D, Green A (2016) An international database for pesticide risk assessments and management. Hum Ecol Risk Assess 22:1050–1064
Ying G-G (2006) Fate, behavior and effects of surfactants and their degradation products in the environment. Environ Int 32:417–431
Fizer M, Slivka M (2016) Synthesis of [1,2,4]triazolo[1,5-a]pyrimidine (microreview). Chem Heterocycl Compd 52:155–157
Fizer MM, Slivka MV, Lendel VG (2013) New method of synthesis of 3,5,6,7-tetrahydro-[1,2,4]triazolo[1,5-a] pyrimidine-2(1H)-thione. Chem Heterocycl Compd 49:1243–1245
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc 107:3902–3909
Stewart JJP (1989) Optimization of parameters for semiempirical methods II. Applications. J Comput Chem 10:221–264
Rocha GB, Freire RO, Simas AM, Stewart JJP (2006) RM1: a reparameterization of AM1 for H, C, N, O, P, S, F, Cl, Br, and I. J Comput Chem 27:1101–1111
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104-1–154104-19
Řezáč J, Hobza P (2012) Advanced corrections of hydrogen bonding and dispersion for semiempirical quantum mechanical methods. J Chem Theory Comput 8:141–151
Stewart JJP (2013) Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J Mol Model 19:1–32
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Becke AD (1993) A new mixing of Hartree–Fock and local density functional theories. J Chem Phys 98:1372–1377
Schaefer A, Horn H, Ahlrichs R (1992) Fully optimized contracted Gaussian basis sets for atoms li to Kr. J Chem Phys 97:2571–2577
Schaefer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms li to Kr. J Chem Phys 100:5829–5835
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Zheng J, Xu X, Truhlar DG (2011) Minimally augmented Karlsruhe basis sets. Theor Chem Accounts 128:295–305
Neese F (2003) An improvement of the resolution of the identity approximation for the calculation of the coulomb matrix. J Comput Chem 24:1740–1747
Izsak R, Neese F (2011) An overlap fitted chain of spheres exchange method. J Chem Phys 135:144105-1–144105-11
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4:17
Rappe AK, Casewit CJ, Colwell KS, Goddard IIIWA, Skiff WM (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114:10024–10035
Stewart JJP (2016) MOPAC2016. Stewart Computational Chemistry, Colorado Springs http://OpenMOPAC.net
Neese F (2012) The ORCA program system. WIREs Comput Mol Sci 2:73–78
Nikolaienko TY, Bulavin LA, Hovorun DM (2014) JANPA: an open source crossplatform implementation of the natural population analysis on the java platform. Comput Theor Chem 1050:15–22
Advanced Chemistry Development Inc. (2015) ADC/Chemsketch (freeware). Advanced Chemistry Development Inc., Toronto, Canada http://www.acdlabs.com
Fizer M, Sukharev S, Slivka M, Mariychuk R, Lendel V (2016) Preparation of bisthiourea and 5-Amino-4-benzoyl-1,2,4-triazol-3-thione complexes of copper(II), nickel and zinc and their biological evolution. J Organomet Chem 804:6–12
Bevziuk K, Chebotarev A, Snigur D, Bazel Y, Fizer M, Sidey V (2017) Spectrophotometric and theoretical studies of the protonation of Allura red AC and Ponceau 4R. J Mol Struct 1144:216–224
Cao J, Ren Q, Chen F, Lu T (2015) Comparative study on the methods for predicting the reactive site of nucleophilic reaction. Sci China Chem 58:1845–1852
Fizer M, Sidey V, Tupys A, Ostapiuk Y, Tymoshuk O, Bazel Y (2017) On the structure of transition metals complexes with the new tridentate dye of thiazole series: theoretical and experimental studies. J Mol Struct 1149:669–682
Acknowledgments
M.F. would like to thank DSc. James J. P. Stewart for providing the program MOPAC2016 and for helpful suggestions. O.F. acknowledges support from the International Visegrad Fund (ID 51700627).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 203 kb)
Rights and permissions
About this article
Cite this article
Fizer, O., Fizer, M., Sidey, V. et al. Benchmark of different charges for prediction of the partitioning coefficient through the hydrophilic/lipophilic index. J Mol Model 24, 141 (2018). https://doi.org/10.1007/s00894-018-3692-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3692-x